Back to Journals » OncoTargets and Therapy » Volume 18
A Rare Histologic Transformation from EGFR-Positive Lung Adenocarcinoma to EGFR-Negative Squamous Cell Carcinoma After EGFR-TKIs Resistance: A Case Report
Authors Qiu M, Guo P, Lin J, Wang S, Wang W , Yang J, Huang Z, Cao Y
Received 19 January 2025
Accepted for publication 20 May 2025
Published 18 July 2025 Volume 2025:18 Pages 803—809
DOI https://doi.org/10.2147/OTT.S513879
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Sanjay Singh
Mengli Qiu,1 Peiwen Guo,1 Jieheng Lin,2 Sisi Wang,2 Wenping Wang,2 Jianying Yang,3 Zhongming Huang,4 Yang Cao2
1The First Clinical School, Guangzhou University of Chinese Medicine, Guangzhou, People’s Republic of China; 2Department of Oncology, The First Affiliated Hospital, Guangzhou University of Chinese Medicine, Guangzhou, People’s Republic of China; 3Department of Oncology, The 928rd Hospital of the Chinese People’s Liberation Army Joint Logistic Support Force, Haikou, People’s Republic of China; 4Department of Oncology, Cancer Hospital of Shantou University Medical College, Shantou University, Shantou, People’s Republic of China
Correspondence: Yang Cao, Department of Oncology, The First Affiliated Hospital, Guangzhou University of Chinese Medicine, Guangzhou, 510000, People’s Republic of China, Tel +86 028-13802945884, Email [email protected] Zhongming Huang, Department of Oncology, Cancer Hospital of Shantou University Medical College, Shantou University, Shantou, 515000, People’s Republic of China, Tel +86 028-13060033385, Email [email protected]
Abstract: Lung adenocarcinoma (ADC) harboring epidermal growth factor receptor (EGFR) mutations rarely transforms into squamous cell carcinoma (SCC) following resistance to targeted therapy. Here, we present a case of EGFR-positive ADC that transformed into EGFR-negative SCC after developing resistance to EGFR tyrosine kinase inhibitors (TKIs). The patient experienced progressive disease after one cycle of chemotherapy and subsequently underwent five courses of tislelizumab combined with chemotherapy. Although the primary tumor showed a partial response to this combined regimen, intracranial metastases continued to progress, ultimately leading to the patient’s death. Notably, the patient survived for 8 months after SCC transformation with immuno-chemotherapy, a significantly longer duration than the previously reported median survival of 3.5 months. This case underscores the occurrence of genomic instability, histological transformation, and dissociated response (DR) following treatment with EGFR-TKIs in EGFR-positive lung ADC. We hypothesize that these phenomena may be driven by tumor heterogeneity and the dynamic variability within the tumor microenvironment (TME).
Keywords: immunotherapy, chemotherapy, brain metastases, epidermal growth factor receptor, dissociated response, histological transformation
Introduction
Epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) represent the standard treatment for advanced EGFR-mutant non-small cell lung cancer (NSCLC). While patients typically exhibit marked initial responses, acquired resistance invariably develops within 9–12 months of treatment initiation.1 Histological transformation constitutes a pivotal mechanism underlying acquired resistance, encompassing phenotypic transitions to small cell lung cancer and squamous cell carcinoma (SCC). Emerging evidence underscores that molecular mechanisms including intra-tumoral heterogeneity, clonal evolution, dysregulated cancer stem cell signaling pathways, and inactivation of critical tumor suppressor genes may collectively contribute to this transformation process.2 Notably, EGFR-positive lung adenocarcinoma (ADC) patients undergoing SCC transformation post-EGFR-TKI therapy frequently retain original EGFR mutation profiles while demonstrating poor clinical outcomes.3–6 Therapeutic strategies for SCC-transformed patients remain inadequately investigated, with current expert consensus advocating chemotherapy regimens analogous to those used for primary SCC.7 Although immune checkpoint inhibitors (ICIs) in combination with chemotherapy represent the standard treatment for advanced pulmonary SCC, their efficacy in SCC-transformed patients remains unestablished.
This study presents a case report of particular clinical significance: A patient with primary EGFR exon 19 deletion (19del) mutant ADC receiving EGFR-TKIs underwent histologically confirmed transformation to EGFR wild-type SCC. Following one cycle of chemotherapy, the patient experienced rapid progression of pulmonary lesions. A subsequent switch to immuno-chemotherapy resulted in sustained regression of the pulmonary lesions. However, the patient ultimately succumbed to progressive brain metastases, with an overall survival (OS) of 8 months following SCC transformation.
Case Presentation
In November 2016, a 60-year-old male was admitted to the hospital presenting with cough and sputum production. The patient had no history of tobacco use and no family history of cancer. Chest X-ray revealed a space-occupying lesion in the left lung. A diagnosis of ADC was confirmed through transbronchial needle aspiration combined with biopsy and immunohistochemistry (IHC) of the primary tumor (Figure 1). Next-generation sequencing (NGS) of the tumor tissue revealed an EGFR exon 19 deletion (19del) mutation. Imaging data, including CT scans, demonstrated metastases from the left upper mediastinal tumor to the bilateral lungs, left pleura, S4 segment of the liver, and the T5 and T11 vertebrae. Cranial MRI detected metastases in the left frontal lobe.
The patient began first-line treatment with gefitinib in January 2017. In March 2019, hematological genetic testing showed no detectable mutations. The disease remained stable until May 2020, when CT scans revealed tumor progression (Figures 2A–D). In response to this progression, a repeat NGS testing confirmed the presence of the EGFR T790M mutation. The patient was then switched to osimertinib as second-line therapy. In January 2021, a mass approximately 60×40 mm was detected beneath the left clavicle, and CT scans revealed significant enlargement of the left upper lobe tumor (Figure 2E), along with an increase in the lesion in the left temporal lobe compared to previous scans. Biopsy and IHC of the left clavicular mass found metastatic poorly differentiated SCC, but no EGFR driver mutations appeared through the NGS of the tumor tissue.
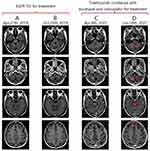
Referring to the NCCN guidelines,8 the patient was treated with carboplatin and docetaxel. However, after one cycle of treatment, the left upper lobe tumor grew from 102×98 mm to 144×115 mm. In February 2021, the patient began receiving tislelizumab coupled with carboplatin and docetaxel. CT scans conducted in April and June 2021 showed a partial response in the pulmonary and left clavicular tumors (Figure 2F and G), but the brain metastases continued to progress (Figure 3). Following multidisciplinary team consultation, the patient underwent palliative whole-brain radiation therapy delivering 30Gy in 10 fractions. Metastatic lesions exceeding 1cm diameter received simultaneous integrated boost (SIB) to 40Gy/10 fractions, while subcentimeter metastases received SIB to 35Gy/10 fractions. The patient underwent a total of five courses of tislelizumab combined with carboplatin and docetaxel. However, this regimen was poorly tolerated due to the patient’s deteriorating physical condition, leading to its discontinuation. The patient was then provided with optimal supportive care for the next three months and died in August 2021, with an OS of 8 months after SCC transformation. Ethics approval for this study was obtained from the First Affiliated Hospital of Guangzhou University of Chinese Medicine (NO: 2023–016). Institutional approval is required for the publication of case details and has been obtained. Written informed consent was obtained from the patient’s wife for publication of this case report and accompanying images due to the patient’s passing.
Discussion
This case represents the first reported instance of EGFR-positive ADC undergoing transformation to EGFR-negative SCC following acquired resistance. While SCC transformation after EGFR-TKI resistance has been previously documented,9 our case offers novel molecular insights and a unique therapeutic paradigm.
According to the NCCN guidelines,8 platinum-based doublet chemotherapy is the standard first-line treatment for stage IV lung SCC without driver mutations. As such, the patient in this case received a combination of docetaxel and carboplatin. However, after one cycle, follow-up CT scans indicated progressive disease, suggesting that platinum-based doublet chemotherapy may be ineffective in patients with transformed lung SCC. Several studies support the efficacy of immuno-chemotherapy over chemotherapy alone in NSCLC patients with brain metastases.10–12 Preclinical evidence suggests that chemotherapy can enhance the effectiveness of immunotherapy by inducing immunogenic cell death and disrupting the tumor’s immune evasion mechanisms.13 Furthermore, immunotherapy has been shown to increase the permeability of the blood-brain barrier,14 thereby facilitating the penetration of chemotherapy drugs into the brain and potentially improving the efficacy of combination treatments.
Moreover, for NSCLC patients with EGFR-TKI resistance, emerging evidence suggests that immunotherapy may offer potential therapeutic benefit, possibly due to variability in the tumor microenvironment (TME). At the time of initial diagnosis, the TME in EGFR-positive NSCLC patients is typically characterized as a “cold” TME, which is less responsive to PD-L1 inhibitors.15 However, EGFR-TKIs have been shown to remodel this environment, converting it from a “cold” to a “hot” TME.16 This shift likely enhances the efficacy of immunotherapy by upregulating PD-L1 expression on tumor cells and increasing the infiltration of effector T cells following EGFR-TKI treatment.17–19 In a study by Zhong et al20 patients with EGFR-sensitive mutations who failed EGFR-TKI therapy demonstrated improved progression-free survival and objective response rates when treated with a combination of tislelizumab and platinum-based doublet chemotherapy. The authors attributed these improvements to changes in the TME induced by the treatment. Based on this rationale and the high level of PD-L1 expression in the present patient (TPS = 75%), we decided to administer immunotherapy on top of chemotherapy given the current patient’s poor response to chemotherapy alone after multidisciplinary therapeutic discussion.
After two courses of immuno-chemotherapy, the patient’s primary lung tumor continued to shrink, while the intracranial metastases showed further progression. This phenomenon, known as “dissociated response” (DR), occurs in approximately 13.2% of NSCLC patients following immunotherapy.21 DR may serve as an independent factor indicative of a better prognosis, and continued immunotherapy may lead to more favorable outcomes in such cases.22 Zhou et al23 demonstrated that, in patients with advanced NSCLC receiving ICIs, those who continued ICIs after experiencing DR had significantly improved OS compared to those who did not continue treatment (10.63 vs 4.33 months, P=0.016). In light of this, the patient in this case was considered for further treatment with immuno-chemotherapy after experiencing a DR. However, despite receiving three additional courses of treatment, the patient’s intracranial metastases continued to grow while the lung tumor remained responsive. Ultimately, the patient could no longer tolerate the treatment due to deteriorating physical condition, leading to discontinuation of therapy. After receiving three months of optimal supportive care, the patient passed away.
Existing data indicate that patients experiencing SCC transformation generally exhibit poor prognosis, with a systematic analysis of 14 patients demonstrating a median OS (mOS) of only 3.5 months.24 A prior case report documented sustained remission in a patient with SCC transformation following osimertinib resistance, achieved with pembrolizumab monotherapy, ultimately resulting in a progression-free survival (PFS) and OS of 9 and 47 months, respectively.25 Notably, previous case reports of SCC-transformed patients predominantly employed chemotherapy or targeted therapies. However, this case demonstrates rapid progression of pulmonary lesions following one cycle of chemotherapy; A subsequent switch to immuno-chemotherapy resulted in sustained regression of pulmonary lesions, despite concurrent suboptimal control of brain metastases. Although this patient’s survival (8 months) proved substantially shorter than the 47-month reported in the aforementioned case, this discrepancy likely reflects the unfavorable prognostic consequences of uncontrolled cerebral metastases. These collective findings underscore the potential therapeutic utility of immuno-chemotherapy for managing pulmonary lesions in EGFR-TKI-resistant SCC transformations. Furthermore, the discordant treatment responses between pulmonary and cerebral metastases provide critical clinical insights into tumor heterogeneity and microenvironmental evolution during histological transformation. Future research should further investigate strategies for controlling brain metastases to improve overall patient outcomes.
This patient’s case provides valuable insights into the complexities of cancer treatment, particularly in the context of targeted therapies and metastases. The genomic changes (conversion from EGFR-positive to EGFR-negative status), pathological type conversion (shift from ADC to SCC), and DR (incongruent response to immunotherapy between primary lung and intracranial metastases) after receiving EGFR-TKIs are indicative of the dynamic nature of cancer progression. Here, tumor heterogeneity and TME variability play a pivotal role in shaping treatment outcomes. Tumor heterogeneity is a critical factor in the failure of targeted therapies like EGFR-TKIs. Tumor evolution often leads to clonal diversification, where genetically distinct subclones coexist within a single tumor. As these clones evolve under selective treatment pressure, they can acquire different resistance mechanisms, which can manifest as discordant responses in different metastases.26,27 For example, while the primary lung tumor may initially respond to EGFR-TKI treatment in this case, certain subclones may develop resistance mechanisms, leading to progression in the brain metastases, despite the drug being effective in other regions. The TME variability also significantly impacts the effectiveness of cancer therapies.28 Different metastases, particularly in organs like the brain, can have distinct TMEs that influence tumor behavior and response to treatment. Brain metastases, for instance, are known to exhibit unique features such as blood-brain barrier challenges, altered immune cell infiltration, and an immune-suppressive environment. Studies29,30 have shown that NSCLC brain metastases often have lower CD8+ T-cell infiltration and distinct PD-L1 expression profiles compared to primary lung tumors, which can contribute to their resistance to immunotherapy. These findings may explain the observed discrepancy in efficacy between extracranial and intracranial response in patients with brain metastases receiving PD-L1 blockade therapy.
These observations underscore the importance of considering tumor heterogeneity and TME variability in clinical treatment decisions. In particular, treatments targeting EGFR may be effective for some subclones but not for others, depending on the genetic profile and the specific TME of each metastases. This highlights the need for more personalized treatment strategies that can account for both intra-tumor genetic heterogeneity and the unique characteristics of metastatic sites.
Conclusion
This case report describes the first documented transformation of EGFR-positive ADC to EGFR-negative SCC following acquired resistance. Notably, while pulmonary lesions demonstrated rapid progression during first-line chemotherapy post-SCC transformation, subsequent implementation of an immuno-chemotherapy combination regimen induced sustained tumor regression, accompanied by an 8-month survival duration post-transformation, underscoring the potential therapeutic value of this approach for managing SCC-transformed lesions following EGFR-TKI resistance. Although the patient ultimately succumbed to progressive brain metastases, the observed differential therapeutic response between pulmonary lesions and intracranial metastases provides critical clinical insights into tumor heterogeneity and the dynamic evolution of TME during histological transformation. These findings enhance our understanding of resistance mechanisms and inform the development of novel therapeutic strategies. Future investigations should focus on optimizing targeted therapies, radiation modalities, and immunotherapeutic approaches for brain metastasis management to improve overall survival. Furthermore, large-scale multicenter prospective clinical trials are warranted to validate the efficacy of immuno-chemotherapy in EGFR-TKI-resistant patients with SCC transformation.
Data Sharing Statement
All inquiries can be directed to the corresponding author (Y Cao and ZM Huang).
Ethics Approval and Consent to Participate
Ethics approval for this study was obtained from the First Affiliated Hospital of Guangzhou University of Chinese Medicine (NO: 2023-016). All procedures performed in this study were in accordance with the ethical standards of the institutional research committee and with the Declaration of Helsinki. Written informed consent was obtained from the patient’s wife for publication of this case report and accompanying images due to the death of this patient.
Acknowledgments
The authors would like to thank this patient and his family in this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; and gave final approval for the version to be published. All authors have agreed on the journal to which the article has been submitted, and agree to be accountable for all aspects of the work.
Funding
This study was funded by the Guangdong Basic and Applied Basic Research Foundation (Grant number: 2022A1515220052), and the Basic and Applied Basic Research Project of the Guangzhou Municipal Science and Technology Bureau City-University (Institute) Joint Funding Program (Grant number: 202201020553).
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Riely GJ, Yu HA. EGFR: the paradigm of an oncogene-driven lung cancer. Clin Cancer Res. 2015;21(10):2221–2226. doi:10.1158/1078-0432.Ccr-14-3154
2. Tan N, Li Y, Ying J, Chen W. Histological transformation in lung adenocarcinoma: insights of mechanisms and therapeutic windows. J Transl Internal Med. 2024;12(5):452–465. doi:10.1515/jtim-2024-0019
3. Bruno R, Proietti A, Alì G, et al. Squamous cell transformation and EGFR T790M mutation as acquired resistance mechanisms in a patient with lung adenocarcinoma treated with a tyrosine kinase inhibitor: a case report. Oncol Lett. 2017;14(5):5947–5951. doi:10.3892/ol.2017.6913
4. Shinohara S, Ichiki Y, Fukuichi Y, et al. Squamous cell carcinoma transformation from adenocarcinoma as an acquired resistance after the EGFR TKI therapy in (EGFR-mutated) non-small cell lung cancer. J Thoracic Dis. 2018;10(7):E526–e531. doi:10.21037/jtd.2018.06.83
5. Ji XZ, Liu ZD, Ye YP, et al. Advanced lung Adenocarcinoma with EGFR 19-del mutation transformed into SCC after EGFR-tyrosine Kinase inhibitors treatment: a case report. World J Clin Cases. 2024;12(20):4405–4411. doi:10.12998/wjcc.v12.i20.4405
6. Liao J, Li Y, Liu C, Long Q, Wang J. Case report: EGFR-positive early-stage lung Adenocarcinoma transforming to Squamous cell carcinoma after TKI treatment. Front Oncol. 2021;11:696881. doi:10.3389/fonc.2021.696881
7. Passaro A, Leighl N, Blackhall F, et al. ESMO expert consensus statements on the management of EGFR mutant non-small-cell lung cancer. Ann Oncol. 2022;33(5):466–487. doi:10.1016/j.annonc.2022.02.003
8. Riely GJ, Wood DE, Ettinger DS, et al. Non-small cell lung cancer, version 4.2024, NCCN clinical practice guidelines in oncology. J Nat Comprehensive Cancer Network. 2024;22(4):249–274. doi:10.6004/jnccn.2204.0023
9. Levin PA, Mayer M, Hoskin S, Sailors J, Oliver DH, Gerber DE. Histologic transformation from adenocarcinoma to Squamous cell Carcinoma as a mechanism of resistance to EGFR inhibition. J Thoracic Oncol. 2015;10(9):e86–e88. doi:10.1097/jto.0000000000000571
10. Powell SF, Rodríguez-Abreu D, Langer CJ, et al. Outcomes with pembrolizumab plus platinum-based chemotherapy for patients with NSCLC and stable brain metastases: pooled analysis of KEYNOTE-021, −189, and −407. J Thoracic Oncol. 2021;16(11):1883–1892. doi:10.1016/j.jtho.2021.06.020
11. Liang X, Chen X, Li H, Li Y. Tislelizumab plus chemotherapy is more cost-effective than chemotherapy alone as first-line therapy for advanced non-squamous non-small cell lung cancer. Front Public Health. 2023;11:1009920. doi:10.3389/fpubh.2023.1009920
12. Liang X, Chen X, Li H, Liu X, Li Y. Sugemalimab plus chemotherapy vs. chemotherapy for metastatic non-small-cell lung cancer: a cost-effectiveness analysis. Front Public Health. 2023;11:1054405. doi:10.3389/fpubh.2023.1054405
13. Zhou S, Xie J, Huang Z, et al. Anti-PD-(L)1 immunotherapy for brain metastases in non-small cell lung cancer: mechanisms, advances, and challenges. Cancer Lett. 2021;502:166–179. doi:10.1016/j.canlet.2020.12.043
14. Peng J, Hamanishi J, Matsumura N, et al. Chemotherapy induces programmed cell death-ligand 1 overexpression via the nuclear factor-κB to foster an immunosuppressive tumor microenvironment in ovarian cancer. Cancer Res. 2015;75(23):5034–5045. doi:10.1158/0008-5472.Can-14-3098
15. Yang L, He YT, Dong S, et al. Single-cell transcriptome analysis revealed a suppressive tumor immune microenvironment in EGFR mutant lung adenocarcinoma. J ImmunoTher Cancer. 2022;10(2):e003534. doi:10.1136/jitc-2021-003534
16. Jia Y, Li X, Jiang T, et al. EGFR-targeted therapy alters the tumor microenvironment in EGFR-driven lung tumors: implications for combination therapies. Int J Cancer. 2019;145(5):1432–1444. doi:10.1002/ijc.32191
17. Kim H, Kim SH, Kim MJ, et al. EGFR inhibitors enhanced the susceptibility to NK cell-mediated lysis of lung cancer cells. J Immunother. 2011;34(4):372–381. doi:10.1097/CJI.0b013e31821b724a
18. Kumai T, Matsuda Y, Oikawa K, et al. EGFR inhibitors augment antitumour helper T-cell responses of HER family-specific immunotherapy. Br J Cancer. 2013;109(8):2155–2166. doi:10.1038/bjc.2013.577
19. Dominguez C, Tsang KY, Palena C. Short-term EGFR blockade enhances immune-mediated cytotoxicity of EGFR mutant lung cancer cells: rationale for combination therapies. Cell Death Dis. 2016;7(9):e2380. doi:10.1038/cddis.2016.297
20. Zhong H, Zhang X, Tian P, et al. Tislelizumab plus chemotherapy for patients with EGFR -mutated non-squamous non-small cell lung cancer who progressed on EGFR tyrosine kinase inhibitor therapy. J ImmunoTher Cancer. 2023;11(8):e006887. doi:10.1136/jitc-2023-006887
21. Guan Y, Feng D, Yin B, Li K, Wang J. Immune-related dissociated response as a specific atypical response pattern in solid tumors with immune checkpoint blockade. Therapeut Adv Med Oncol. 2022;14:17588359221096877. doi:10.1177/17588359221096877
22. Sato Y, Morimoto T, Hara S, et al. Dissociated response and clinical benefit in patients treated with nivolumab monotherapy. Invest New Drugs. 2021;39(4):1170–1178. doi:10.1007/s10637-021-01077-7
23. Zhou H, Sun Y, Xiu W, et al. Overall survival benefit of continuing immune checkpoint inhibitors treatment post dissociated response in patients with advanced lung cancer. J Cancer Res Clin Oncol. 2020;146(11):2979–2988. doi:10.1007/s00432-020-03282-y
24. Roca E, Pozzari M, Vermi W, et al. Outcome of EGFR-mutated adenocarcinoma NSCLC patients with changed phenotype to squamous cell carcinoma after tyrosine kinase inhibitors: a pooled analysis with an additional case. Lung Cancer. 2019;127:12–18. doi:10.1016/j.lungcan.2018.11.016
25. Takahashi S, Sato Y, Sato Y, et al. Long-term efficacy of immune checkpoint inhibitor for Squamous cell Carcinoma lesion transformed from EGFR-mutated adenocarcinoma after Osimertinib treatment: a case report. JTO Clin Res Rep. 2024;5(2):100639. doi:10.1016/j.jtocrr.2024.100639
26. Burrell RA, McGranahan N, Bartek J, Swanton C. The causes and consequences of genetic heterogeneity in cancer evolution. Nature. 2013;501(7467):338–345. doi:10.1038/nature12625
27. Swanton C. Intratumor heterogeneity: evolution through space and time. Cancer Res. 2012;72(19):4875–4882. doi:10.1158/0008-5472.Can-12-2217
28. Junttila MR, de Sauvage FJ. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature. 2013;501(7467):346–354. doi:10.1038/nature12626
29. Zhou J, Gong Z, Jia Q, Wu Y, Yang ZZ, Zhu B. Programmed death ligand 1 expression and CD8(+) tumor-infiltrating lymphocyte density differences between paired primary and brain metastatic lesions in non-small cell lung cancer. Biochem Biophys Res Commun. 2018;498(4):751–757. doi:10.1016/j.bbrc.2018.03.053
30. Mansfield AS, Aubry MC, Moser JC, et al. Temporal and spatial discordance of programmed cell death-ligand 1 expression and lymphocyte tumor infiltration between paired primary lesions and brain metastases in lung cancer. Ann Oncol. 2016;27(10):1953–1958. doi:10.1093/annonc/mdw289
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Pathologic Complete Response Prediction to Neoadjuvant Immunotherapy Combined with Chemotherapy in Resectable Locally Advanced Esophageal Squamous Cell Carcinoma: Real-World Evidence from Integrative Inflammatory and Nutritional Scores
Feng J, Wang L, Yang X, Chen Q, Cheng X
Journal of Inflammation Research 2022, 15:3783-3796
Published Date: 6 July 2022
Nanoparticles for Chemoimmunotherapy Against Triple-Negative Breast Cancer
Liu S, Li J, Gu L, Wu K, Xing H
International Journal of Nanomedicine 2022, 17:5209-5227
Published Date: 7 November 2022
Pembrolizumab in Lymphopenic Metastatic Breast Cancer Patients Treated with Metronomic Cyclophosphamide: A Clinical and Translational Prospective Study
Mery B, Ménétrier-Caux C, Montané L, Heudel PE, Ray-Coquard I, Bachelot T, Derbel O, Augereau P, Treilleux I, Berthet J, Nkodia A, Bardin-Dit-Courageot C, Attignon V, Ferrari A, Garin G, Perol D, Caux C, Dubois B, Trédan O
Breast Cancer: Targets and Therapy 2023, 15:311-325
Published Date: 27 April 2023
Systemic Treatment-Decision Algorithms in Muscle-Invasive Bladder Cancer: Clinical Complexities and Navigating for Improved Outcomes
Giles M, Crabb SJ
Research and Reports in Urology 2023, 15:321-331
Published Date: 7 July 2023
Breast Cancer: An Overview of Current Therapeutic Strategies, Challenge, and Perspectives
Wang J, Wu SG
Breast Cancer: Targets and Therapy 2023, 15:721-730
Published Date: 20 October 2023