Back to Journals » Orthopedic Research and Reviews » Volume 16
3D Printing for Personalized Solutions in Cervical Spondylosis
Authors Wu LN, Zhang ZF, Li RJ, Xin DQ, Wang JF
Received 10 July 2024
Accepted for publication 7 October 2024
Published 17 October 2024 Volume 2024:16 Pages 251—259
DOI https://doi.org/10.2147/ORR.S486438
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Professor Clark Hung
Li-Na Wu, Zhi-Feng Zhang, Ru-Jun Li, Da-Qi Xin, Jun-Feng Wang
Orthopaedic Clinical Research Center, The Second Affiliated Hospital of Inner Mongolia Medical University, Hohhot, Inner Mongolia Autonomous Region, People’s Republic of China
Correspondence: Da-Qi Xin; Jun-Feng Wang, The Second Affiliated Hospital of Inner Mongolia Medical University, No. 59, Horqin South Road, Saihan District, Hohhot, Inner Mongolia Autonomous Region, People’s Republic of China, Email [email protected]; [email protected]
Abstract: In the context of the digital revolution, 3D printing technology brings innovation to the personalized treatment of cervical spondylosis, a clinically common degenerative disease that severely impacts the quality of life and increases the economic burden of patients. Although traditional surgeries, medications, and physical therapies are somewhat effective, they often fail` to meet individual needs, thus affecting treatment adherence and outcomes. 3D printing, with its customizability, precision, material diversity, and short production cycles, shows tremendous potential in the treatment of cervical spondylosis. This review discusses the multiple applications of 3D printing in the treatment of cervical spondylosis, including the design, manufacture, and advantages of 3D-printed cervical collars, the role of 3D models in clinical teaching and surgical simulation, and the application of 3D-printed scaffolds and implants in cervical surgery. It also discusses the current challenges and future directions.
Keywords: 3D printing, cervical spondylosis, cervical collar, orthopedic implants, biomaterials
A Letter to the Editor has been published for this article.
Introduction
Cervical spondylosis is a common global spinal disease, primarily caused by degenerative changes in the cervical intervertebral discs, osteophyte growth, and joint degeneration. Not only does it manifest symptoms such as neck pain, upper limb numbness, nausea, and dizziness,1 but it also leads to radiculopathy and myelopathy,2 severely affecting patients’ quality of life and functional abilities. Long-term pain may lead to psychological issues such as anxiety and depression, profoundly affecting patients and their families. With aging populations and changes in modern lifestyles, the incidence of cervical spondylosis is on the rise and showing trends of affecting younger populations.2
The causes of cervical spondylosis are diverse and complex, including age-related structural changes to the cervical spine and disc degeneration. Additionally, long-term poor posture, repetitive occupational injuries, congenital spinal stenosis, and participation in strenuous activities may accelerate the progression of the disease.3 Diagnosis depends on clinical symptoms and radiological aids. Patients may exhibit symptoms such as discomfort behind the eye sockets and reduced neck mobility. When the spinal cord and nerve roots are affected, pain and numbness may radiate to the shoulders and arms.4 Although magnetic resonance imaging is highly sensitive in detecting spinal pathologies, many asymptomatic individuals also show degenerative anomalies on it scans, so it should not be the primary diagnostic tool.5 X-ray films are usually sufficient for initial examinations, while computed tomography combined with intrathecal contrast injection can more accurately assess the sites and extent of nerve compression.6
The treatment of cervical spondylosis includes both surgical and conservative methods, which aim to relieve pain, improve symptoms, and restore cervical function. Surgical treatments are generally indicated for patients who do not respond to conservative management or who present with neurological deficits or spinal cord compression. The natural progression of myelopathic cervical spondylosis tends to worsen over time,7 so early surgery is recommended. However, surgical procedures, such as foraminotomy, anterior cervical discectomy and fusion, laminectomy, or laminoplasty, can be invasive and carry inherent risks. Moreover, these procedures are not universally suitable for all types of cervical spondylosis and may not meet the specific needs of every patient.6 Pharmacological treatments, including the use of steroids to reduce inflammation, NSAIDs for pain relief, and anticonvulsants or antidepressants for neuropathic pain,8 are effective but often result in significant side effects. Consequently, long-term use of these medications is not advisable. Physical therapies such as traction help alleviate nerve compression, while acupuncture, massage, and electrotherapy can relieve pain and activate muscles. Sling exercise training, proprioceptive neuromuscular facilitation, and muscle energy techniques can increase muscle strength, improve flexibility, and enhance mobility.9 However, the lengthy treatment duration and high costs limit many patients’ accesses to treatment and long-term benefits. The complexity and heterogeneity of cervical spondylosis further complicate treatment effectiveness, underscoring the need for more personalized approaches. In this context, 3D printing technology offers substantial promise due to its customizability, precision, material diversity, and rapid production capabilities.10 These features allow for more tailored treatment solutions that can potentially address the limitations of existing methods and improve patient outcomes.
This review aims to systematically expound on the applications of 3D printing technology in the treatment of cervical spondylosis, including the customization of cervical supports, surgical applications of bone implants, clinical teaching and surgery simulation through 3D models, analysis of material selection, and future prospects, providing references for clinicians to understand and apply this new technology, promoting the application and development of 3D printing technology in the treatment of cervical spondylosis, and ultimately enhancing treatment outcomes and quality of life for patients.
Introduction to 3D Printing Technology and Its Application in the Medical Field
Development of 3D Printing Technology
3D printing, also known as additive manufacturing, is a process that creates three-dimensional solid objects from a digital model by adding material layer by layer.11 The primary steps include 3D modeling, slicing, printing, and post-processing. Based on the materials and techniques used, 3D printing can be divided into Fused Deposition Modeling (FDM), Stereolithography (SLA), Selective Laser Sintering (SLS), and Direct Ink Writing (DIW).10 FDM is known for its cost-effectiveness, using heated polymers to construct objects. However, its use of thermosetting materials limits sterilization processes, making it unsuitable for surgical applications and more commonly used for patient-specific drug delivery.12 SLA and SLS utilize lasers to solidify or sinter materials, providing higher precision,13 and making them suitable for creating sterilizable implant materials.14 DIW is highly versatile, employing a syringe-like device to extrude gel materials, making it adaptable for various medical applications.15 For a comprehensive overview of these 3D printing methods, their development, and medical applications, please refer to Figure 1.10
Since its inception in the 1980s with stereolithography, 3D printing’s application in the medical field has expanded to include the creation of surgical models, personalized implants, and tissue engineering scaffolds.10 The rise of 3D printing technology is attributed to its capability in complex and precise manufacturing; the diversity of materials has broadened its application scope, and the decreasing cost of 3D printing has made it more prevalent.
Applications of 3D Printing Technology in the Medical Field
3D printing has become a leading manufacturing technology in the medical field, revolutionizing tissue engineering, regenerative medicine, the pharmaceutical industry, and rehabilitation sectors.16 Specific applications include: (1) Bioprinting of tissues and organs.17 Bioprinting technology holds the potential for future breakthroughs in organ transplants, currently applied to skin, bone implants, and soft tissues.18,19 (2) Surgical planning and simulation. 3D printed anatomical bio-similar models assist surgeons in understanding lesion sites, formulating detailed surgical plans, and conducting preoperative simulations to shorten surgery time and optimize outcomes, especially in vascular diseases, cardiac conditions, and oncological surgeries.20,21 (3) Medical education and training. Color-printed models enable more intuitive and realistic learning and training for students and medical staff, enhancing educational effectiveness and training quality.22,23 (4) Drug development and customization. 3D printing is used for tissue models in drug discovery, allowing precise control over drug dosage and release rates.24,25 (5) Customized medical devices. Personalized medical devices, prosthetics, and rehabilitation braces are fabricated based on specific patient needs and anatomical structures.26–29
Application of 3D Printing in the Treatment of Cervical Spondylosis
Personalized 3D Printed Cervical Collars
The human cervical spine is the most flexible but least stable joint. Repetitive movements beyond the normal range can lead to mechanical compression, increasing the risk of secondary injuries.11 Wearing a cervical collar effectively provides support, alleviates pain and discomfort, improves the biomechanical load distribution between intervertebral discs, and promotes proper posture and functional recovery.30–32 However, common cervical collars often fail to fully meet patient needs. 3D printed cervical collars are designed and manufactured based on the morphological and pathological characteristics of the patient’s cervical spine through 3D scanning, modeling, and printing technologies. 3D scanning technologies like structured light capture the patient’s neck shape in a natural standing or sitting position. This method offers more accurate cervical spine representations compared to CT scans, which are taken in a supine position. Combining with CT data enhances the precision of 3D-printed models and devices.33 Cervical collars are beneficial in perioperative periods, acute trauma, and the management of chronic degenerative diseases.34,35 Compared to common cervical collars, 3D printed personalized cervical collars offer numerous advantages, including precise fit to the patient’s cervical spine morphology, enhanced comfort, precise control of the mechanical properties for personalized stable support, lightweight design to reduce the burden on the wearer, breathable material choices to avoid overheating and skin discomfort, and the ability to rapidly modify and produce based on changes in the patient’s condition.36 Figure 2 indicates that 3D printed cervical collars can improve patient compliance, ensuring continuity of treatment and enhancing overall satisfaction.
The design and manufacturing of personalized 3D-printed cervical collars require multidisciplinary collaboration, tailored to the individual anatomical and pathological features of a patient’s cervical spine to create comfortable, effective, and safe personalized brace. The process begins with data collection and processing. Data can be gathered using 3D scanning technology, which captures the shape of the patient’s neck non-invasively to generate a high-precision 3D model. Common 3D scanning technologies include structured light scanning and laser scanning, known for their speed, high precision, and non-invasiveness.37 Imaging data is then registered with 3D scanning data to create a comprehensive 3D model that includes both the internal and external morphology of the cervical spine. Based on this, virtual design and simulation are carried out to assess the fit, support effectiveness, and range of motion restriction of the collar. During the design, cervical spine biomechanical analysis is conducted. Professional CAD software and finite element analysis algorithms help in designing supports that conform to ergonomics and biomechanical principles, optimizing the collar design scheme.38,39
3D Printing Models, Devices, and Implants for Cervical Spine Surgery
Accurate 3D printed models of the cervical spine can assist in pre-surgical visualization and planning, providing surgeons with accurate simulation models that offer tactile feedback on varying bone densities.40 These models aid in understanding disc herniation, nerve root or spinal cord compression, and joint fusion. They help determine the surgical approach and enable simulations to ensure optimal screw trajectories and precise implant placement,41 reducing intraoperative risks and enhancing surgical success rates.42,43
3D-printed pedicle screw guides improve the accuracy and number of screw placements,44 define safe screw trajectories, reduce vascular or nerve damage, shorten surgery time, and lessen radiation exposure,45 and are suitable for upper, middle, and lower cervical spine surgeries.46–48 In cases of cervical spine disease with cancer, preoperative use of 3D models to assess the extent of the tumor and its proximity to critical structures is highly valuable.49
Customized 3D printed implants enhance biological deficiencies for patients with abnormal anatomical structures or spinal deformities, avoiding damage to specific anatomical features, thus limiting trauma and complications.50 The porosity and mechanical properties of 3D printed implants are comparable to cancellous bone, maximizing the reduction of stress shielding, and the microporous ultrastructure is conducive to bone induction, thus effectively preventing displacement or settlement.51 3D printing has improved the current manufacturing processes of implants and orthopedic surgeries, making significant advancements in printing bone graft substitutes and cartilage materials,52 especially suitable for complex oncological pathologies and atypical bone defects.50 Figure 3 shows the 3D printed models and implants used for cervical spine surgery.
In summary, 3D printing technology improves the treatment of cervical spondylosis in many aspects, including prevention, surgery, and protection. Figure 4 summarizes the applications of 3D printing in the prevention and treatment of cervical spondylosis.
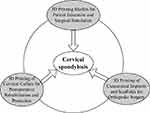 |
Figure 4 The Role of 3D Printing Technology in the Prevention and Treatment of Cervical Spondylosis. |
Challenges and Future Prospects of 3D Printing
While 3D printing offers promising avenues for personalized treatment in cervical spondylosis, it also presents several challenges. Table 1 below outlines the main advantages and limitations observed in the use of this technology. 3D printing technology presents challenges in terms of material selection and process optimization. While the variety of available 3D printing biomaterials is extensive, they still cannot fully replicate the complex structures and functions of human tissues. To address this, researchers are developing multi-material printing technologies and more advanced bio-ink formulations to better mimic the heterogeneity of human tissues.10 The development of high-performance biomaterials requires not only appropriate chemical and mechanical properties but also biocompatibility and bioactivity. This necessitates a comprehensive consideration of various parameters to find the most suitable printable materials for biomedical applications.53,54
 |
Table 1 Advantages and Limitations of 3D Printing in Cervical Spondylosis Treatment |
Polylactic Acid exhibits good mechanical properties,55,56 and compressive strength similar to bone,57 often used for manufacturing rigid orthotics and support braces. However, the release of lactic acid byproducts during degradation may cause tissue inflammation, leading to poor long-term biocompatibility.58 One solution is to combine polylactic acid with calcium phosphate to neutralize the acidity and buffer within physiological pH ranges.59 Although acrylonitrile butadiene styrene has high strength and toughness, its biocompatibility is poor and it is non-degradable.60 Improvements can be made by surface treatment or coating with biocompatible materials such as polyvinyl alcohol or polylactic acid.61 Polycaprolactone is low-cost, with good rheological and viscoelastic properties, providing support for tissue regeneration or healing.62 It is an appropriate material for producing scaffolds, though its biodegradation time is lengthy. Its degradation rate can be adjusted by adding enzymes.63 Ceramic materials are widely used in orthopedic surgery due to their mineral-like compatibility with bone.64 However, current 3D printing methods are mainly limited to direct ceramic printing. A potential solution is to use ceramic materials as powder additives in other materials.65 Hydrogels, capable of absorbing and retaining significant amounts of water, exhibit good biocompatibility and minimal immune response elicitation.66 However, hydrogels exhibit poor stability, which can be enhanced through chemical and photo-crosslinking.67 Bio-inks containing cells or biochemical molecules can affect cell growth, proliferation, and differentiation,64 but cells in bio-inks may face reduced survival rates during the printing and crosslinking process.68 Cell viability can be improved using extrusion-based bioprinting technologies.69
Future research needs to delve deeper into the interactions between 3D-printed implants and human organs and tissues to better serve clinical needs. Multi-material printing technologies will enable 3D-printed cervical collars and orthopedic implants to have more complex functions and superior performance.70 The application of shape-memory alloys is expected to enable adaptive adjustment and precise control of support intensity in cervical collars, aiding tissue repair in implants.71 Integrated sensors in cervical collars can monitor cervical activity, muscle status, and physiological parameters in real-time, providing crucial data support for personalized treatment plans.71 Combined with artificial intelligence and big data technologies, a comprehensive evaluation system can be established, customizing optimal brace design and rehabilitation plans based on patient data, thereby enhancing treatment outcomes and quality of life for patients.72 Furthermore, the personalized customization inherent in 3D printing technology requires stringent quality control standards. Machine learning methods can be effectively employed to model and predict surface roughness in additive manufacturing processes, significantly enhancing quality control and ensuring greater accuracy and improved outcomes in the production of these customized medical models.73 Integrating response surface methodology with machine learning techniques has also shown promise in optimizing manufacturing and surface coating processes. This approach can improve the biocompatibility and mechanical properties of 3D-printed bone plates, contributing to the development of safer and more effective orthopedic implants.74 Aspect of benefits and limitations are listed in Table 1.
Conclusion
3D printing technology, with its personalization and precision, offers new hope for the prevention and treatment of cervical spondylosis. Personalized cervical collars better meet patient needs, providing effective support and correction functions, while 3D-printed implants and surgical models increase the success rate and safety of surgeries. However, the application of 3D printing technology in the treatment of cervical spondylosis still faces challenges due to the insufficient diversity of biomaterials. Future research will focus on developing higher-performance biomaterials, multi-material printing technologies, integrating artificial intelligence and big data technologies to promote the widespread application of 3D printing technology in the treatment of cervical spondylosis, improving patient treatment outcomes and quality of life.
Funding
This work was supported by the “Grassland Talent” High-Level Talent Program of Inner Mongolia (Grant No. CYYC012068) and the Inner Mongolia Autonomous Region Science and Technology Plan (Grant No. 2021GG0138).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Jitin B. Cervical spondylosis and atypical symptoms. Neurol India. 2021;69(3):602–603. doi:10.4103/0028-3886.319240
2. Theodore N. Degenerative cervical spondylosis. N Engl J Med. 2020;383(2):159–168. doi:10.1056/NEJMra2003558
3. Singh S, Kumar D, Kumar S. Risk factors in cervical spondylosis. J Clinl Orthopaedics Trauma. 2014;5(4):221–226. doi:10.1016/j.jcot.2014.07.007
4. Srivastav Y, Prajapati A, Kumar M, Verma A. A short overview of cervical spondylosis, including its diagnosis and current treatment strategies. J Adv Med Med Res. 2023;35(22):170–188. doi:10.9734/jammr/2023/v35i225258
5. Brinjikji W, Luetmer PH, Comstock B, et al. Systematic literature review of imaging features of spinal degeneration in asymptomatic populations. Am J Neuroradiol. 2015;36(4):811–816. doi:10.3174/ajnr.A4173
6. Kuo DT, Tadi P. Cervical Spondylosis. In: StatPearls. StatPearls Publishing; 2024.
7. Takagi I, Eliyas JK, Stadlan N. Cervical spondylosis: an update on pathophysiology, clinical manifestation, and management strategies. Disease-a-Month. 2011;57(10):583–591. doi:10.1016/j.disamonth.2011.08.024
8. Mazanec D, Reddy A. Medical management of cervical spondylosis. Neurosurgery. 2007;60(1 Supp1 1):S43–50.
9. Chen Q, Wang Z, Zhang S. Exploring the latest advancements in physical therapy techniques for treating cervical spondylosis patients: a narrative review. Biomol Biomed. 2023;23(5):752–759. doi:10.17305/bb.2023.9049
10. Pugliese R, Beltrami B, Regondi S, Lunetta C. Polymeric biomaterials for 3D printing in medicine: an overview. Anna 3D Printed Medicine. 2021;2:100011. doi:10.1016/j.stlm.2021.100011
11. McCormick JR, Sama AJ, Schiller NC, Butler AJ, Donnally CJ. Cervical spondylotic myelopathy: a guide to diagnosis and management. J Am Board Family Med. 2020;33(2):303–313. doi:10.3122/jabfm.2020.02.190195
12. Long J, Gholizadeh H, Lu J, Bunt C, Seyfoddin A. Application of fused deposition modelling (FDM) method of 3D printing in drug delivery. Curr. Pharm. Des. 2017;23(3):433–439. doi:10.2174/1381612822666161026162707
13. Malik HH, Darwood AR, Shaunak S, et al. Three-dimensional printing in surgery: a review of current surgical applications. J Surg Res. 2015;199(2):512–522. doi:10.1016/j.jss.2015.06.051
14. Garg B, Mehta N. Current status of 3D printing in spine surgery. J Clin Orthop Trauma. 2018;9(3):218–225. doi:10.1016/j.jcot.2018.08.006
15. Saadi M, Maguire A, Pottackal NT, et al. Direct ink writing: a 3D printing technology for diverse materials. Adv Mater. 2022;34(28):2108855. doi:10.1002/adma.202108855
16. Liaw CY, Guvendiren M. Current and emerging applications of 3D printing in medicine. Biofabrication. 2017;9(2):024102. doi:10.1088/1758-5090/aa7279
17. Pennarossa G, Arcuri S, De Iorio T, Gandolfi F, Brevini TA. Current advances in 3D tissue and organ reconstruction. Int J Mol Sci. 2021;22(2):830. doi:10.3390/ijms22020830
18. Kadakia RJ, Wixted CM, Allen NB, Hanselman AE, Adams SB. Clinical applications of custom 3D printed implants in complex lower extremity reconstruction. 3D Print Med. 2020;6(1):29. doi:10.1186/s41205-020-00083-4
19. Sutradhar A, Park J, Carrau D, Nguyen TH, Miller MJ, Paulino GH. Designing patient-specific 3D printed craniofacial implants using a novel topology optimization method. Med Biol Eng Comput. 2016;54(7):1123–1135. doi:10.1007/s11517-015-1418-0
20. Silberstein JL, Maddox MM, Dorsey P, Feibus A, Thomas R, Lee BR. Physical models of renal malignancies using standard cross-sectional imaging and 3-dimensional printers: a pilot study. Urology. 2014;84(2):268–272. doi:10.1016/j.urology.2014.03.042
21. Zein NN, Hanouneh IA, Bishop PD, et al. Three-dimensional print of a liver for preoperative planning in living donor liver transplantation. Liver Transpl. 2013;19(12):1304–1310. doi:10.1002/lt.23729
22. Loke YH, Harahsheh AS, Krieger A, Olivieri LJ. Usage of 3D models of tetralogy of Fallot for medical education: impact on learning congenital heart disease. BMC Med Educ. 2017;17(1):54. doi:10.1186/s12909-017-0889-0
23. Sun Z. Clinical applications of patient-specific 3D printed models in cardiovascular disease: current status and future directions. Biomolecules. 2020;10(11):1577. doi:10.3390/biom10111577
24. Peng W, Datta P, Ayan B, Ozbolat V, Sosnoski D, Ozbolat IT. 3D bioprinting for drug discovery and development in pharmaceutics. Acta Biomater. 2017;57:26–46. doi:10.1016/j.actbio.2017.05.025
25. Ma X, Liu J, Zhu W, et al. 3D bioprinting of functional tissue models for personalized drug screening and in vitro disease modeling. Adv Drug Deliv Rev. 2018;132:235–251. doi:10.1016/j.addr.2018.06.011
26. Said S, Boulkaibet I, Sheikh M, Karar AS, Alkork S, Naït-Ali A. Machine-learning-based muscle control of a 3D-printed bionic arm. Sensors. 2020;20(11):3144. doi:10.3390/s20113144
27. Chen YJ, Lin H, Zhang X, Huang W, Shi L, Wang D. Application of 3D-printed and patient-specific cast for the treatment of distal radius fractures: initial experience. 3D Print Med. 2017;3(1):11. doi:10.1186/s41205-017-0019-y
28. Wang K, Shi Y, He W, et al. The research on 3D printing fingerboard and the initial application on cerebral stroke patient’s hand spasm. Biomed Eng Online. 2018;17(1):92. doi:10.1186/s12938-018-0522-4
29. Mo S, Leung SHS, Chan ZYS, et al. The biomechanical difference between running with traditional and 3D printed orthoses. J Sports Sci. 2019;37(19):2191–2197. doi:10.1080/02640414.2019.1626069
30. Verbiest H. The management of cervical spondylosis. Neurosurgery. 1973;20(Supplement 1):262–294. doi:10.1093/neurosurgery/20.CN_suppl_1.262
31. Unlu Z, Cerrahoğlu L, Aslan A, Tarhan S. Efficacy of collar treatment for patients with cervical spondylatrosis complaining of vertigo. J Phys Therap Sci. 2001;13(2):115–120. doi:10.1589/jpts.13.115
32. Kumar Y, Sinha AK, Kumar R, Kataria C. Soft vs Philadelphia collar: a short term effect on pain, gait and balance in people with cervical spondylosis; 2019.
33. Zhang S. High-speed 3D shape measurement with structured light methods: a review. Opt Lasers Eng. 2018;106:119–131. doi:10.1016/j.optlaseng.2018.02.017
34. Brannigan JFM, Mowforth OD, Francis JJ, Budu A, Laing RJ, Davies BM. Hard collar immobilisation following elective surgery on the cervical spine: a cross-sectional survey of UK spinal surgeons. Br J Neurosurg. 2022;36(5):627–632. doi:10.1080/02688697.2022.2087861
35. Freeman J, Nikjou D, Maloney J, et al. The role of orthoses in chronic axial spinal conditions. Curr Pain Headache Rep. 2024;28(6):501–506. doi:10.1007/s11916-024-01233-7
36. Choo YJ, Boudier-Revéret M, Chang MC. 3D printing technology applied to orthosis manufacturing: narrative review. Anna Palliat Med. 2020;9(6):4262270–4264270. doi:10.21037/apm-20-1185
37. Haleem A, Javaid M. 3D scanning applications in medical field: a literature-based review. Clin Epidemiol Global Health. 2019;7(2):199–210. doi:10.1016/j.cegh.2018.05.006
38. Cobetto N, Aubin C, Parent S, Barchi S, Turgeon I, Labelle H. Effectiveness of braces designed using computer-aided design and manufacturing (CAD/CAM) and finite element simulation compared to CAD/CAM only for the conservative treatment of adolescent idiopathic scoliosis: a prospective randomized controlled trial. Eur Spine J. 2016;25(10):3056–3064. doi:10.1007/s00586-016-4434-3
39. Xu Y, Li X, Chang Y, et al. Design of personalized cervical fixation orthosis based on 3D printing technology. Appl Bionics Biomech. 2022;2022:8243128. doi:10.1155/2022/8243128
40. Burkhard M, Fürnstahl P, Farshad M. Three-dimensionally printed vertebrae with different bone densities for surgical training. Eur Spine J. 2019;28(4):798–806. doi:10.1007/s00586-018-5847-y
41. Clifton W, Nottmeier E, Edwards S, et al. Development of a Novel 3D printed phantom for teaching neurosurgical trainees the freehand technique of C2 laminar screw placement. World Neurosurg. 2019;129:e812–e820. doi:10.1016/j.wneu.2019.06.038
42. Karlin L, Weinstock P, Hedequist D, Prabhu SP. The surgical treatment of spinal deformity in children with myelomeningocele: the role of personalized three-dimensional printed models. J Pediat Orthop B. 2017;26(4):375–382. doi:10.1097/bpb.0000000000000411
43. Tan LA, Yerneni K, Tuchman A, et al. Utilization of the 3D-printed spine model for freehand pedicle screw placement in complex spinal deformity correction. J Spine Surg. 2018;4(2):319–327. doi:10.21037/jss.2018.05.16
44. Garg B, Gupta M, Singh M, Kalyanasundaram D. Outcome and safety analysis of 3D-printed patient-specific pedicle screw jigs for complex spinal deformities: a comparative study. Spine J. 2019;19(1):56–64. doi:10.1016/j.spinee.2018.05.001
45. Sugawara T, Kaneyama S, Higashiyama N, et al. Prospective multicenter study of a multistep screw insertion technique using patient-specific screw guide templates for the cervical and thoracic spine. Spine. 2018;43(23):1685–1694. doi:10.1097/brs.0000000000002810
46. Sugawara T, Higashiyama N, Kaneyama S, Sumi M. Accurate and simple screw insertion procedure with patient-specific screw guide templates for posterior C1-C2 Fixation. Spine. 2017;42(6):E340–e346. doi:10.1097/brs.0000000000001807
47. Kaneyama S, Sugawara T, Sumi M. Safe and accurate midcervical pedicle screw insertion procedure with the patient-specific screw guide template system. Spine. 2015;40(6):E341–8. doi:10.1097/brs.0000000000000772
48. Li F, Huang X, Wang K, et al. Preparation and assessment of an individualized navigation template for lower cervical anterior transpedicular screw insertion using a three-dimensional printing technique. Spine. 2018;43(6):E348–e356. doi:10.1097/brs.0000000000002341
49. Xiao JR, Huang WD, Yang XH, et al. En bloc resection of primary malignant bone tumor in the cervical spine based on 3-dimensional printing technology. Orthop Surg. 2016;8(2):171–178. doi:10.1111/os.12234
50. Wallace N, Schaffer NE, Aleem IS, Patel R. 3D-printed patient-specific spine implants: a systematic review. Clin Spine Surg. 2020;33(10):400–407. doi:10.1097/BSD.0000000000001026
51. Xu N, Wei F, Liu X, et al. Reconstruction of the upper cervical spine using a personalized 3D-printed vertebral body in an adolescent with Ewing sarcoma. Spine. 2016;41(1):E50–4. doi:10.1097/brs.0000000000001179
52. Rosenzweig DH, Carelli E, Steffen T, Jarzem P, Haglund L. 3D-Printed ABS and PLA scaffolds for cartilage and nucleus pulposus tissue regeneration. Int J Mol Sci. 2015;16(7):15118–15135. doi:10.3390/ijms160715118
53. Mobaraki M, Ghaffari M, Yazdanpanah A, Luo Y, Mills D. Bioinks and bioprinting: a focused review. Bioprinting. 2020;18:e00080.
54. Skardal A. Perspective: “Universal” bioink technology for advancing extrusion bioprinting-based biomanufacturing. Elsevier. 2018;2018:e00026.
55. Valerga AP, Batista M, Fernandez-Vidal SR, Gamez AJ. Impact of chemical post-processing in fused deposition modelling (FDM) on polylactic acid (PLA) surface quality and structure. Polymers. 2019;11(3):566. doi:10.3390/polym11030566
56. Guzzi EA, Tibbitt MW. Additive manufacturing of precision biomaterials. Adv Mater. 2020;32(13):1901994. doi:10.1002/adma.201901994
57. Liu F, Zhang DZ, Zhang P, Zhao M, Jafar S. Mechanical properties of optimized diamond lattice structure for bone scaffolds fabricated via selective laser melting. Materials. 2018;11(3):374. doi:10.3390/ma11030374
58. Da Silva D, Kaduri M, Poley M, et al. Biocompatibility, biodegradation and excretion of polylactic acid (PLA) in medical implants and theranostic systems. Chem Eng J. 2018;340:9–14. doi:10.1016/j.cej.2018.01.010
59. Schiller C, Epple M. Carbonated calcium phosphates are suitable pH-stabilising fillers for biodegradable polyesters. Biomaterials. 2003;24(12):2037–2043. doi:10.1016/S0142-9612(02)00634-8
60. Casavola C, Cazzato A, Moramarco V, Renna G. Mechanical behaviour of ABS-Fused Filament Fabrication compounds under impact tensile loadings. Materials. 2019;12(8):1295. doi:10.3390/ma12081295
61. McCullough EJ, Yadavalli VK. Surface modification of fused deposition modeling ABS to enable rapid prototyping of biomedical microdevices. J Mater Process Technol. 2013;213(6):947–954. doi:10.1016/j.jmatprotec.2012.12.015
62. Eshraghi S, Das S. Mechanical and microstructural properties of polycaprolactone scaffolds with one-dimensional, two-dimensional, and three-dimensional orthogonally oriented porous architectures produced by selective laser sintering. Acta Biomater. 2010;6(7):2467–2476. doi:10.1016/j.actbio.2010.02.002
63. Van Haaften E, Duijvelshoff R, Ippel B, et al. The degradation and performance of electrospun supramolecular vascular scaffolds examined upon in vitro enzymatic exposure. Acta Biomater. 2019;92:48–59. doi:10.1016/j.actbio.2019.05.037
64. Groll J, Burdick JA, Cho D-W, et al. A definition of bioinks and their distinction from biomaterial inks. Biofabrication. 2018;11(1):013001. doi:10.1088/1758-5090/aaec52
65. Esslinger S, Grebhardt A, Jaeger J, et al. Additive manufacturing of β-tricalcium phosphate components via fused deposition of ceramics (FDC). Materials. 2020;14(1):156. doi:10.3390/ma14010156
66. Zhang YS, Khademhosseini A. Advances in engineering hydrogels. Science. 2017;356(6337):eaaf3627. doi:10.1126/science.aaf3627
67. Lim KS, Galarraga JH, Cui X, Lindberg GC, Burdick JA, Woodfield TB. Fundamentals and applications of photo-cross-linking in bioprinting. Chem Rev. 2020;120(19):10662–10694. doi:10.1021/acs.chemrev.9b00812
68. Schwab A, Levato R, D’Este M, Piluso S, Eglin D, Malda J. Printability and shape fidelity of bioinks in 3D bioprinting. Chem Rev. 2020;120(19):11028–11055. doi:10.1021/acs.chemrev.0c00084
69. Chang CC, Boland ED, Williams SK, Hoying JB. Direct‐write bioprinting three‐dimensional biohybrid systems for future regenerative therapies. J Biomed Mater Res Part B. 2011;98(1):160–170. doi:10.1002/jbm.b.31831
70. Yu Z, Voumard B, Shea K, Stanković T. Exploration of the influence of different biomimetic designs of 3D printed multi-material artificial spinal disc on the natural mechanics restoration. Mater Des. 2021;210:110046. doi:10.1016/j.matdes.2021.110046
71. Ramaraju H, Akman RE, Safranski DL, Hollister SJ. Designing biodegradable shape memory polymers for tissue repair. Adv Funct Mater. 2020;30(44):2002014. doi:10.1002/adfm.202002014
72. Chia HN, Wu BM. Recent advances in 3D printing of biomaterials. J Biol Eng. 2015;9(1):4. doi:10.1186/s13036-015-0001-4
73. Xia C, Pan Z, Polden J, Li H, Xu Y, Chen S. Modelling and prediction of surface roughness in wire arc additive manufacturing using machine learning. J Intell Manuf. 2022;33(5):1467–1482. doi:10.1007/s10845-020-01725-4
74. Sharma S, Gupta V, Mudgal D. Response surface methodology and machine learning based tensile strength prediction in ultrasonic assisted coating of poly lactic acid bone plates manufactured using fused deposition modeling. Ultrasonics. 2024;137:107204. doi:10.1016/j.ultras.2023.107204
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




