Back to Journals » Journal of Inflammation Research » Volume 18
A Case Report of Wolf’s Post-Herpetic Isotopic Response: Herpetiform Pemphigus
Authors Tang S , Wang L , Liu Y, Liu L , Deng J, Guo G, Liu H, Wen C, Wu R
Received 11 February 2025
Accepted for publication 1 July 2025
Published 15 July 2025 Volume 2025:18 Pages 9275—9282
DOI https://doi.org/10.2147/JIR.S522003
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Tara Strutt
Shanshan Tang,1,* Lanying Wang,2,* Yuetong Liu,2 Lanxin Liu,2 Jiqing Deng,2 Guixian Guo,2 Haiyan Liu,2 Changhui Wen,1 Ran Wu1
1Department of Dermatology, The First Affiliated Hospital of Guizhou University of Traditional Chinese Medicine, Guiyang, Guizhou, 550001, People’s Republic of China; 2Guizhou University of Traditional Chinese Medicine, Guiyang, Guizhou, 550002, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Ran Wu, Department of Dermatology, The First Affiliated Hospital of Guizhou University of Traditional Chinese Medicine, Guiyang, Guizhou, 550001, People’s Republic of China, Email [email protected]
Purpose: To report a rare case of Wolf’s post-herpetic isotopic response (PHIR) manifesting as herpetiform pemphigus (HP) after herpes zoster (HZ) infection and explore its potential pathogenesis.
Patients and Methods: A 76-year-old male patient with a history of HZ on the right lateral waist, abdomen, and back. Fifteen days after the cure of HZ, annular erythema, blisters, and pruritus appeared at the original site of the same skin lesions and then spread throughout the body. Retrospective analysis was performed through clinical examination, histopathology, direct immunofluorescence, and autoantibody detection.
Results: The patient was diagnosed with PHIR presenting as HP based on medical history and test results. Treatment with methylprednisolone (initial dose 40 mg/day) and antibiotics resolved lesions within 2 weeks, with no recurrence during 18 months of follow-up. The pathogenesis was hypothesized to involve local and systemic immune microenvironment changes induced by varicella-zoster virus (VZV) infection.
Conclusion: HP developing after HZ may represent a novel manifestation of PHIR, providing new evidence for this phenomenon. Clinicians should consider immune-related bullous diseases in post-HZ skin lesions. Larger studies are needed to validate the association between viral infection and immune imbalance in pathogenesis.
Keywords: Wolf’s post-herpetic isotopic response, herpes zoster, herpetiform pemphigus, varicella-zoster virus infection
Introduction
Herpes zoster (HZ), caused by reactivation of latent varicella-zoster virus (VZV) in ganglia, is an acute infectious dermatosis characterized by unilateral, dermatomal clusters of vesicles often accompanied by neuralgia. Wolf’s isotopic response (WIR) refers to developing a new, unrelated skin disorder at the site of a previously healed cutaneous condition. HZ is WIR’s most frequently reported antecedent disease, followed by herpes simplex, varicella, and thrombophlebitis. When occurring after HZ, this phenomenon is termed Wolf’s post-herpetic isotopic response (PHIR). Manifestations of PHIR include granulomatous reactions, primary/metastatic tumours, leukemic/lymphomatous infiltrates, dysimmune disorders, secondary infections, acne, and epidermal cysts. The interval between the two conditions varies widely (days to years), and the pathogenesis remains unclear, with viral, immune, neural, and vascular hypotheses proposed. Herpetiform pemphigus (HP) is a rare autoimmune bullous disorder characterized by clinical features mimicking dermatitis herpetiformis and histopathological changes typical of pemphigus. Its core mechanism involves abnormal immune attacks on intercellular junction structures in the skin, leading to intraepidermal blister formation—notably, no PHIR presenting as HP has been documented in the literature. Herein, we describe a case of PHIR manifesting as HP. Identifying this case may give clinicians a new perspective, emphasizing the need to consider immune-mediated bullous diseases in new skin lesions developing after herpes zoster resolution.
Case Presentation
The patient was a 76-year-old male who presented to our department on March 14, 2023, due to “scattered erythema, blisters, and itching all over the body for 15 days”. One month prior, the patient had consulted our department for “herpes zoster (HZ) on the right lateral waist, abdomen, and back” and received 14 days of treatment, including antiviral therapy, nerve nutrition, and pain relief, after which the rash healed and the patient was discharged on February 27, 2023. Post-discharge, the patient occasionally experienced distending pain in the herpes-affected skin area of the right lateral waist, abdomen, and back, where large pigmentation spots remained. Fifteen days before the current presentation, the patient noticed small blisters and papules scattered along the edges of the previously healed HZ lesion area on the right lateral waist, abdomen, and back. These blisters recurred repeatedly within the original HZ lesion area. They gradually involved the entire body after 7 days, predominantly affecting the right lateral waist, abdomen, back, and proximal regions of both lower limbs, accompanied by intense itching, frequent scratching, and no pain. Thus, the patient revisited our hospital and was admitted to the dermatology department with a tentative diagnosis of “bullous pemphigoid (BP)?”. The patient had a history of hypertension for over one year and was treated with oral Irbesartan 0.15g QD with well-controlled blood pressure. The patient also had a history of smoking and alcohol consumption for over 20 years. There was no history of drug allergies, family history of hereditary diseases, or similar skin diseases. On admission, the patient’s vital signs were stable Dermatological examination (Figures 1–3) revealed scattered annular erythemas varying across the entire body, most prominent on the right lateral waist, abdomen, back, and proximal lower limbs. Some erythemas coalesced into patchy formations with slightly elevated edges, where millet- to mung bean-sized blisters were observable. A few blisters were arranged in a circular or semi-circular configuration with clear blister fluid. The central part of the erythemas showed erosion, exudation, and crusting, and the Nikolsky sign was negative. The right lateral waist, abdomen, and back displayed extensive pigmented macules in a horizontal band-shaped distribution.
 |
Figure 1 Right lumboabdominal skin lesions. |
 |
Figure 2 Right lower back skin lesions. |
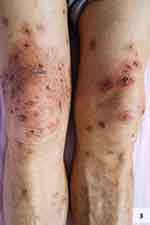 |
Figure 3 Lesions of proximal lower limbs. |
Initially diagnosed with bullous pemphigoid, the patient was treated with oral minocycline, nicotinamide, and topical ointments after full communication with the patient and family and obtaining informed consent. After five days of treatment, existing skin lesions showed signs of regression, but new blisters continued to develop, predominantly on the right thoracoabdominal region and proximal thighs. Laboratory and auxiliary examinations showed: Blood cell analysis showed elevated eosinophil percentage (15.7%, normal range: 0.4–8%) and absolute eosinophil count (0.94×109/L, normal range: 0.02–0.52×109/L), with red and white blood cell counts and differential classifications within normal limits. Blood biochemistry showed low total protein levels (57.99g/L, normal range: 65–85g/L) and albumin 3 (4.10g/L, normal range: 40–55g/L). Blood glucose, electrolytes, liver and kidney function, C-reactive protein, routine stool and urine tests, hepatitis B surface antigen and antibody, and HIV antibody all showed normal results. Serum varicella-zoster virus (VZV) DNA polymerase chain reaction (PCR) testing revealed a negative result for VZV. Enzyme-linked immunosorbent assays (ELISA)for IgG and IgE autoantibodies against Desmoglein(Dsg)1, Dsg3 and BP antigen II (BP180), BP antigen I (BP230) were all negative. The histopathological findings of the skin lesion on the right thigh (Figure 4) revealed the formation of an intraepidermal blister. A large number of eosinophils and a small number of lymphocytes and neutrophils were observed within the blisters. In the superficial to deep layers of the dermis, perivascular infiltration of numerous eosinophils, scattered lymphocytes, and a few neutrophils was noted, with the infiltration predominantly in the superficial layer. Direct immunofluorescence(DIF) showed deposition of immunoglobulin G (IgG) and Complement component 3 (C3) at the intercellular portion of the epidermis (Figure 5), and immunoglobulin A (IgA), immunoglobulin M (IgM) and Fibrin (Fib) were negative.
 |
Figure 4 Histopathology of right thigh skin lesions (HE × 40). |
 |
Figure 5 (a and b) DIF showed reticular lattice deposition of C3 and IgG between Interspinous epidermal cells. |
Five days after admission, the final diagnosis was confirmed as the post-herpetic isotopic response (PHIR) in herpetiform pemphigus based on clinical manifestations, laboratory results, and histopathological findings. Consequently, with the patient’s informed consent, the treatment regimen was adjusted: minocycline and nicotinamide were discontinued, and an intravenous drip of methylprednisolone sodium succinate (40mg/day) was initiated for immunosuppression, along with cefuroxime sodium (1.5 g every 8 hours) for infection prevention, supplemented by symptomatic and supportive measures. After one week of treatment, the patient’s original vesicles were absorbed, dried, and crusted, with no new vesicles. The patient reported no significant generalized itching, and the methylprednisolone dosage was reduced to 32 mg/day. After five days of clinical observation, the patient was discharged with no new cutaneous lesions (including erythema and vesicles) and no itching or pain. At discharge, the patient had numerous scattered dark brown scabs and pigmented macules on the trunk and limbs, with large pigmented macules and scars on the right lateral waist and back. The patient was instructed to return for regular follow-ups and gradually taper off corticosteroids. During the 18-month follow-up, the corticosteroid dosage was gradually tapered and eventually discontinued, with the patient achieving complete remission and remaining relapse-free.
Discussion
Wolf’s isotopic response(WIR) refers to the occurrence of another unrelated or differently-natured skin disease at the site of a previously healed skin condition. First reported by neurologist Wyburn-Mason in 1955,1 this concept was first summarized and defined as “isotopic response” by Wolf et al in 1995.2 In 2017, the definition was expanded to include new skin diseases arising at sites of pigmentation changes or scars from prior dermatoses.3 HZ is WIR’s most common antecedent disease, followed by herpes simplex, varicella, and thrombophlebitis.4 Clinical manifestations are diverse and categorized into granulomatous reactions, primary/metastatic tumours, leukemic/lymphomatous infiltrations, immune disorders, infections, acne, skin cysts, etc.5,6 In this case, the patient had no previous history of HP. Notably, the initial lesions of HP were strictly distributed along the pigmented area left by HZ, a feature highly consistent with the core definition of WIR that “primarily involves the original lesion area”, providing a fundamental basis for the diagnosis. Although the lesions generalized to the whole body 7 days after onset, the clinical manifestation broke through the typical “localized to the original site” pattern, which may represent a special case of post-herpetic isotopic response and belongs to the extended variant of WIR. The unique evolution of this case provides a new observational dimension for the clinical spectrum of isotopic response, making it academically valuable for reporting.
HP, a rare autoimmune bullous disorder first described by Jablonska et al in 1975,6 is a pemphigus subtype characterized by clinical features mimicking dermatitis herpetiformis and immunopathological characteristics of pemphigus, comprising approximately 7% of all pemphigus cases. Its etiology remains incompletely understood, with proposed associations with infections, trauma, diet, and medications.7,8 HP typically presents as annular erythema with or without blisters, urticarial lesions, or edematous erythema with crusting, featuring negative Nikolsky’s sign and predilection for the trunk and extremities, often sparing oral mucosa. Histopathology shows intraepidermal blisters with eosinophilic/neutrophilic infiltration and intraepidermal clefts (with or without acantholysis). Direct immunofluorescence (DIF) reveals IgG ± C3 deposition between keratinocytes. Specific autoantibody testing detects anti-Dsg1/3 and anti-desmocollin (Dsc) 1–3 antibodies by ELISA.9,10 Recently, cases of HP with isolated anti-Dsc antibodies (negative for anti-Dsg) have been reported.11 In this case, HP diagnosis was supported by clinical, histological, and immunofluorescence findings despite negative anti-Dsg1/3 antibodies. Reports indicate anti-Dsc antibodies may have pathogenic potential in atypical pemphigus, interfering with desmosomal assembly in vivo,12 though their precise role remains unclear. The patient’s pemphigus likely resulted from anti-Dsc autoantibodies. Dscs, belonging to the cadherin superfamily, provide structural support to desmosomes and are crucial for intercellular adhesion. Approximately 60% of HP patients have Dsc rather than Dsg antibodies; thus, Dsc-related studies are warranted when HP is suspected, but Dsg antibodies are negative.13 Further research on Dsc is needed in this case.
In this case, HP developed 15 days after the HZ resolution, with a very short interval between the two episodes, necessitating differentiation from Disseminated herpes zoster (DHZ). HZ is characterized by clustered vesicles distributed along sensory nerve branches, with histopathology showing intraepidermal vesicles accompanied by ballooning degeneration of keratinocytes and eosinophilic intranuclear inclusions (Cowdry type A). DHZ is a systemic hematogenous virus that spreads, manifesting as more than 20 varicella-like lesions throughout the body or visceral involvement.14 The patient did not receive antiviral therapy yet demonstrated a favourable response to glucocorticoid treatment, suggesting an immune-mediated disease rather than a viral infection from a therapeutic perspective. Additionally, histopathological and immunofluorescence findings clearly distinguished it from viral infection. Furthermore, HP is often misdiagnosed as bullous pemphigoid (BP) due to clinical similarities, but pathological differentiation is key: BP features subepidermal blisters, whereas this case showed intraepidermal blisters. Therefore, based on this observation, the diagnosis of bullous pemphigoid can be excluded. HP typically follows a chronic course, and currently, there is no standardized treatment protocol. Dapsone and/or glucocorticoids remain the preferred treatments.8 Mild cases can be managed with low-dose glucocorticoids or dapsone alone; for cases with disease progression or ineffective glucocorticoid treatment, combination therapy with medications such as cyclophosphamide, Sulfapyridine, azathioprine, methotrexate, and minocycline can be considered. Plasma exchange and intravenous immunoglobulin administration also effectively control the disease.9,10 In the realm of biologics, there exists a documented case report wherein a severe case of HP was successfully managed with rituximab.15 Compared with other types of pemphigus, HP has a favourable prognosis, with a few cases progressing to pemphigus vulgaris, pemphigus foliaceous, or pemphigus erythematosus. In this case, minocycline and nicotinamide showed some limited therapeutic effect. However, the administration of glucocorticoids resulted in satisfactory clinical outcomes.
The etiopathogenesis of PHIR has not been comprehensively clarified. It is likely associated with the pathophysiological reactions induced by VZV infection, encompassing viral, immunological, vascular, and neurological elements. Mahajan et al postulated that the Wolf’s isotopic response stems from neuropeptides and neural signals from damaged nerve terminals.16 Some scholars have defined skin areas that are more vulnerable either genetically or through acquired means as “immunocompromised (or immunologically unstable) districts(ICDs)”. This concept elucidates why previously injured skin sites are susceptible to opportunistic infections, tumours, and heightened immune responses. Potentially, this is due to local lymphatic drainage obstruction, which impedes the regular migration of immunocompetent cells and/or causes damage to the sensory nerve fibres that secrete immune-related peptides. This situation, in turn, leads to local immune dysregulation and the localized onset of immune-related rashes or skin diseases.17,18 HZ typically affects the elderly, immunosuppressed individuals, or those with immune deficiencies, and its occurrence is closely linked to the body’s immune state. HZ is caused by VZV reactivation, a contagious neurotropic double-stranded DNA virus that damages peripheral sensory nerve fibres and skin nerve terminals, disrupts neuromodulator release, induces neuroimmune instability, and alters local immune mechanisms. Local skin lesions in herpes-infected areas often exhibit severe neuroimmune dysfunction, making them preferential sites for subsequent immune-related skin diseases.19 Tabosa et al suggested that post-HZ Wolf’s isotopic responses predominantly occur in immunocompromised skin regions.20 Goon et al reported the first instance of pemphigus vulgaris developing 17 days after the resolution of varicella lesions. They hypothesized an etiopathogenesis in which varicella infection exposes intraepidermal epitopes to the immune system, resulting in the production of anti-intercellular antibodies or cross-reactivity between the initial immune response triggered by varicella infection and intraepidermal autoantigens, thus triggering autoimmune diseases.21 Additionally, literature reports indicate that VZV infection can initiate or exacerbate pemphigus progression, possibly through non-specific immune activation by viral infections.22–24 Increased humoral and cellular factors activate immune responses, leading to autoimmune diseases in genetically predisposed individuals.25–27 As a subtype of pemphigus, the development of HP isotopic response after HZ in this patient is hypothesized to be related to VZV infection and subsequent local and systemic skin immune impairment. However, due to the lack of relevant case reports for reference, the data analysis of this case has certain limitations.
Conclusion
This case provides new case evidence for the clinical diversity of WIR. It highlights the need for clinicians to consider immune-related bullous diseases in new skin lesions following HZ healing. It suggests a potential association between viral infection and local immune imbalance. Due to the limited research, the specific relationship between viral infection and local/systemic immune dysregulation in pathogenesis requires further validation through more cases and in-depth molecular and immunological studies.
Ethical Approval
The patient gave written informed consent to publish all the images and data included in this article. The Declaration of Helsinki conducted the study (as revised in 2013). The Institutional Review Board (IRB) waived ethical review and approval due to the study’s retrospective nature and the complete anonymization of patient data.
Consent to Publish
The patient provided informed consent to publish their case details and accompanying images.
Funding
This research was supported by the Science and Technology Fund Project of the Health Commission of Guizhou Province in 2024 (Grant No. GZWKJ 2024-133).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wyburn-Mason R. Malignant change arising in tissues affected by herpes. Br Med J. 1955;2(4948):1106–1109. doi:10.1136/bmj.2.4948.1106
2. Wolf R, Brenner S, Ruocco V, et al. Isotopic response. Int J Dermatol. 1995;34(5):341–348. doi:10.1111/j.1365-4362.1995.tb03616.x
3. Wolf R, Wolf D. “Wolf’s isotopic response”: the originators speak their mind and set the record straight. Clin Dermatol. 2017;35(4):416–418. doi:10.1016/j.clinderma-tol.2017.02.003
4. Chun SH, Kim BY, Kim CM, Park JB, Ryu HJ. A case of wolf’s isotopic response presenting as bullous pemphigoid. Ann Dermatol. 2017;29(4):499–500. doi:10.5021/ad.2017.29.4.499
5. Ruocco V, Ruocco E, Brunetti G, Russo T, Gambardella A, Wolf R. Wolf’s post-herpetic isotopic response: infections, tumors, and immune disorders arising on the site of healed herpetic infection. Clin Dermatol. 2014;32(5):561–568. doi:10.1016/j.clindermatol.2014.04.003
6. Wang T, Zhang M, Zhang Y, et al. Wolf’s isotopic response after herpes zoster infection: a study of 24 new cases and literature review. Acta Derm Venereol. 2019;99(11):953–959. doi:10.2340/00015555-3269
7. Kridin K. Pemphigus group: overview, epidemiology, mortality, and comorbidities. Immunol Res. 2018;66(2):255–270. doi:10.1007/s12026-018-8986-7
8. Kasperkiewicz M, Kowalewski C, Jabłońska S. Pemphigus herpetiformis: from first description until now. J Am Acad Dermatol. 2014;70(4):780–787. doi:10.1016/j.jaad.2013.11.043
9. Peterman CM, Vadeboncoeur S, Schmidt BA, Gellis SE. Pediatric pemphigus herpetiformis: case report and review of the literature. Pediatr Dermatol. 2017;34(3):342–346. doi:10.1111/pde.13152
10. Costa LMC, Cappel MA, Keeling JH. Clinical, pathologic, and immunologic features of pemphigus herpetiformis: a literature review and proposed diagnostic criteria. Int J Dermatol. 2019;58(9):997–1007. doi:10.1111/ijd.14395
11. Ansai O, Shimomura Y, Fujimoto A, et al. Case of pemphigus herpetiformis with immunoglobulin G autoantibodies against desmocollin-3. J Dermatol. 2017;44(1):104–105. doi:10.1111/1346-8138.13451
12. Ishii N. Significance of anti-desmocollin autoantibodies in pemphigus. J Dermatol. 2023;50(2):132–139. doi:10.1111/1346-8138.16660
13. Nakashima H, Fujimoto M, Watanabe R, et al. Herpetiform pemphigus without anti-desmoglein 1/3 autoantibodies. J Dermatol. 2010;37(3):264–268. PMID: 20507392. doi:10.1111/j.1346-8138.2009.00786.x
14. Drone E, Ganti L. A case of disseminated zoster in an immunocompetent patient. Cureus. 2019;11(12). doi:10.7759/cureus.6286
15. Marniquet ME, Joly P, Dansette D, Fenot M. A case of pemphigus herpetiformis successfully treated with rituximab. J Eur Acad Dermatol Venereol. 2022;36(9):e686–e688. doi:10.1111/jdv.18122
16. Mahajan R, De D, Saikia UN. Wolf’s isotopic response: report of a case and review of literature. Indian J Dermatol. 2014;59(3):275–282. doi:10.4103/0019-5154.131401
17. Ruocco V, Ruocco E, Piccolo V, Brunetti G, Guerrera LP, Wolf R. The immunocompromised district in dermatology: a unifying pathogenic view of the regional immune dysregulation. Clin Dermatol. 2014;32(5):569–576. doi:10.1016/j.clindermatol.2014.04.004
18. Ruocco V, Sangiuliano S, Brunetti G, Ruocco E. Beyond zoster: sensory and immune changes in zoster-affected dermatomes: a review*. Acta Derm Venereol. 2012;92(4):378–382. doi:10.2340/00015555-1284
19. Seo JK, Jeong KH, Shin MK. A case of post-herpetic nevoid comedones. Ann Dermatol. 2019;31(Suppl):S36–S38. doi:10.5021/ad.2019.31.S.S36
20. Tabosa GVBS, Stelini RF, Souza EM, Velho PENF, Cintra ML, Florence MEB.Immunocompromised cutaneous district, isotopic, and isopathic phenomena-systematic review. J Cosmet Dermatol. 2021;20(2):410–416.
21. Goon AT, Tay YK, Tan SH. Pemphigus vulgaris following varicella infection. Clin Exp Dermatol. 2001;26(8):661–663. doi:10.1046/j.1365-2230.2001.00912.x
22. Murata T, Morita K. Unsuspected varicella-zoster infection complicating mucosal- dominant pemphigus vulgaris. Int J Dermatol. 2013;52(11):1453–1454. doi:10.1111/j.1365-4632.2011.05393.x
23. Douard PA, Delaumenie S, Pittoni J, et al. Reactivation of pemphigus by varicella zoster virus after anti-CD20 treatment. Int J Dermatol. 2020;59(3):e52–e53. doi:10.1111/ijd.14711
24. Fernandez-Flores A, Cassarino D. Varicella zoster with pemphigus-like reaction. Am J Dermatopathol. 2022;44(7):e75–e78. doi:10.1097/DAD.0000000000002178
25. Hanmei Z. Feng Suying.Pemphigus complicated by herpes simplex virus infection. Chin Jl of Dermatol. 2022;55(6):545–548. doi:10.35541/cjd.20200248
26. Abreu Velez AM, Smoller BR, Gao W, et al. Varicella-zoster virus (VZV) and alpha 1 antitrypsin: a fatal outcome in a patient affected by endemic pemphigus foliaceus. Int J Dermatol. 2012;51(7):809–816. doi:10.1111/j.1365-4632.2011.05296
27. Ruocco V, Ruocco E, Lo Schiavo A, Brunetti G, Guerrera LP, Wolf R. Pemphigus: etiology, pathogenesis, and inducing or triggering factors: facts and controversies. Clin Dermatol. 2013;31(4):374–381. doi:10.1016/j.clindermatol.2013.01.004
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

