Back to Journals » Clinical Ophthalmology » Volume 18
A Novel Technique Using Lyophilized Amniotic Membrane Patch (LAMPatch) as Primary Procedure in Patients with Myopic Traction Maculopathy with Macular Detachment
Authors Ramirez-Estudillo A , Rojas-Juarez S, Ramirez-Galicia X, Garcia-Vasquez A, Medina-Medina S , Gulias-Cañizo R
Received 20 March 2024
Accepted for publication 15 August 2024
Published 2 September 2024 Volume 2024:18 Pages 2473—2480
DOI https://doi.org/10.2147/OPTH.S469801
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Abel Ramirez-Estudillo,1,2 Sergio Rojas-Juarez,2 Ximena Ramirez-Galicia,1 Angel Garcia-Vasquez,2 Silvia Medina-Medina,2 Rosario Gulias-Cañizo1,3
1Centro Oftalmológico Mira, Mexico City, Mexico; 2Retina Department, Fundación Hospital Nuestra Señora de la Luz, Mexico City, Mexico; 3Centro de Investigación en Ciencias de la Salud, Universidad Anahuac, Estado de Mexico, Mexico
Correspondence: Abel Ramirez-Estudillo, Rosario Gulias-Cañizo, Centro Oftalmológico Mira, Taxco 35, Col. Roma Sur, C.P. 06760, Mexico City, Mexico, Email [email protected]; [email protected]
Introduction: Maculopathy secondary to pathologic myopia (PM) is increasingly causing visual impairment and blindness worldwide. PM is associated with tractional maculopathy that ranges from macular foveoschisis to macular hole. These disorders are treated with different options that offer variable results, reflecting the need for new techniques that address myopic maculopathy with consistent outcomes.
Methods: Since human amniotic membrane (HAM) has been reported to be safe for intraocular use and to promote retinal healing, it was incorporated as an adjuvant in pars plana vitrectomy in patients with different tractional disorders related to myopia. This work presents a prospective, consecutive case series of seven patients with high myopia who underwent a 25-gauge vitrectomy with ILM peeling and HAM transplantation.
Results and Discussion: Our six-month results show that this novel technique delivers functional success related to tissue permanence without recurrence of traction that translates into visual acuity gain and maintenance that are superior to other techniques.
Keywords: retinal surgery, macular surgery, lyophilized amniotic membrane, amniotic membrane patch, LAMPatch, myopic traction maculopathy, macular hole, retinal detachment
Introduction
Maculopathy secondary to pathologic myopia (PM) is increasingly causing visual impairment and blindness worldwide. PM is an ocular disorder characterized by a spherical equivalent (SE) of more than – 6.0 diopters (D) or by an axial length (AL) of more than 26.5 millimeters (mm). PM is associated with myopic maculopathy (MM) or myopic tractional maculopathy (MTM), a disorder that encompasses a spectrum of retinal abnormalities in patients with high myopia, including macular foveoschisis and macular hole.
Myopic foveoschisis consists of the progressive separation of the layers of the retina, which are connected by Müller cells. However, when foveoschisis progresses, it can lead to a macular hole. Several classifications and stages that help understand this entity, not within the scope of this work, are explained in detail in an excellent review on the subject published in 2018 by Ruiz-Medrano et al.1 In this work, the authors introduced a novel ATN grading system that included three critical components of myopic maculopathy, namely atrophy (A), traction (T), and neovascularization (N). The T component mainly includes foveoschisis (FS), foveal detachment (FD), full-thickness macular hole (MH), and MH with retinal detachment (MHRD).1 Although the ATN system considers clinical characteristics, Parolini et al2 developed another staging system that complements the structural aspects based on optical coherence tomography (OCT) imaging. This system includes four stages: Stage 1: inner/outer maculoschisis, Stage 2: predominantly outer maculoschisis, Stage 3: maculoschisis/macular detachment, and Stage 4: macular detachment. Both staging systems are very useful in determining the appropriate timing for surgical intervention in MTM. When necessary, treatment can be performed using two approaches, external or internal. The ab externo approach is used to counteract the perpendicular centrifugal forces on the retina, while the ab interno is more suitable for addressing tangential forces. Centrifugal forces refer to anteroposterior elongation that stretches retinal fibers, causing them to “fray like a fabric”. On the other hand, tangential forces are those exerted by tissues, especially the hyaloid and epiretinal membranes, that pull the retina from within. Thus, the selection of each approach will depend on the characteristics and severity of MTM, and sometimes it is even necessary to combine both.3
Due to the tractional component of MTM and MHRD, one of the first surgeries performed was pars plana vitrectomy (PPV), which over time has diversified into various techniques and variants along with technological advances. For example, 25G or 27G vitrectomy has been considered equally effective,4 with or without ILM peeling, gas, or air tamponade. Several authors state that vitrectomy with ILM peeling is as effective as tamponade vitrectomy, but that tamponade accelerates results.5–7 The use of gas versus air tamponade has also been compared, reporting the use of air in patients with foveoschisis <=400 mm but claiming that C3F8 is more effective in cases with foveoschisis > 400 mm.8 There is no consensus on the matter, which continues to be controversial. For example, a systematic review with meta-analysis concluded that vitrectomy with ILM peeling achieves better results than without ILM peeling and that the use of gas tamponade does not improve anatomical and visual outcomes and is associated with more complications compared to not using tamponade.9 Still, other authors state that the results are similar with or without ILM peeling.10,11 However, there are reports of the need to re-operate, including ILM peeling in the second vitrectomy due to foveoschisis persistence.12
Another essential component in myopic traction maculopathy is the presence of a posterior staphyloma (PS). This disorder is treated with macular buckling, which aims to reduce the complex anteroposterior and tangential tractions resulting from PS in highly myopic eyes by modifying the macular shape from a concave to a convex profile. An extensive review of the literature reported that macular buckling is more effective in achieving complete resolution of foveoschisis, reattachment, and macular hole closure compared with vitrectomy13–15 with reports of variations of this technique, such as adjustable macular buckling in eyes with foveoschisis and macular hole with good surgical success,16 the use of different materials to induce indentation such as cross-linked and stabilized hyaluronic acid,17 a 3-armed silicone capsule,18 a polytetrafluoroethylene vascular graft,19 or along with vitrectomy placing an L-shaped titanium plate with a silicon sponge;20 however, despite the higher success rates for macular buckling, there are concerns regarding the effects of the chronic compression induced by indentation.13,15
Finally, there have been attempts to place various tissues in areas where there is a loss of continuity of the macula, such as autologous ILM transplantation21 or anterior lens capsule transplantation for refractory macular holes,22 or the ILM flap as primary treatment of myopic macular holes.23
This diversity of treatments with variable results reflects the need for new techniques that address myopic maculopathy with consistent results. In addition, good preoperative visual acuity and the absence of preoperative foveal detachment are significant predictors of good visual prognosis, which is why surgical intervention before the development of macular complications may improve visual outcomes. However, this is not the reality in the clinical setting, where patients present with advanced cases, foveal detachment, and poor visual acuity.
It has been reported that the human amniotic membrane (HAM) is safe for intraocular use and to promote retinal healing;24 other authors proposed the use of a thin layer of lyophilized amniotic membrane (LAM) as a patch (LAMPatch) to temporarily block retinal breaks, reporting good tolerance on the retinal surface.25,26 Based on this, we present a novel technique using the lyophilized amniotic membrane as an adjuvant in pars plana vitrectomy in patients with FD, MH, and MHRD.
Methods
This study was approved by the Ethics Committee of the Fundación Hospital Señora de la Luz (CC-RET-20221006) and adhered to the latest tenets of the Declaration of Helsinki. An informed consent form was obtained from all subjects before conducting any study procedures. This prospective, consecutive case series was conducted in two centers.
Patient Selection
The inclusion criteria for patient selection were as follows:
- A diagnosis of high myopia, defined as an axial length (AL) ≥26 mm or a myopic refractive error ≥6.0 diopters (D),
- Age >18 years old,
- Diagnosis of FD, MH, or MHRD based on the ATN classification system evaluated by spectral-domain optical coherence tomography (Spectralis SD-OCT, Heidelberg Eye Explorer version 1.10.2.0, Heidelberg Engineering) with a 55° widefield lens.
The exclusion criteria were the following:
- Previous vitrectomy,
- History or actual ocular or severe systemic disease, including dense cataract, glaucoma, diabetic retinopathy, or autoimmune diseases,
- Media opacity or poor central fixation leading to poor-quality OCT images (image quality <60) or inability to locate the lowest point of the ocular wall at the fovea.
Lyophilized Amniotic Membrane
We used sterilized, lyophilized amniotic membrane (MAFIX, Top Health, Mexico; not procured from prisoners) provided as a 2×2 cm layer that is cut before surgery inside a laminar flow hood into a 2×2 mm fragment placed in a bag that is later deposited inside another bag for sterilization with ethylene oxide.
Surgical Procedure
Each patient underwent phacoemulsification (due to the high refractive errors) and a 25-gauge vitrectomy (Alcon ConstellationTM Vision System, Alcon Laboratories, Fort Worth, TX, USA) with ILM staining (Membrane Blue-Dual®, D.O.R.C. Zuidland, The Netherlands) and peeling (one disc diameter of the foveal center) for tangential removal with assisted traction. After ILM peeling, we performed a complete air-fluid exchange with a bimanual technique to keep the foveal surface completely dry. Then, a 1.5–2 mm diameter lyophilized amniotic membrane patch (LAMPatch) was inserted through the 25-gauge valved trocar using a 25-gauge ILM clamp, extending it over the foveal area. Finally, we performed fluid air exchange and administration of 20% SF6 as tamponade. The trocar incisions were sutured with 7–0 vicryl.
Results
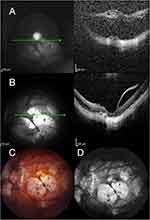
We describe the results of seven patients who completed the 6-month follow-up (Table 1). Representative images of the cases are depicted in Figures 1 and 2 for MHRD (Case 2), Figure 3 for FD (Case 4), and Figure 4 for MH (Case 7).
 |
Table 1 MHRD, FD, and MH Cases That Underwent LAMPatch |
 |
Figure 1 (Case 2): Thickness map change report. (A) Foveoschisis (April 2021); (B) MHRD (February 2023); (C) One month after surgery; (D) Six months after surgery. |
 |
Figure 2 (Case 2): Color images of LAMPatch steps (Case 2). (A) Vitrectomy; (B) Membrane Blue injection; (C) Limitorhexis; (D) Air-fluid exchange; (E) Amniotic membrane; (F) End of surgery. |
Discussion
In 2004, the term MTM was introduced27 to describe MM-related alterations such as retinal or foveal detachment, foveoschisis, lamellar or complete macular hole, among others. Overall, MTM is a complex condition that represents different stages of the same disease, and the staging systems developed1,2 provide data that facilitate the identification of both clinical and structural conditions. Both classifications are complementary, as they consider different aspects. For example, Parolini et al hypothesize, through OCT, that myopic tractional changes in the retina and fovea are not simply different types of MTM, but evolutionary stages of the same disease. The usefulness of both classifications in the context of our study is because they place our patients among those at risk of presenting recurrence.
Tractional myopic degeneration with foveoschisis and concurrent tangential traction represents a significant challenge since the standard of care, PPV plus limitorhexis, fails to maintain the retina attached to the posterior pole. For this purpose, several scleral buckling options have been developed, as previously mentioned, but at least in our country, these options are not readily accessible. For example, amniotic membrane can be found in three presentations: fresh, cryopreserved and freeze-dried. The fresh variety is difficult to obtain, and as a human-derived tissue, entails infectious risks and its regulatory and logistics management are complex. These disadvantages gave rise to the cryopreserved membrane, which is widely available and is the one that has been broadly used in ophthalmology, including as a plug transplanted into the subretinal space.28 On the other hand, LAM is equivalent to cryopreserved membrane, and was used for the first time in ophthalmology in 2004.29 Subsequently, other authors have validated LAM advantages, such as its ease of use and availability, among others.30
Due to the above, LAMPatch is a valuable option. It reduces tangential traction by using an amniotic membrane graft approved and used for other types of ocular surgeries, especially of the anterior segment. The utility of this graft is that it works as a patch that maintains the adherence of the posterior pole to the choroid and sclera; specifically, it serves as a scaffold to increase the adhesion surface without which tangential traction would again pull the retina, causing retinal detachment.
It is relevant to mention that in addition to this advantage, this technique is not more complicated than conventional vitrectomy plus limitorhexis since it only adds one step: the amniotic membrane placement. However, amniotic membrane handling requires a learning curve since it is essential to keep the media dry to avoid folding. Specifically, the vitreous cavity must be completely free of fluid to insert the amniotic membrane so that it does not fold, and the surgeon can unfold it over the desired anatomical location.
Finally, although the anatomical results in our case series are like those observed after PPV plus limitorhexis, the great differentiator of this technique is that the permanence of tissue at six months is very stable, unlike the recurrence of traction with conventional surgery. This functional success translates into a gain and maintenance of visual acuity superior to vitrectomy plus limitorhexis; up to now, the results we report from the 6-month follow-up are excellent both anatomically and functionally.
Conclusions
Lyophilized amniotic membrane as an additional step after vitrectomy with limitorhexis, provides structural and clinical stabilization by OCT at the 6-month follow-up in a series of cases with FD, MH, and MHRD. Although longer-term structural and functional follow-up is required, our results laid the groundwork for a prospective, randomized, controlled clinical study.
Disclosure
The authors declare no competing interests in this work.
References
1. Ruiz-Medrano J, Montero JA, Flores-Moreno I, Arias L, Garcia-Layana A, Ruiz-Moreno JM. Myopic maculopathy: current status and proposal for a new classification and grading system (ATN). Prog Retin Eye Res. 2019;69:80–115. doi:10.1016/j.preteyeres.2018.10.005
2. Parolini B, Palmieri M, Finzi A, et al. The new myopic traction maculopathy staging system. Eur J Ophthalmol. 2021;31(3):1299–1312. doi:10.1177/1120672120930590
3. Anderson WJ, Akduman L. Management of myopic maculopathy: a review. Turk J Ophthalmol. 2023;53(5):307–312. doi:10.4274/tjo.galenos.2023.59844
4. Jiang X, Zhang S, Zhang Z, Zhou X, Wei Y. Comparative study of 27-gauge versus 25-gauge vitrectomy with air tamponade in the treatment of myopic foveoschisis. Ophthalmic Surg Lasers Imaging Retina. 2018;49(10):e135–e42. doi:10.3928/23258160-20181002-16
5. Kim KS, Lee SB, Lee WK. Vitrectomy and internal limiting membrane peeling with and without gas tamponade for myopic foveoschisis. Am J Ophthalmol. 2012;153(2):320–6e1. doi:10.1016/j.ajo.2011.07.007
6. Uchida A, Shinoda H, Koto T, et al. Vitrectomy for myopic foveoschisis with internal limiting membrane peeling and no gas tamponade. Retina. 2014;34(3):455–460. doi:10.1097/IAE.0b013e3182a0e477
7. Zhang T, Zhu Y, Jiang CH, Xu GZ. Long-term follow-up of vitrectomy in patients with pathologic myopic foveoschisis. Int J Ophthalmol. 2017;10(2):277–284. doi:10.18240/ijo.2017.02.16
8. Jiang J, Yu X, He F, et al. Treatment of myopic foveoschisis with air versus perfluoropropane: a retrospective study. Med Sci Monit. 2017;23:3345–3352. doi:10.12659/MSM.901758
9. Meng B, Zhao L, Yin Y, et al. Internal limiting membrane peeling and gas tamponade for myopic foveoschisis: a systematic review and meta-analysis. BMC Ophthalmol. 2017;17(1):166. doi:10.1186/s12886-017-0562-8
10. Gui J, Ai L, Huang T. Vitrectomy with or without internal limiting membrane peeling for myopic foveoschisis. BMC Ophthalmol. 2020;20(1):83. doi:10.1186/s12886-020-01354-8
11. Qi Y, Duan AL, Meng X, Wang N. Vitrectomy without inner limiting membrane peeling for macular retinoschisis in highly myopic eyes. Retina. 2016;36(5):953–956. doi:10.1097/IAE.0000000000000826
12. Sayanagi K, Ikuno Y, Tano Y. Reoperation for persistent myopic foveoschisis after primary vitrectomy. Am J Ophthalmol. 2006;141(2):414–417. doi:10.1016/j.ajo.2005.09.009
13. Alkabes M, Mateo C. Macular buckle technique in myopic traction maculopathy: a 16-year review of the literature and a comparison with vitreous surgery. Graefes Arch Clin Exp Ophthalmol. 2018;256(5):863–877. doi:10.1007/s00417-018-3947-3
14. Bures-Jelstrup A, Alkabes M, Gomez-Resa M, Rios J, Corcostegui B, Mateo C. Visual and anatomical outcome after macular buckling for macular hole with associated foveoschisis in highly myopic eyes. Br J Ophthalmol. 2014;98(1):104–109. doi:10.1136/bjophthalmol-2013-304016
15. Zhao X, Li Y, Ma W, et al. Macular buckling versus vitrectomy on macular hole associated macular detachment in eyes with high myopia: a randomised trial. Br J Ophthalmol. 2022;106(4):582–586. doi:10.1136/bjophthalmol-2020-317800
16. Cacciamani A, Lazzeri S, Rossi T, et al. ADJUSTABLE MACULAR BUCKLING FOR FULL-THICKNESS MACULAR HOLE WITH FOVEOSCHISIS IN HIGHLY MYOPIC EYES: long-term anatomical and functional results. Retina. 2016;36(4):709–716. doi:10.1097/IAE.0000000000000802
17. El Rayes EN. Supra choroidal buckling in managing myopic vitreoretinal interface disorders: 1-year data. Retina. 2014;34(1):129–135. doi:10.1097/IAE.0b013e31828fcb77
18. Liu B, Ma W, Li Y, et al. Macular buckling using a three-armed silicone capsule for foveoschisis associated with high myopia. Retina. 2016;36(10):1919–1926. doi:10.1097/IAE.0000000000001014
19. Wu PC, Sheu JJ, Chen YH, et al. Gore-tex vascular graft for macular buckling in high myopia eyes. Retina. 2017;37(7):1263–1269. doi:10.1097/IAE.0000000000001376
20. Xiong SQ, Jiang HB, Li FL, et al. Treatment of myopic foveoschisis via macular buckling and vitrectomy. Int J Ophthalmol. 2017;10(5):815–818. doi:10.18240/ijo.2017.05.26
21. Morizane Y, Shiraga F, Kimura S, et al. Autologous transplantation of the internal limiting membrane for refractory macular holes. Am J Ophthalmol. 2014;157(4):861–9e1. doi:10.1016/j.ajo.2013.12.028
22. Chen SN, Yang CM. Lens capsular flap transplantation in the management of refractory macular hole from multiple etiologies. Retina. 2016;36(1):163–170. doi:10.1097/IAE.0000000000000674
23. Chen SN. Large semicircular inverted internal limiting membrane flap in the treatment of macular hole in high myopia. Graefes Arch Clin Exp Ophthalmol. 2017;255(12):2337–2345. doi:10.1007/s00417-017-3808-5
24. Rizzo S, Giansanti F, Finocchio L, et al. Vitrectomy with internal limiting membrane peeling and air tamponade for myopic foveoschisis. Retina. 2019;39(11):2125–2131. doi:10.1097/IAE.0000000000002265
25. Saravia M, Zeman L, Berra A. Lyophilized amniotic membrane patch (LAMPatch) as a replacement of tamponades in the treatment of primary rhegmatogenous retinal detachment. Int J Retina Vitreous. 2020;6(1):58. doi:10.1186/s40942-020-00264-7
26. Caporossi T, De Angelis L, Pacini B, Rizzo S. Amniotic membrane for retinal detachment due to paravascular retinal breaks over patchy chorioretinal atrophy in pathologic myopia. Eur J Ophthalmol. 2020;30(2):392–395. doi:10.1177/1120672119891415
27. Panozzo G, Mercanti A. Optical coherence tomography findings in myopic traction maculopathy. Arch Ophthalmol. 2004;122(10):1455–1460. doi:10.1001/archopht.122.10.1455
28. Rizzo S, Caporossi T, Tartaro R, et al. A human amniotic membrane plug to promote retinal breaks repair and recurrent macular hole closure. Retina. 2019;39:S95–S103. doi:10.1097/IAE.0000000000002320
29. Nakamura T, Yoshitani M, Rigby H, et al. Sterilized, freeze-dried amniotic membrane: a useful substrate for ocular surface reconstruction. Invest Ophthalmol Vis Sci. 2004;45(1):93–99. doi:10.1167/iovs.03-0752
30. Garcin T, Gain P, Thuret G. Epiretinal large disc of blue-stained lyophilized amniotic membrane to treat complex macular holes: a 1-year follow-up. Acta Ophthalmol. 2022;100(2):e598–e608. doi:10.1111/aos.14909
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.