Back to Journals » Clinical Ophthalmology » Volume 18
Accuracy of Axial Length Measurements by Two Swept-Source Optical Coherence Tomography Biometers in Macula-off Rhegmatogenous Retinal Detachment Eyes
Authors Helaly HA , Elnaggar OR, Abou Shousha M, Elhady AM
Received 15 March 2024
Accepted for publication 14 November 2024
Published 19 November 2024 Volume 2024:18 Pages 3321—3334
DOI https://doi.org/10.2147/OPTH.S469094
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Hany Ahmed Helaly,* Osama Ramadan Elnaggar,* Mohsen Abou Shousha, Amr Mohamed Elhady
Ophthalmology Department, Faculty of Medicine, Alexandria University, Alexandria, Egypt
*These authors contributed equally to this work
Correspondence: Hany Ahmed Helaly, Email [email protected]
Purpose: To assess the accuracy of axial length (AXL) measurements using two swept-source optical coherence biometers, IOLMaster 700 (Carl Zeiss Meditec AG, Jena, Germany) and ARGOS (Alcon, Inc. Fort Worth, TX), for macula-off rhegmatogenous retinal detachment (RRD).
Methods: This retrospective study included 100 eyes with phakic primary macula-off RRD. Preoperative AXL measurements were performed using different methods: applanation A-scan ultrasound (U/S) biometry, combined applanation vector A/B-scan biometry, and optical biometry measurements were obtained using IOLMaster 700 (Carl Zeiss, Meditec, Jena, Germany) and ARGOS (Alcon, Inc. Fort Worth, TX). All patients underwent pars plana phacovitrectomy. At 8– 10 weeks postoperatively, optical biometry was performed to record AXL.
Results: Mean preoperative AXL measured using vector-A/B-scan ultrasonography was higher than that of postoperative AXL measured using IOLMaster (p < 0.05). Mean AXL measured by the standard mode of the ARGOS optical biometer was lower than mean AXL measured by both enhanced retina visualization (ERV) mode and user-adjusted method (p < 0.05). Mean same-eye AXL measured using IOLMaster was lower than that measured using ARGOS (p < 0.05). The least difference was observed with combined vector-A/B-scan ultrasound (on the positive side), followed by fellow eye AXL measured using IOLMaster optical biometry (on the negative side).
Conclusion: Optical biometry of fellow eye in macula-off RRD was noted to be highly correlating with postoperative optical biometry of same eye using IOL Master 700 in eyes without anisometropia. IOLMaster 700 showed less accuracy in the AXL measurements for same eye. The ARGOS optical biometer may have a good potential for measuring same eye AXL. Using ERV mode or a user-adjusted method for the ARGOS optical biometer may improve accuracy of AXL measurements. Most accurate method for measuring AXL in same eye was vector-A/B-scan ultrasound.
Keywords: macula-off, optical biometry, fellow eye, RRD, IOL calculation, ARGOS, IOLMaster
Introduction
More than 50% of phakic patients who undergo phacovitrectomy for rhegmatogenous retinal detachment (RRD) require subsequent cataract surgery within one year.1,2 Combined phacovitrectomy is encouraged in patients aged ≥ 50 years because of the lower cost of surgery, faster visual rehabilitation, and better visual outcomes.3–6 The advantages of vitreoretinal surgeons include thorough and safe shaving of the vitreous base without fear of lenticular touch and a better view of the retina for delicate maneuvers, such as internal limiting membrane peeling and detection of small breaks. Posterior capsule rupture or lens drop are of little concern. Phacoemulsification in non-vitrectomized eyes is easier because cataract in vitrectomized eyes tend to be more difficult. In addition, the anterior chamber tends to fluctuate during phaco, owing to the absence of vitreous support, which leads to a higher possibility of posterior capsule rupture and decreased nuclear fragments. There might be some disadvantages to combined phacovitrectomy; for example, a slightly higher chance of anterior segment inflammation in eyes with diabetic tractional detachment.3–6
Intraocular lens (IOL) power calculations are challenging during phacovitrectomy. Significant postoperative increase in axial length (AXL) was noted only after scleral buckling. Poor fixation of the patient might interfere with the scan alignment. Potential mechanisms of postoperative myopic shift include the underestimation of AXL in the detached macula, changes in vitreous cavity properties after vitreous removal, and anterior displacement of the IOL caused by gas tamponade.7–10
A-scan ultrasound biometry measures the AXL from the corneal vertex to the vitreoretinal interface, leading to a falsely measured shorter AXL in the detached macula. AXL measurements correlate with retinal detachment height, and the level of error is difficult to predict because of the dynamic nature of RRD. Optical biometry measures AXL from the tear film to the retinal pigment epithelium by adjusting retinal thickness in healthy adults. Theoretically, if retinal pigment epithelium is preserved, changes in the macula should not affect AXL measurements using optical biometry.7–10
Multiple options are available for determining the AXL in macula-off RRD. First, biometry can be performed on the same eye using either A-scan ultrasound or optical biometry (OB).9 Second, fellow eye biometry can be performed either A-scan ultrasonography or optical biometry.11,12 Third, user-adjusted biometry is an available option for the IOLMaster 500 and ARGOS optical biometers.13 Forth, a combined vector-A/B-scan biometry can be performed.14 Fifth, delayed cataract surgery and sequential procedures can be performed.15 Currently, there is no consensus regarding the optimal method.
This study aimed to assess the accuracy of AXL measurements using two swept-source optical coherence biometers, IOLMaster 700 (Carl Zeiss Meditec AG, Jena, Germany) and ARGOS (Alcon, Inc., Fort Worth, TX), in macula-off rhegmatogenous retinal detachment.
Material and Methods
This was a retrospective study included 100 eyes from 100 patients. Included patients were > 18 years of age, phakic patients with primary macula-off RRD with recent presentation with no previous retinal surgery. The included patients underwent a standard, uneventful, 23-gauge pars plana phacovitrectomy. The patients were recruited for a final follow-up visit and signed an informed consent form in which they agreed to participate in the study. This study was approved by the local ethics committee of the Faculty of Medicine of Alexandria University, Alexandria, Egypt. This ethical code is based on the tenets of the Declaration of Helsinki. Patients were excluded if they had a history of anisometropia, scleral buckling surgery, recurrent retinal detachment, or any other ocular problems affecting biometric measurements, such as corneal scarring and lens dislocation. Patients were also excluded if they had silicone oil tamponade, scleral buckling, or any intraoperative complications that affected postoperative biometric measurements. The medical records of the patients (from January 2021 to July 2023) were reviewed. Demographic data, such as age and sex, were recorded. In addition, biometric data (different methods of measuring AXL) were recorded.
Preoperative AXL measurements were performed using different methods. Applanation A-scan ultrasound (U/S) biometry was performed using EZ Scan AB5500+ (Sonomed Inc., NY, USA). Combined applanation vector A/B-scan biometry was performed using an EZ Scan AB5500 ultrasonic biometer. Optical biometry (OB) measurements were performed using an IOLMaster 700 (Carl Zeiss, Meditec, Jena, Germany) swept-source optical coherence tomography (SS-OCT) biometer that uses a 1050 nm wavelength version 1.88.1.64861. ARGOS (Alcon, Inc., Fort Worth, TX), a new SS-OCT biometer with a wavelength of 1060 nm, was used to obtain AXL measurements.
ARGOS has an enhanced retinal visualization (ERV) mode, in which the optical path length is measured to minimize the effect of attenuation and by changing the optical coherence tomography (OCT)-sensitive position to the retinal side. The AXL in ERV mode was calculated by adding the optical path length to the anterior segment information up to the posterior surface of the crystalline lens measured in standard mode. The ARGOS SS-OCT biometer allows the user-adjusted measurement of AXL by checking the signal spikes in the analysis window, allowing the user to manually move the cursor to match the point of the highest spike. This is useful in detached macula, where measurements could sometimes be falsely lower due to confusion between the detached retina spike and the original RPE spike.16,17 Unfortunately, the new version of IOLMaster 700 does not allow this user-adjusted mode, which is available in the older version of IOLMaster 500. A horizontal axial B-scan image was acquired using the combined application vector A/B-scan biometry technique. The vector-A-scan was then adjusted to pass through the middle of the cornea and the anterior and posterior lens echoes. This alignment ensured that the vector-A-scan intersected the retina at the approximate center of the macula. This technique has the advantage of being able to directly visualize what is being measured to avoid the fallacies of A-scan biometry.14
All cases were examined by the same experienced operator (H.A.H). The average of the three high-quality scans was recorded. The following preoperative AXL measurements were recorded: same-eye applanation A-scan in the sitting position (same AXL-U/S sitting), same-eye applanation A-scan in the supine position (same AXL-U/S supine), same-eye IOLMaster optical biometry (AXL-IOLMaster), fellow eye IOLMaster optical biometry (fellow AXL-IOLMaster), same eye ARGOS optical biometry using the standard mode (same AXL-ARGOS), same eye ARGOS optical biometry using the ERV mode (same AXL-ARGOS ERV), same eye ARGOS optical biometry using user adjustment (same AXL-ARGOS user-adjusted), fellow eye ARGOS optical biometry (fellow AXL-ARGOS), and same eye combined vector-A/B-scan biometry (AXL-B-scan).
All patients underwent standard uneventful 23-gauge pars plana implantation of a foldable hydrophobic acrylic IOL and were followed-up postoperatively. At 8–10 weeks postoperatively (to ensure absorption of any gas tamponade), optical biometry using an IOLMaster 700 SS-OCT biometer was performed to record the postoperative AXL measurement (postoperative. AXL-IOLMaster). Only cases that did not have silicone oil injection were included.
Data analysis was performed using the statistical package for social sciences (SPSS) for Windows (version 26.0; SPSS Inc., Chicago, IL, USA). Quantitative data were described using the range, mean, and standard deviation. Normality of the data was evaluated using the Kolmogorov–Smirnov test. ANOVA test was used to compare different means. A paired t-test was used to compare the mean preoperative and postoperative measurements of the same individual. The agreement between the preoperative and postoperative measurements was analyzed using a Bland–Altman plot. Differences were considered statistically significant when the associated p value was less than 0.05.
Results
This retrospective study included 100 eyes of 100 patients. The study included 65 males and 35 females. The mean age was 39.5 ± 13.2 (range 21–66) years. Forty-eight eyes were right and 52 were left. All included cases were phakic with primary macula-off RRD and had a recent presentation. Proliferative vitreoretinopathy (PVR) was defined as minimal grade A (pigment proliferation in the vitreous) or grade B (partial-thickness retinal wrinkles and rolled edges on retinal breaks due to preretinal membrane contractions).18
Table 1 shows the preoperative and postoperative AXL measurements obtained using different methods. Preoperative IOLMaster’s eye measurements were unsuccessful in 37 patients. The ANOVA revealed a statistically significant difference between the means of the measured AXLs (p < 0.05). A paired t-test was used to compare different preoperative AXL measurements with postoperative IOLMaster AXL measurements, and there was a statistically significant difference (p < 0.05). The mean preoperative AXL measured by vector-A/B-scan ultrasound was only higher than that of the postoperative AXL measured using IOLMaster (p < 0.05). The mean same-eye AXL measured using A-scan biometry in the supine position was higher than that in the sitting position (p < 0.05). ARGOS optical biometry measured lower mean fellow AXL than that measured using IOLMaster optical biometry (P < 0.05). The mean AXL measured using the standard mode of the ARGOS optical biometer was lower than the mean AXL measured using both the ERV and user-adjusted methods (p < 0.05). The mean same-eye AXL measured using the IOLMaster optical biometer was lower than that measured using the ARGOS optical biometer (p < 0.05).
 |
Table 1 Preoperative and Postoperative Axial Length of the Included Eyes Measured by Different Methods (n=100, Except for Same AXL-IOLMaster; n=63) |
The mean difference between the preoperative AXL measurements and postoperative IOLMaster AXL was highest with the same eye A-scan biometry in the sitting position, followed by the same eye AXL measured using ARGOS optical biometry. The least difference was observed with combined vector-A/B-scan ultrasound (on the positive side), followed by fellow eye AXL measured using IOLMaster optical biometry (on the negative side). This was followed by the mean difference in the same eye AXL measured by ARGOS optical biometry using the ERV mode (on the negative side). Figures 1–9 show the Bland-Altman plots of different preoperative AXL measurements and postoperative AXL measured by the IOLMaster. The Bland-Altman plot showed good agreement between preoperative AXL measurements and postoperative AXL measured by IOLMaster.
 |
Figure 1 Bland-Altman plot (same eye axial length by ultrasound in sitting position). |
 |
Figure 2 Bland-Altman plot (same eye axial length by ultrasound in supine position). |
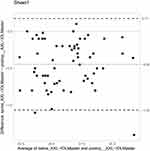 |
Figure 3 Bland-Altman plot (same eye axial length by IOLMaster). |
 |
Figure 4 Bland-Altman plot (fellow eye axial length by IOLMaster). |
 |
Figure 5 Bland-Altman plot (same eye axial length by ARGOS). |
 |
Figure 6 Bland-Altman plot (same eye axial length by ARGOS: ERV mode). |
 |
Figure 7 Bland-Altman plot (same eye axial length by ARGOS: user adjusted method). |
 |
Figure 8 Bland-Altman plot (fellow eye axial length by ARGOS). |
 |
Figure 9 Bland-Altman plot (same eye axial length by combined A-vector/B scan). |
Discussion
Calculating the IOL power in a detached macula is problematic. To date, no established method has been considered as the gold standard for measurements. Ultrasound A-scan biometry has the problem of falsely measuring a shorter AXL due to reflection of the sound waves from the vitreoretinal interface, which moves forward in cases of macula-off RRD, leading to a shorter measured AXL than the actual one that results in a higher IOL power and a myopic surprise. Theoretically, optical biometry is a better alternative as it measures beyond the detached macula to reflect the actual AXL (as it measures till the retinal pigment epithelium). However, due to multiple factors, such as lower reflectivity, denser cataracts, and lack of fixation, there might be some fallacies in the measurements. In 10–17% of the cases of macula-off RRD, it was impossible to obtain AXL measurements using IOLMaster because of machine limitations, such as dense media opacity, poor fixation by the patients, or even the lack of the machine itself13,19,20 Rahman et al10 found that IOLMaster could not provide AXL measurements in ¼ of the cases. Therefore, the idea is to depend on the AXL of the fellow eye. This idea was encouraged because multiple articles have published comparable measurements of fellow eye AXL.12,21,22 Also, many studies that deal with myopic shifts after phacovitrectomy have excluded cases in which preoperative IOLMaster measurement of AXL could not be obtained. In the current study, the authors included these patients (37 of 100 did not have successful preoperative IOLMaster AXL measurements).
ARGOS is a new SS-OCT biometer that uses a fast-sweeping near-infrared laser with a wavelength of 1060 nm and excellent acquisition rates. It is 1.5 x faster than the IOLMaster with a measurement capture speed of less than one second. Longer wavelengths and rapid measurement speeds reduce light scattering from opaque media, allowing greater penetration through denser cataracts and increased acquisition rates.16,17,23 The main difference from other SS-OCT biometers is the concept of sum-of-segments in AXL measurement. The ARGOS biometer uses different refractive indices for different parts of the AXL. Calculation of segmented AXL equals the sum of 4 true physical distances calculated by dividing the optical distance by corresponding individual refractive index (cornea 1.375, aqueous 1.336, lens 1.41, vitreous 1.336) in contrast to a composite refractive index of 1.3549 that IOLMaster uses. The AXL measured using the ARGOS biometer tended to be shorter in long eyes (AXL > 26.0 mm) and longer in short eyes (AXL < 22.5 mm). This could be explained by the fact that longer eyes have a relatively larger proportion of vitreous in the total AXL, and shorter eyes have a larger proportion of crystalline lenses in the total AXL.16,17,23
Postoperative AXL measurement was performed using the IOLMaster 700 SS-OCT biometer and was considered the benchmark for comparison with different preoperative AXL measurements. The postoperative AXL measurement was taken 8–10 weeks after the surgery to allow time for inflammation to resolve, any corneal edema or wound gapping to resolve, and gas tamponade absorption. Patients who required silicone oil tamponade were excluded from the study to avoid fallacies in postoperative AXL measurements due to the presence of silicone oil. All AXL measurements were performed by the same experienced operator (H.A.H). to avoid measurement variation errors with a reproducible technique, and the average of three quality scans was used.
Applanation A-scan ultrasound biometry was used in the current study rather than the immersion method, because it is the most commonly used method in clinical practice. Immersion A-scan biometry requires an additional coupling fluid, is more difficult to perform, and not all operators are trained to perform this technique. Applanation A-scan biometry has the problem of variable corneal compression, which can further reduce AXL measurements.24,25 In the current study, corneal compression was minimal during AXL measurements (there was no statistically significant difference between anterior chamber depth measured by applanation A-scan biometry and optical biometry as shown in Table 2). AXL measurements by A-scan ultrasound biometry were performed in both sitting and supine positions. The sitting position was used to mimic the optical biometry measurement position and the supine position was used to mimic the immersion ultrasound biometry position. In the current study, the supine position yielded a longer mean AXL measurement than the sitting position did. This could be explained by the fact that the supine position may somehow disperse the subretinal fluid away from the macular area, whereas in the sitting position, the detached retina moves away from retinal pigment epithelium. Pongsachareonnont and Tangjanyatam26 compared the accuracy of AXL measurements obtained using immersion A-scan ultrasound and IOLMaster 500. They reported a longer mean AXL with immersion ultrasound biometry and explained this by the same idea of dispersing the subretinal fluid in the supine position. They explained the lower accuracy of IOLMaster measurements by interference of the detached retina with retinal pigment epithelium light reflectivity, leading to the misinterpretation of signals from the detached macula rather than the retinal pigment epithelium. In the current study, the mean preoperative AXL measured using the IOLMaster 700 was lower than the mean of both positions of the A-scan AXL measurements, which confirms the above-mentioned explanation. However, A-scan ultrasound biometry showed a higher standard deviation than IOLMaster measurements. Moussa et al15 assessed the refractive outcomes and accuracy of biometry in phacovitrectomy and sequential operations in patients with retinal detachment compared with routine cataract surgery. They reported comparable refractive outcomes when the IOLMaster 700 was used and inferior refractive outcomes when ultrasound biometry was used.
 |
Table 2 Preoperative Anterior Chamber Depth Measurements |
The use of fellow-eye biometry was suggested because of errors in same-eye biometry. There was little difference in the AXL measurements between both eyes (82.5% had a < 0.3 mm difference between eyes). Some conditions may hinder the use of fellow eye biometry, such as significant anisometropia, or fellow eye conditions that affect AXL readings, such as poor fixation, media opacity, or macular edema. This is a good tool for comparing fellow eye biometry with the known refraction.12 In the current study, the mean AXL of fellow eyes measured by IOLMaster was 0.17 mm shorter than that of postoperative AXL measured by IOLMaster. This was the closest result obtained using optical biometry for both the same and fellow eyes. ARGOS biometry for the fellow eyes yielded shorter mean AXL as expected due to the nature of the measurement technique of segmented AXL (0.49 mm shorter than the mean postoperative AXL-IOLMaster and 0.32 mm shorter than the mean preoperative fellow eye AXL-IOLMaster). It should be noted that both fellow eye measurements showed a high standard deviation. A difference of 0.3 mm in the AXL results in around 0.75 D change in the IOL (0.5 D in refraction), which is very acceptable El-Khayat et al12 concluded that IOL power calculations using fellow eye biometry for phacovitrectomy in macula-off RRD were more accurate and better than those from the same eye biometry.
The ARGOS SS-OCT biometer in the standard mode showed the shortest mean AXL among the preoperative optical biometry measurements for both the same and fellow eyes. This was second to applanation A-scan biometry in the sitting position. ARGOS measurements showed high variability in macula-off eyes and the measurements were repeated several times to obtain reliable readings. In contrast to IOLMaster, no eyes could not be measured. The user-adjusted method for ARGOS is an option for the macula-off RRD. This study is the first to publish this technique for biometry of macula-off RRD. The ARGOS biometer allows the operator to analyze the image formed and to check the location of the spikes in the cornea, lens, and retina. The operator can verify that the measured AXL distance reaches the RPE. If the measurement is falsely short, the user can simply move the cursor backward until it reaches the RPE level and measure the correct AXL. The user-adjusted method improved the accuracy of ARGOS AXL measurements. Table 3 shows a representative case for different methods used to measure the AXL.
 |
Table 3 Representative Case |
The ERV mode in ARGOS yielded the best results for preoperative AXL measurements using optical biometry in the same eye. The mean AXL was 0.33 mm shorter than the mean postoperative AXL-IOLMaster, which was the closest measurement considering that the ARGOS biometer measured a shorter AXL than IOLMaster. As mentioned above, the ERV mode operates by changing the OCT-sensitive position to the retinal side, which enhances the retinal spike. This is useful in cases of macula-off RRD, and could explain why this mode yielded the best results in optical biometry of the same eyes.
The combined vector-A/B-scan ultrasound yielded the closest results for the mean AXL. The mean AXL measured by this method was 0.10 mm longer than that of the postoperative AXL measured by IOLMaster. The results show the lowest standard deviation. This method was first described in 2016 by the authors to measure AXL in cases of macula-off RRD.
Conclusions
In conclusion, optical biometry of the fellow eye in macula-off RRD was noted to be highly correlating with the postoperative optical biometry of the same eye using IOL Master 700 in eyes without anisometropia. IOLMaster 700 showed less accuracy in the AXL measurements for the same eye. The ARGOS optical biometer may have a good potential for measuring the same eye AXL. Using the ERV mode or a user-adjusted method for the ARGOS optical biometer may improve the accuracy of AXL measurements. The most accurate method for measuring AXL in the same eye was vector-A/B-scan ultrasound.
Abbreviations
AXL, axial length; OCT, optical coherence tomography; ERV, enhanced retina visualization; RRD, rhegmatogenous retinal detachment; IOL, intraocular lens; OB, optical biometry; SS-OCT, swept source optical coherence tomography; U/S, ultrasound.
Data Sharing Statement
Available upon request from the authors.
Ethics and Consent to Participate
This study was approved by the local ethics committee of the Faculty of Medicine at Alexandria University, Egypt. The tenets of the Declaration of Helsinki were followed for this study. All the included patients were recalled for the final follow-up visit and signed an informed consent form.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Fajgenbaum MA, Neffendorf JE, Wong RS, Laidlaw DA, Williamson TH. Intraoperative and postoperative complications in phacovitrectomy for epiretinal membrane and macular hole: a clinical audit of 1000 consecutive eyes. Retina. 2018;38(9):1865–1872.
2. Pinarci EY, Bayar SA, Sizmaz S, Yesilirmak N, Akkoyun I, Yilmaz G. Anterior segment complications after phacovitrectomy in diabetic and nondiabetic patients. European J Ophtha. 2013;23(2):223–229.
3. Le NT, Marshall B, Houser KH, Khandelwal SS. Combined Pars Plana Vitrectomy, Phacoemulsification and Intraocular Lens Implantation: a review on the Advantages and Limitations of Phacovitrectomy. 2022;1.
4. Kim MS, Woo SJ, Park KH. Phacovitrectomy versus lens-sparing vitrectomy for rhegmatogenous retinal detachment repair according to the surgical experience. Retina. 2021;41(8):1597–1604.
5. Demir G, Çakmak S, Ş Ö, Güneş H, Arıcı M, Alkın Z. Comparison of the Long-term Outcomes of Combined Phacovitrectomy and Sequential Surgeries for Macular Hole and Cataract. Europe Arch f Medical R. 2020;36(4).
6. Valmaggia C, Kostadinov F, Lang C, Guber J. Comparative study of combined vitrectomy with phacoemulsification versus vitrectomy alone for primary full-thickness macular hole repair. BMC Ophthalmology. 2021;21:1–6.
7. Hipólito-Fernandes D, Elisa Luís M, Maleita D, et al. Intraocular lens power calculation formulas accuracy in combined phacovitrectomy: an 8-formulas comparison study. Int J Retin Vitreous. 2021;7:1–8.
8. Shiraki N, Wakabayashi T, Sakaguchi H, Nishida K. Optical biometry-based intraocular lens calculation and refractive outcomes after phacovitrectomy for rhegmatogenous retinal detachment and epiretinal membrane. Sci Rep. 2018;8(1):11319.
9. Manvikar SR, Allen D, Steel DH. Optical biometry in combined phacovitrectomy. J Cataract Refract Surg. 2009;35(1):64–69.
10. Sato T, Korehisa H, Shibata S, Hayashi K. Prospective comparison of intraocular lens dynamics and refractive error between phacovitrectomy and phacoemulsification alone. Ophthalmol Retina. 2020;4(7):700–707.
11. Kohnen T, Kaiser K, Bucur J, Jandeworth T, Lwowski C Fellow Eye Data for IOL Calculation in Eyes Undergoing Combined Phacovitrectomy.
12. El-Khayat AR, Brent AJ, Peart SA, Chaudhuri PR. Accuracy of intraocular lens calculations based on fellow-eye biometry for phacovitrectomy for macula-off rhegmatogenous retinal detachments. Eye. 2019;33(11):1756–1761.
13. Rahman R, Kolb S, Bong CX, Stephenson J. Accuracy of user-adjusted axial length measurements with optical biometry in eyes having combined phacovitrectomy for macular-off rhegmatogenous retinal detachment. J Cataract Refract Surg. 2016;42(7):1009–1014.
14. Abou-Shousha M, Helaly HA, Osman IM. The accuracy of axial length measurements in cases of macula-off retinal detachment. Can J Ophthalmol. 2016;51(2):108–112.
15. Moussa G, Sachdev A, Mohite AA, Hero M, SW C, Andreatta W. Assessing refractive outcomes and accuracy of biometry in phacovitrectomy and sequential operations in patients with retinal detachment compared with routine cataract surgery. Retina. 2021;41(8):1605–1611.
16. Higashiyama T, Mori H, Nakajima F, Ohji M. Comparison of a new biometer using swept-source optical coherence tomography and a conventional biometer using partial coherence interferometry. PLoS One. 2018;13(4):e0196401.
17. Tañá-Rivero P, Aguilar-Córcoles S, Tañá-Sanz P, Tañá-Sanz S, Montés-Micó R. Axial length acquisition success rates and agreement of four optical biometers and one ultrasound biometer in eyes with dense cataracts. Eye and Vision. 2023;10(1):35.
18. Di Lauro S, Kadhim MR, Charteris DG, Pastor JC. Classifications for proliferative vitreoretinopathy (PVR): an analysis of their use in publications over the last 15 years. J Ophthal. 2016.
19. Rahman R, Bong CX, Stephenson J. Accuracy of intraocular lens power estimation in eyes having phacovitrectomy for rhegmatogenous retinal detachment. Retina. 2014;34(7):1415–1420.
20. Kim YK, Woo SJ, Hyon JY, Ahn J, Park KH. Refractive outcomes of combined phacovitrectomy and delayed cataract surgery in retinal detachment. Can J Ophthalmol. 2015;50(5):360–366.
21. De Bernardo M, Zeppa L, Forte R, et al. Can we use the fellow eye biometric data to predict IOL power? Semin Ophthalmol. 2017;432(3):363–370.
22. Liu R, Li H, Li Q. Differences in axial length and IOL power based on alternative A-scan or fellow-eye biometry in macula-off rhegmatogenous retinal detachment eyes. Ophthal Therapy. 2022;1:1–8.
23. Huang J, Chen H, Li Y, et al. Comprehensive comparison of axial length measurement with three swept-source OCT-based biometers and partial coherence interferometry. J Refract Surg. 2019;35(2):115–120.
24. Ademola-Popoola DS, Nzeh DA, Saka SE, Olokoba LB, Obajolowo TS. Comparison of ocular biometry measurements by applanation and immersion A-scan techniques. J Curr Ophthal. 2015;27(3–4):110.
25. Sanchis-Gimeno JA, Lleo A, Herrera M, et al. Quantitative ocular anatomy in vivo: comparison of axial length and anterior chamber depth values obtained by a single observer by means of optical biometry and immersion and applanation ultrasound biometry. Eur J Anat. 2024;10(1):27–29.
26. Pongsachareonnont P, Tangjanyatam S. Accuracy of axial length measurements obtained by optical biometry and acoustic biometry in rhegmatogenous retinal detachment: a prospective study. Clin Ophthalmol. 2018;23:973–980.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

