Back to Journals » Clinical Ophthalmology » Volume 18
A Randomized Pilot Study of Four Dosing Schemes of Sublingual Methazolamide in Glaucoma Patients
Authors Tan NE , Patnaik JL , McWilliams S , Seibold LK, Kahook MY
Received 15 September 2024
Accepted for publication 2 December 2024
Published 21 December 2024 Volume 2024:18 Pages 3893—3901
DOI https://doi.org/10.2147/OPTH.S496420
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Nicholas E Tan, Jennifer L Patnaik, Sara McWilliams, Leonard K Seibold, Malik Y Kahook
Sue-Anschutz Rodgers Eye Center at the University of Colorado, Aurora, CO, USA
Correspondence: Malik Y Kahook, Sue Anschutz-Rodgers Eye Center, 1675 Aurora Court, Aurora, CO, 80045, USA, Email [email protected]
Purpose: To evaluate the safety and efficacy of sublingual methazolamide in patients with open-angle glaucoma (OAG) and inform future trial design.
Methods: Fourteen participants (28 eyes) aged 50 to 90 years with bilateral OAG and intraocular pressure (IOP) between 18 and 35 mmHg after medication washout were included. Participants were randomized to receive either 25 mg or 50 mg of sublingual methazolamide once daily for one week, followed by twice-daily administration during the second week. The primary outcome was change in IOP from baseline to days 7 and 14. Secondary outcomes included changes in serum methazolamide levels, serum electrolytes, urine pH and electrolytes, and side effects.
Results: After randomization, exclusion, and two dropouts, four patients in the 25 mg group and ten in the 50 mg group completed the study in full. Both doses of sublingual methazolamide resulted in significant reductions in IOP from the post-washout baseline at all follow-up points (all p < 0.05). Lowest mean IOPs were recorded 8 hours post-dose; after a week of daily dosing, the 25 mg and 50 mg groups achieved reductions of 6.6 mmHg (− 26.5%) and 4.2 mmHg (− 19.3%), respectively (both p < 0.001). Twice-daily dosing resulted in significantly lower morning IOPs compared to once-daily in each group (p = 0.05 for 25 mg; p = 0.003 for 50 mg). Serum methazolamide levels correlated with dose amount and frequency. Serum electrolyte levels were stable throughout, while urinary pH and urinary electrolytes fluctuated based on time since last dose. Side effects of mild headaches and/or fatigue were reported by 3 out of 14 (21.4%) participants, with no serious adverse events.
Conclusion: Sublingual methazolamide demonstrated effective IOP reduction with a favorable safety profile. Twice-daily dosing may offer more sustained IOP control. These findings support further investigation into sublingual methazolamide as an alternative glaucoma treatment.
Keywords: IOP, carbonic anhydrase inhibitor, pressure, compounding
Introduction
Glaucoma is a leading cause of irreversible blindness, affecting millions of people worldwide.1 Current interventions focus on the only modifiable risk factor, intraocular pressure (IOP).2 Eye drops are the most utilized vehicle for delivering IOP-reducing therapy.2,3 However, patients often struggle with consistent drop usage due to factors such as difficulties with administration, inconvenience, forgetfulness and local reactions.4–6 Poor adherence may in turn compromise the effectiveness of treatment. As a result, there has been significant interest in alternative methods of achieving pressure reduction, such as through intracameral, sustained-release implants.7,8 Concerns with such alternatives, however, relate to their procedural nature, duration of effectiveness, and potential for serious complications.9
A third path for glaucoma pharmacotherapy is provided by systemically absorbed carbonic anhydrase inhibitors (CAIs). CAIs are among the oldest medications for IOP reduction.10 CAIs accomplish ocular hypotensive effects by decreasing aqueous humor production. Oral acetazolamide is used sparingly for IOP lowering due to its systemic side effects and less favorable long-term profile compared to topical therapies.11 Methazolamide, another oral CAI, precipitates fewer and less intense systemic side effects than acetazolamide with the tradeoff of more gradual IOP reduction.12–15 The dose of oral methazolamide used in clinical practice is typically 50–100 mg three times per day.12
While methazolamide is FDA-approved for IOP reduction, the sublingual formulation is only available through compounding pharmacies and has not been extensively studied. However, the sublingual approach comes with advantages. Sublingual administration tends to bypass gastric absorption, leading to less first-pass metabolism by the liver.16 Furthermore, onset of action tends to be faster with direct translocation into the venous system.16 In sum, this should allow for lower dosing amounts to achieve similar efficacy, which in turn may translate to fewer side effects. Also, at the University of Colorado, we have observed a rise in patient engagement with compounding pharmacies in order to receive customized therapies.
Given the potential for sublingual methazolamide to help fill a patient-centered need in glaucoma pharmaceuticals, we performed a pilot study of its safety and efficacy. We specifically evaluated dosages of 25 mg or 50 mg, once or twice daily. Efficacy was evaluated through serial IOP readings and methazolamide serum levels, while safety was assessed through serum electrolytes, urine studies, and subjective side effects. The ultimate goal is for our current study to provide preliminary information for designing future trials.
Methods
The current study was a single-site, proof-of-concept randomized controlled trial approved by the University of Colorado institutional review board (approval #22-0721). Written informed consent was obtained from all enrollees, and the study was conducted in accordance with the Declaration of Helsinki. Participants were assigned to receive either 25 mg or 50 mg of sublingual methazolamide, administered once daily for one week and twice daily for the second week. No placebo group was used. The clinicaltrials.gov registration number is: NCT05498103.
The principal investigator (MYK) and collaborating research staff enrolled participants at the University of Colorado Eye Center’s glaucoma service from February 2023 through July 2023. Research technicians performed simple randomization for each eligible participant with binary random number generation for a goal of parallel 1:1 assignment. Assignments were unknown to technicians until enrollment, while clinical investigators were masked throughout follow-up and outcomes assessment. Patients were not masked; the 50 mg group took two pills per dosing, while the 25 mg group took one. All sublingual methazolamide was compounded by the same pharmacy, which has years of experience producing the sublingual formulation.
Eligible participants were between 50 and 90 years old, could read and write in English, and had open-angle glaucoma (OAG) bilaterally. Additionally, they were required to demonstrate an untreated IOP between 18 and 35 mmHg after washout. Exclusion criteria encompassed non-OAG, severe or end-stage glaucoma, change in IOP-modifying medications within the past 30 days, renal or hepatic dysfunction, adrenocortical insufficiency, electrolyte imbalances, intolerance to carbonic anhydrase or sulfa medications, diabetes mellitus, active steroid therapy, history of intraocular injections, and pregnancy or breastfeeding. All IOP readings in the study were obtained via Goldmann applanation tonometry.
At the screening visit, initial data including demographics and IOP were obtained. After screening, enrollees underwent standardized washouts ranging from 5 to 28 days depending on the glaucoma medications they were using (Supplementary Table 1). At the post-washout visit (day 1), repeat IOP was obtained to confirm inclusion; randomization followed. An advancing enrollee next completed a standardized side effects questionnaire (Supplementary Table 2) to establish symptom baseline that same visit. Blood draws and urine test samples were obtained and assessed for pre-dose electrolytes, methazolamide levels, and urinary pH. Subsequently, the first dose of methazolamide was provided at approximately 8 AM in clinic. Education on technique was provided, including drinking water 10 to 15 minutes prior, sublingual application, avoiding food or drink for 30–45 minutes post-dose, and avoiding teeth-brushing or tobacco use within 2 hours of dosing. Blood and urine were drawn again in clinic at 1 hour and 3 hours post-dosing. IOP was repeated at 4 and 8 hours post-dosing, after which the participant returned home. Pills and instructions were provided for subsequent home doses.
Study participants took methazolamide for a total of two weeks. Patients were informed to record any side effects observed using the aforementioned question inventory both at home and at clinic follow-up. At the end of a participant’s first week (day 7), an early morning clinic appointment was scheduled. During that appointment the participant had their urine, blood, and IOP obtained prior to the morning dose. The sublingual methazolamide was then self-administered in-office at 8 AM. Blood draws and urine collection were repeated at 1 and 3 hours post-dosing, and IOP at 4 and 8 hours. The participant was instructed to increase dosing frequency to twice daily at 8AM and 8PM, starting the night of the day 7 visit. Repeat measurements were performed upon return to clinic at the end of week 2 (day 14). Afterwards, the participant discontinued methazolamide and returned to pre-enrollment therapy. The symptom questionnaire was presented over phone 1 week after study completion to monitor for post-therapy side effects.
The primary outcome measure was change in IOP over time, incorporating data from the intake, day 1, day 7, and day 14 visits. IOP changes were compared both between groups (25 mg versus 50 mg) and within groups (once daily versus twice daily). Secondary outcomes included changes in plasma methazolamide, plasma electrolytes, urinary pH, and urinary electrolytes, as well as adverse events throughout the study.
Being a pilot study, the initial enrollment goal was up to 30 participants diagnosed with OAG; no a priori power analysis was conducted. Data were analyzed by an epidemiologist (JLP). Statistical comparisons for baseline values between groups were obtained via the t-test for age, Fisher’s exact tests for categorical variables, and Wilcoxon rank sum tests for laboratory values. IOP comparisons were performed using generalized estimating equations with an exchangeable correlation structure to account for patients having two eyes included in the study. Mean IOP and standard errors (SE) are presented at pre-study washout, and at three time points (pre-treatment, 4 and 8 hours post-treatment) over three days (baseline, day 7 and day 14). Laboratory results were evaluated with descriptive statistics and comparison to reference levels. Data analyses were performed only for participants who had completed the trial in full. Statistical significance was set at p < 0.05.
Results
A total of 17 participants contributing 34 eyes were enrolled at the time recruitment was terminated. Study termination was due to resource constraints within the study group staff and difficulties with enrolling patients in a timely manner due to the burden of several extended visits over a short period of time. Seven participants were randomized to the 25 mg group, and ten to the 50 mg group (Figure 1). Two participants in the 25mg group exited the study prior to receiving methazolamide. The first failed post-washout screening due to an IOP of 49 in the left eye, while the second voluntarily withdrew, citing blood pressure concerns. During follow up, one patient in the 25 mg group voluntarily withdrew midway through the second week of treatment because of concerns regarding a mild skin rash that was present prior to and stable since enrollment. All other participants received the intended treatment dosings as assigned and completed the study. There were no participants lost to follow-up. Enrollment started in February 2023, and the last follow-up visit was completed on September 2023. In total, 14 participants contributing 28 eyes completed the study in full.
 |
Figure 1 Enrollment, Randomization, and Losses. |
Baseline data is compiled in Table 1. There were no significant demographic differences between the two study groups. The average age of the sample was 66 years, with a near equal distribution of males and females (Table 1). Most participants identified as being of white race (n = 12, 85.7%). Average serum chemistry values and urinary pH were similar between groups, and no participants in either group had detectable levels of methazolamide prior to initiating the study medication. Urinary sodium, potassium, and chloride were each numerically higher in the 25 mg group, but not to an extent that reached statistical significance (p = 0.13, 0.08, and 0.31 respectively).
 |
Table 1 Baseline Demographics and Lab Values by Methazolamide Dosage Group |
IOP changes over the course of the study are described in Table 2. Prior to medication washout, mean IOPs were similar between groups (18.1 ± 2.0 mmHg vs 17.6 ± 0.9 mmHg, p = 0.81). After washout, the 25 mg group had a numerically higher mean IOP of 24.9 ± 2.1 mmHg compared to the 50 mg group’s 21.8 ± 0.6 mmHg, although this difference was not statistically significant (p = 0.16). At all timepoints after the first methazolamide dose, pressures in both groups were reduced compared to the post-washout baseline values (Table 2; p < 0.05 for all). On all three days of monitoring, pressures were lowest 8 hours after sublingual methazolamide administration, regardless of daily or twice daily dosing. The lowest pressures achieved were 17.7 ± 1.2 mmHg in the 25 mg group (baseline day) and 17.0 ± 0.8 mmHg in the 50 mg group (day 14). When compared to the IOPs obtained prior to medication washout, the 25 mg group generally had higher follow-up pressures with exception to the values obtained at 8 hours post-dose (Table 2). The 50 mg group, in contrast, demonstrated no follow-up IOPs that were significantly higher than the mean pre-washout reading (p ≥ 0.05 for all). Day 7 and day 14 IOPs obtained prior to the morning methazolamide dose were also compared within groups in order to examine the effects of daily versus twice daily dosing. In the 25 mg cohort, the twice daily pre-treatment IOP was 1.2 mmHg less than the once daily equivalent (p = 0.05). In the 50 mg cohort, the difference was 1.7 mmHg (p = 0.003).
 |
Table 2 Mean Intraocular Pressures Before and After Sublingual Methazolamide |
Serum sodium, potassium, chloride, and bicarbonate levels at each follow-up timepoint are charted in Figure 2. Electrolyte values were similar between groups. Over time, including after the second week’s doubling in dose, there was minimal to no change compared to baseline. As for urinary electrolytes, levels of sodium, chloride, and potassium were generally lowest at 3 hours post-dose, and highest prior to dosing. Urinary pH curves were similar between groups, with peak pHs of around 7 being observed at 3 hours post-dose. Figure 3 illustrates how serum methazolamide levels increased in correspondence with dose amount and dose frequency. With once daily dosing of either 25 mg or 50 mg, serum methazolamide levels continued to increase from 1 to 3 hours post-dose. However, with twice daily dosing, peak concentration was achieved before the 1-hour post-dose blood draw and remained stable at 3 hours. Twice daily dosing also resulted in higher pre-dose levels of methazolamide in each group. Overall, 3 out of 14 patients (21.4%) reported side effects that were surveyed as “uncomfortable” or “bothersome.” In the 25 mg group, one patient (25.0%) complained of an uncomfortable headache that occurred once when receiving twice daily dosing. In the 50 mg cohort, two patients (20.0%) noted side effects. Both complained of uncomfortable or bothersome fatigue present during both once daily and twice daily dosing, and one noted a standalone, light headache that occurred while on twice daily dosing. No patients reported any intolerable side effects, nor did any report serious adverse events related to the study medication.
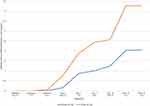 |
Figure 3 Serum Methazolamide Levels throughout Follow-up. Pre Tx = Level obtained prior to the morning methazolamide dose. |
Discussion
In our pilot trial of sublingual methazolamide, the drug demonstrated effective IOP control for glaucoma patients. Both the 25 mg and 50 mg groups achieved statistically significant reductions in post-washout IOPs at every follow-up timepoint. The lowest IOPs were recorded at 8 hours post-dose, suggesting an interval requirement for peak efficacy. Mean IOP after 7 days, 8 hours of daily 25 mg administration was reduced by 6.6 mmHg (26.5% decrease) compared to the post-washout baseline. At the same timepoint, daily 50 mg administration achieved a mean reduction of 4.2 mmHg (19.3% decrease) versus baseline. Clinically relevant reductions from the post-washout baseline and similarities to pre-washout IOPs support the use of once daily sublingual methazolamide as an ocular hypotensive. However, the main weakness of once daily dosing was in IOP fluctuation. Generally, the morning pre-dose IOPs on the once daily schemes were higher than those recorded while on previous treatment. The 25 mg group’s mean IOPs in the morning and at 4 hours post-dose compared especially poorly to the pre-washout mean, potentially reflecting a relative advantage of 50 mg dosing in accomplishing earlier pressure reduction. However, the transition to twice daily administration in the second week led to lower pre-dose IOPs in both cohorts, indicating a longer duration of therapeutic effect. For comparison, consider acetazolamide, the most common oral CAI used in glaucoma.17 A dose of 500 mg of acetazolamide achieves a quicker peak onset of action 2–4 hours after administration.18 One prospective study found that 500 mg of oral acetazolamide decreased IOP by 17.19% at 2 hours after administration, while other studies have reported a range of 15–34% from varying doses.17,19–21 Per our data, sublingual methazolamide may yield similar reductions.
The safety profile of sublingual methazolamide was good. Average serum sodium, potassium, chloride, and bicarbonate values remained stable across two weeks and stayed within the University of Colorado’s reference lab ranges. Urinary pH rose temporarily after dosing in a manner consistent with known CAI renal effects and never exceeded the reference bounds.22 Urine electrolytes, similarly, undulated in accordance to methazolamide’s diuretic effects.22 Further interpretations of urine electrolytes are limited, as both the University of Colorado and other sources only list reference ranges for 24-hour collections, not spot measurements.23 There was no evidence of the hypokalemia or metabolic acidosis observed with oral CAI use.11 No patients reported uncomfortable or bothersome side effects while on 25 mg once daily dosing. One patient from each cohort reported mild headache while on twice daily dosing, which was transient and of unclear relation to the study drug. Two patients in the 50 mg group reported fatigue. The fatigue persisted from daily through twice daily dosing, providing evidence for causation. Fatigue is also a well-documented dose-dependent effect of oral CAIs.11,24 There were no reports in our sample of other bothersome or intolerable symptoms linked with CAIs, such as paresthesias, gastrointestinal discomfort, or urinary frequency. No patient had a serious adverse event. The current study’s 21.4% rate of side effects compares reasonably well to the 30–40% frequency reported in patients taking different classes of CAIs for various indications.25 When compared to acetazolamide dosed specifically for glaucoma, it has been noted that as many as 50% of patients may discontinue the agent due to side effects.11 In our brief trial, no patient dropped out because of a new symptom that arose while on sublingual methazolamide.
To our knowledge, our paper is the first to study the sublingual approach to methazolamide administration in glaucoma patients. Our review of the literature reveals no prior published studies on sublingual methazolamide for IOP lowering. The study’s key strengths include its prospective enrollment and effective randomization with no significant baseline differences between groups. No enrollee in either group was lost to follow-up. To minimize risk of confounding from pretrial drugs, washouts of ocular hypotensive agents were standardized and followed the recommendations borne from pharmacologic studies.26–28 IOP and electrolyte measurements at several timepoints captured the granular effects of four dosing schemes. A comprehensive questionnaire that covered known side effects of CAIs and allowed for free-text entry was used; this enabled wide capture of any potential adverse events throughout follow-up, with the tradeoff of possible over-reporting of unrelated complaints.
Our pilot trial has additional limitations. First, the small sample size limits internal validity, and may have contributed to differences such as the numerically higher post-washout baseline IOP in the 25 mg group. There was differential participation; all three participants who either were excluded after enrollment or dropped out were in the 25 mg group. Having only four participants in the 25 mg group contributed to wider standard errors in IOP measurements, increased variability in urine electrolyte levels, lessened statistical power, and limited inferences from side effects reports. The short two-week follow-up may have failed to capture gradual systemic effects, such as electrolyte shifts. As part of the protocol, patients were thoroughly educated on correct sublingual administration. However, in a non-study setting, patients may take the drug in a manner that interferes with absorption. Though the washout process was comprehensive, pre-washout IOPs were not measured at specific times relative to eye drop administration, adding inherent variability to those measurements that impacted comparisons with follow-up IOPs. Another limitation is a lack of patient diversity that reduces generalizability. Most participants were of white race, and the remainder were Latino. Lastly, though a single pharmacy supplied the agent for our study, other compounding pharmacies may use differing techniques that could affect absorption.
Conclusion
Overall, if one were to design future trials of sublingual methazolamide based on the current study’s findings, the two most clinically useful interventions may be 25 mg twice daily and 50 mg twice daily. Twice daily demonstrated more sustained pressure reductions and did not substantially increase side effect frequency compared to one dose daily. The 25 mg twice daily dose may cause less fatigue with the tradeoff of decreased early-phase pressure reduction. With the limitations of a small sample and brief follow-up in mind, the favorable outcomes of sublingual methazolamide across multiple doses make it a promising alternative to not only oral CAIs, but also topical drops. Such drops are linked with adherence challenges and can contribute to worsening of ocular surface disease.4,29 If safety and efficacy are confirmed by larger-scale trials, sublingual methazolamide may offer another tool for effectively treating glaucoma patients.
Abbreviations
IOP, Intraocular Pressure; OAG, Open-Angle Glaucoma; CAIs, Carbonic Anhydrase Inhibitors; FDA, Food and Drug Administration.
Data Sharing Statement
Data is available upon reasonable request of the corresponding author.
Disclosure
Author Leonard K. Seibold reports the following: Consultant to New World Medical, Allergan, and Oculus Surgical. Author Malik Y. Kahook reports the following: Consultant to New World Medical, SpyGlass Pharma and Zeiss. Patent Royalties: Alcon, SpyGlass Pharma, New World Medical and Zeiss. Ownership: SpyGlass Pharma. All authors report no other conflicts of interest in this work.
References
1. Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081–2090. doi:10.1016/j.ophtha.2014.05.013
2. Jonas JB, Aung T, Bourne RR, Bron AM, Ritch R, Panda-Jonas S. Glaucoma. Lancet. 2017;390(10108):2183–2193. doi:10.1016/S0140-6736(17)31469-1
3. Gedde SJ, Vinod K, Wright MM, et al. Primary open-angle glaucoma preferred practice pattern®. Ophthalmology. 2021;128(1):P71–p150. doi:10.1016/j.ophtha.2020.10.022
4. Wolfram C, Stahlberg E, Pfeiffer N. Patient-reported nonadherence with glaucoma therapy. J Ocul Pharmacol Ther. 2019;35(4):223–228. doi:10.1089/jop.2018.0134
5. Quaranta L, Riva I, Gerardi C, Oddone F, Floriani I, Konstas AG. Quality of life in glaucoma: a review of the literature. Adv Ther. 2016;33(6):959–981. doi:10.1007/s12325-016-0333-6
6. Schwartz GF, Hollander DA, Williams JM. Evaluation of eye drop administration technique in patients with glaucoma or ocular hypertension. Curr Med Res Opin. 2013;29(11):1515–1522. doi:10.1185/03007995.2013.833898
7. Sirinek PE, Lin MM. Intracameral sustained release bimatoprost implants (Durysta). Semin Ophthalmol. 2021;36:1–6. doi:10.1080/08820538.2021.1894889
8. Sarkisian SR, Ang RE, Lee AM, et al. Travoprost intracameral implant for open-angle glaucoma or ocular hypertension: 12-month results of a randomized, double-masked trial. Ophthalmol Ther. 2024;13(4):995–1014. doi:10.1007/s40123-024-00898-y
9. Al-Qaysi ZK, Beadham IG, Schwikkard SL, Bear JC, Al-Kinani AA, Alany RG. Sustained release ocular drug delivery systems for glaucoma therapy. Expert Opin Drug Deliv. 2023;20(7):905–919. doi:10.1080/17425247.2023.2219053
10. Berson FG, Epstein DL, Grant WM, Hutchinson BT, Dobbs PC. Acetazolamide dosage forms in the treatment of glaucoma. Arch Ophthalmol. 1980;98(6):1051–1054. doi:10.1001/archopht.1980.01020031041005
11. Gulati S, Aref AA. Oral acetazolamide for intraocular pressure lowering: balancing efficacy and safety in ophthalmic practice. Expert Rev Clin Pharmacol. 2021;14(8):955–961. doi:10.1080/17512433.2021.1931123
12. Lichter PR, Musch DC, Medzihradsky F, Standardi CL. Intraocular pressure effects of carbonic anhydrase inhibitors in primary open-angle glaucoma. Am J Ophthalmol. 1989;107(1):11–17. doi:10.1016/0002-9394(89)90807-6
13. Becker B. Use of methazolamide (neptazane) in the therapy of glaucoma; comparison with Acetazolamide (diamox). Am J Ophthalmol. 1960;49:1307–1311. doi:10.1016/0002-9394(60)91346-5
14. Campbell DA. Effect of neptazane on intra-ocular pressure in relation to its systemic action and its clinical application. Br J Ophthalmol. 1960;44(7):415–429. doi:10.1136/bjo.44.7.415
15. Dahlen K, Epstein DL, Grant WM, Hutchinson BT, Prien EL Jr, Krall JM. A repeated dose-response study of methazolamide in glaucoma. Arch Ophthalmol. 1978;96(12):2214–2218. doi:10.1001/archopht.1978.03910060516009
16. Goswami T, Jasti B, Li X. Sublingual drug delivery. Crit Rev Ther Drug Carrier Syst. 2008;25(5):449–484. doi:10.1615/CritRevTherDrugCarrierSyst.v25.i5.20
17. Loiselle AR, de Kleine E, van Dijk P, Jansonius NM. Intraocular and intracranial pressure in glaucoma patients taking Acetazolamide. PLoS One. 2020;15(6):e0234690. doi:10.1371/journal.pone.0234690
18. Yano I, Takayama A, Takano M, et al. Pharmacokinetics and pharmacodynamics of acetazolamide in patients with transient intraocular pressure elevation. Eur J Clin Pharmacol. 1998;54(1):63–68. doi:10.1007/s002280050422
19. Zinkernagel MS, Ebneter A. Acetazolamide influences ocular pulse amplitude. J Ocul Pharmacol Ther. 2009;25(2):141–144. doi:10.1089/jop.2008.0077
20. Alm A, Berggren L, Hartvig P, Roosdorp M. Monitoring acetazolamide treatment. Acta Ophthalmol. 1982;60(1):24–34. doi:10.1111/j.1755-3768.1982.tb05778.x
21. Friedland BR, Mallonee J, Anderson DR. Short-term dose response characteristics of Acetazolamide in man. Arch Ophthalmol. 1977;95(10):1809–1812. doi:10.1001/archopht.1977.04450100111014
22. Purkerson JM, Schwartz GJ. The role of carbonic anhydrases in renal physiology. Kidney Int. 2007;71(2):103–115. doi:10.1038/sj.ki.5002020
23. Ferri FF. Laboratory values and interpretation of results. In: Ferri’s Best Test.
24. Dominelli PB, McNeil CJ, Vermeulen TD, et al. Effect of acetazolamide and methazolamide on diaphragm and dorsiflexor fatigue: a randomized controlled trial. J Appl Physiol. 2018;125(3):770–779. doi:10.1152/japplphysiol.00256.2018
25. Swenson ER. Safety of carbonic anhydrase inhibitors. Expert Opin Drug Saf. 2014;13(4):459–472. doi:10.1517/14740338.2014.897328
26. Quaranta L, Gandolfo F, Turano R, et al. Effects of topical hypotensive drugs on circadian IOP, blood pressure, and calculated diastolic ocular perfusion pressure in patients with glaucoma. Invest Ophthalmol Vis Sci. 2006;47(7):2917–2923. doi:10.1167/iovs.05-1253
27. Stewart WC, Holmes KT, Johnson MA. Washout periods for brimonidine 0.2% and latanoprost 0.005%. Am J Ophthalmol. 2001;131(6):798–799. doi:10.1016/S0002-9394(00)00930-2
28. Hong YJ, Shin DH, Ahn BH, McCarty B. Intraocular pressure after a two-week washout following long-term timolol or levobunolol. J Ocul Pharmacol Ther. 1995;11(2):107–112. doi:10.1089/jop.1995.11.107
29. Anwar Z, Wellik SR, Galor A. Glaucoma therapy and ocular surface disease: current literature and recommendations. Curr Opin Ophthalmol. 2013;24(2):136–143. doi:10.1097/ICU.0b013e32835c8aba
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


