Back to Journals » Journal of Inflammation Research » Volume 18
Achieving Therapeutic Success with Spesolimab: Two Cases of Drug-Induced Generalized Pustular Psoriasis Initially Mimicking Acute Generalized Exanthematous Pustulosis
Authors Chen J, Xia Y , Zhou F, Tao J , Zhou N
Received 8 February 2025
Accepted for publication 11 July 2025
Published 19 July 2025 Volume 2025:18 Pages 9581—9585
DOI https://doi.org/10.2147/JIR.S520767
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Ning Quan
Jianxia Chen,1,* Yuting Xia,2,3,* Faqiong Zhou,1 Juan Tao,2,3 Nuoya Zhou2,3
1Department of Dermatology, The Central Hospital of Enshi Tujia and Miao Autonomous Prefecture, Enshi, Hubei, People’s Republic of China; 2Department of Dermatology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, People’s Republic of China; 3Hubei Engineering Research Center of Skin Disease Theranostics and Health, Wuhan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Nuoya Zhou, Department of Dermatology, Union Hospital, Tongji Medical College, No. 1277 Jiefang Avenue, Wuhan, Hubei, 430022, People’s Republic of China, Email [email protected]
Background: Patients with newly diagnosed generalized pustular psoriasis (GPP) lacking a history of psoriasis but with recent medication exposure may present with clinical features overlapping with acute generalized exanthematous pustulosis (AGEP). Accurate differentiation is critical for treatment and prognosis.
Case Summary: We report two cases of drug-induced GPP initially with AGEP-like manifestations. Both patients received systemic glucocorticoids and cyclosporine yet exhibited recurrent pustular flares with lakes of pus and a prolonged disease course exceeding two months. Genetic testing confirmed IL36RN mutations in both cases. Subsequent spesolimab therapy achieved rapid resolution of pustules and sustained remission.
Conclusion: In patients with first-onset drug-induced pustulosis resembling AGEP, the presence of lakes of pus, prolonged duration, and recurrent flares despite conventional therapies should be diagnosed as GPP promptly. Early intervention with spesolimab demonstrates efficacy in controlling disease activity and improving outcomes.
Keywords: generalized pustular psoriasis, acute generalized exanthematous pustulosis, IL36RN gene, spesolimab
Background
Generalized pustular psoriasis (GPP) and acute generalized exanthematous pustulosis (AGEP) exhibit striking clinical similarities, including cutaneous erythema, widespread sterile pustules, and systemic symptoms such as fever and leukocytosis. Beyond these shared manifestations, these two dermatoses demonstrate overlapping genetic mechanisms and dysregulation of the IL-36/IL-17 inflammatory axis.1,2 Histologically, both conditions exhibit subcorneal pustules and neutrophilic dermal infiltrates, while GPP is typically associated with more pronounced psoriasiform epidermal hyperplasia and large pustules.3
Key distinctions emerge in disease course and prognosis. AGEP, typically triggered by drugs or infections, manifests abruptly and resolves spontaneously within 2 weeks after drug withdrawal.4 In contrast, GPP, which may also be drug-induced, follows a protracted course marked by recurrent flares, lakes of pus, and potential life-threatening complications.5 Persistent pustules, poor response to glucocorticoids/cyclosporine, and recurrent flares should raise suspicion for GPP.
The prognosis of these two diseases is strikingly different. AGEP generally has a good prognosis with spontaneous resolution. In contrast, GPP can be fatal if not treated adequately, with reported mortality rates ranging from 1% to 32%.5,6 Timely recognition of GPP not only prevents fatal outcomes but also underscores the necessity of precision medicine in optimizing therapeutic efficacy. In this report, we present two cases of drug-induced GPP mimicking AGEP in their initial episodes, ultimately requiring reclassification and successful treatment with spesolimab.
Case Presentation
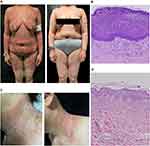
Patient 1 was a 46-year-old female with no personal or familial history of psoriasis (Table S1). Due to a 20-year history of atopic dermatitis (AD), she received three subcutaneous dupilumab injections (initial dose: 600 mg, followed by 300 mg every two weeks). Within 24 hours after each administration, pruritic erythema and papules developed on her neck, trunk, and flexural areas, with progressive worsening after subsequent doses (Figure S1A–C). Following the third injection, extensive erythema and papules erupted in her intertriginous regions, rapidly disseminating to the entire body, accompanied by widespread pustules (Figures 1A and S1D–E).
Upon admission, routine laboratory tests revealed leukocytosis (12.8 × 10⁹/L) with elevated white blood cell (19.19 × 10⁹/L) and neutrophil counts (15.21 × 10⁹/L), along with an elevated C-reactive protein (CRP) level (14.92 mg/L). Histopathological examination demonstrated hyperkeratosis, parakeratosis, neutrophilic microabscesses in the upper epidermis, proliferation and dilation of superficial dermal blood vessels, and dense perivascular infiltration of neutrophils, lymphocytes, histiocytes, and rare eosinophils (Figure 1B). Using the European Study Group on Severe Cutaneous Adverse Reactions (EuroSCAR) criteria, the patient’s diagnostic score was 7, comprising: pustules (+2), erythema (+2), distribution/pattern (+2), postpustular desquamation (+1), absent mucosal involvement (0), absent acute onset (0), resolution ≤15 days (−4), absent fever ≥38°C (0), polymorphonuclear neutrophils ≥7000/μL (+1), and histology (+3)—fulfilling criteria for “possible AGEP.” Dupilumab was discontinued due to the suspected drug-induced reaction.
The patient received intravenous dexamethasone (10 mg/day) and intravenous immunoglobulin (IVIG, 25 g/day for 5 days), but demonstrated inadequate clinical response. Cyclosporine (3.5 mg/kg/day) was added to the regimen. After 12 days of combined therapy, pustules and erythema partially subsided with no new lesions, prompting discharge with a tapering schedule of oral cyclosporine and prednisolone. Unfortunately, post-discharge skin lesions failed to improve, and new eruptions occurred intermittently, leading to a prolonged, two-month course characterized by recurrent flares. Based on the recurrence of pustules, the diagnosis of GPP was made. Whole-exome sequencing (WES) performed on peripheral blood samples identified a pathogenic mutation in the IL36RN gene.
The patient received an initial intravenous dose of spesolimab (900 mg), followed by a second 900 mg dose one week later. Acitretin (20 mg/day) and cyclosporine (3.5 mg/kg/day) were concurrently administered with the first spesolimab infusion. Within two weeks, rapid regression of pustules and significant improvement in erythema were observed, with no adverse events reported during or after treatment. Cyclosporine was subsequently tapered by 0.5 mg/kg every three weeks, while acitretin was reduced by 10 mg every two weeks until discontinuation. At present, cyclosporine is maintained at 1.5 mg/kg/day. During the four-month follow-up period, complete clinical remission was sustained (Figures 1A and S1F).
Patient 2 was a 51-year-old female with a history of widespread erythema and pustules following suspected intravenous drug exposure two decades prior (Table S1). Three months before admission, she received intravenous levofloxacin and dexamethasone for diabetic foot management. Within 48 hours, she developed a generalized rash characterized by erythema and pustules. Laboratory tests revealed mild anemia (RBC 3.41×10¹²/L, Hb 105 g/L) and an elevated C-reactive protein (CRP) level (48.3 mg/L). Histopathological examination revealed epidermal hyperkeratosis with neutrophils aggregation in the stratum corneum and upper spinous layer, interspinous edema, and mild perivascular infiltration of lymphocytes, histiocytes, and neutrophils in the superficial dermis (Figure 1D). Using the EuroSCAR criteria, her diagnostic score was 10—pustules (+2), erythema (+2), distribution/pattern (+2), postpustular desquamation (+1), absent mucosal involvement (0), absent acute onset (0), resolution ≤15 days (0), absent fever ≥38°C (0), polymorphonuclear neutrophils ≥7000/μL (+1), and histology (+2)—fulfilling criteria for AGEP. She was subsequently treated with intravenous methylprednisolone (50 mg daily) combined with cyclosporine (3.5 mg/kg daily).
After a 15-day hospitalization, the pustules and erythema demonstrated moderate improvement. She was discharged with a tapering regimen of oral prednisolone at 40 mg daily and cyclosporine at 3.5 mg/kg/d. Nevertheless, within three months post-discharge, she experienced two cycles of relapse and remission. Three months after the initial hospitalization, spontaneous relapse of erythema occurred on her neck, trunk, and limbs, accompanied by the appearance of pustules on her neck (Figures 1C and S1G–I). Laboratory tests revealed normal C-reactive protein levels (<3.05 mg/L). Upon readmission, the diagnosis was revised to GPP. Whole-exome sequencing on peripheral blood samples confirmed a mutation in the IL36RN gene. A single intravenous dose of spesolimab (900 mg) was administered, leading to rapid improvement in erythema. Notably, pustules resolved particularly rapidly within one day (Figure 1C). Complete remission was achieved within one week, and no recurrence was observed during the subsequent four-month follow-up period.
Discussion
GPP is a rare, severe inflammatory skin disorder characterized by cutaneous erythema and macroscopically visible sterile pustules. It may present with or without systemic symptoms, concomitant psoriasis subtypes, or laboratory abnormalities. With a reported prevalence ranging from 1.8 to 124 cases per million individuals.7 GPP is notable for its potential to trigger life-threatening multiorgan complications during flares, underscoring the critical need for rapid and accurate diagnosis. Nevertheless, the disease’s rarity and clinical overlap with other pustular dermatoses, such as AGEP, pose significant challenges to timely recognition and intervention.
Differentiating drug-induced GPP flares from AGEP poses a clinical challenge, particularly in first-episode GPP patients without a prior psoriasis history. Both conditions share genetic, immunological, and histopathological similarities, including rapid-onset generalized sterile pustules. Nevertheless, key clinical distinctions exist: GPP is characterized by lakes of pus, prolonged duration, and recurrent flares, whereas AGEP typically resolves spontaneously within 2 weeks. Importantly, The European Rare and Severe Psoriasis Expert Network mandates ≥1 relapse or persistence >3 months for GPP diagnosis,8 while the International Psoriasis Council’s consensus permits diagnosis at the first flare without temporal constraints.9
Nevertheless, IL36RN gene alterations are not reliable discriminators, as they have been identified in AGEP cases.10,11 Despite this, IL36RN genetic screening is recommended when feasible, given that variants in this gene—encoding the IL-36 receptor antagonist—disrupt the IL-36 inflammatory pathway, conferring susceptibility to GPP. In our patients, IL36RN mutations likely predisposed them to recurrent generalized pustular reactions, whereby drug hypersensitivity triggered unchecked neutrophilic skin inflammation due to insufficient IL-36Ra-mediated pathway inhibition. Spesolimab, an approved anti-IL-36 receptor monoclonal antibody for GPP flares, rapidly controlled disease activity in both cases, with a favorable safety profile and no recurrences during four months of follow-up.
For patients presenting with first-onset drug-induced pustulosis mimicking AGEP—particularly those with pustular lakes, prolonged duration, and recurrent flares—clinicians should promptly re-evaluate the diagnosis toward GPP and adjust treatment strategies. A proactive approach is critical to avoid the “watch-and-wait” strategy typically reserved for AGEP, which risks overlooking early GPP and exacerbating disease progression. Genetic screening for IL36RN variants is advised when feasible, as affected patients may require intensified monitoring and prioritization for targeted therapies. Given its established efficacy and safety profile, spesolimab represents a promising treatment for GPP, particularly in cases with clinical or genetic features indicating IL-36 pathway dysregulation.
Conclusion
Through these two cases, we highlight the critical need to recognize that first-onset drug-induced pustulosis mimicking AGEP—characterized by pus lakes, prolonged duration, or recurrent flares—warrants timely treatment adjustment according to GPP guidelines. Clinicians should prioritize early initiation of spesolimab for targeted therapy and avoid the “wait-and-watch” approach typically used for AGEP, which may delay appropriate intervention and exacerbate disease severity.
Ethics Approval and Consent to Participate
According to the policies of Ethics Committee of Wuhan Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, ethical approval was waived for anonymized case reports. Written consent to participate in this study was obtained from both patients.
Consent for Publication
Written informed consent was obtained from both patients for publication of this case report and any accompanying images.
Acknowledgments
The authors are immensely grateful to our generous patient for allowing us to publish her case.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by a grant from the National Natural Science Foundation of China (No. 82103769).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Benezeder T, Bordag N, Woltsche J, et al. IL-36-driven pustulosis: transcriptomic signatures match between generalized pustular psoriasis (GPP) and acute generalized exanthematous pustulosis (AGEP). J Allergy Clin Immunol. 2025;18:
2. Song HS, Kim SJ, Park TI, Jang YH, Lee ES. Immunohistochemical comparison of IL-36 and the IL-23/Th17 axis of generalized pustular psoriasis and acute generalized exanthematous pustulosis. Ann Dermatol. 2016;28(4):451–456. doi:10.5021/ad.2016.28.4.451
3. Feldmeyer L, Heidemeyer K, Yawalkar N. Acute generalized exanthematous pustulosis: pathogenesis, genetic background, clinical variants and therapy. Int J Mol Sci. 2016;17:1214. doi:10.3390/ijms17081214
4. Tetart F, Walsh S, Milpied B, et al. Acute generalized exanthematous pustulosis: European expert consensus for diagnosis and management. J Eur Acad Dermatology Venereol. 2024;38:2073–2081. doi:10.1111/jdv.20232
5. Miyachi H, Konishi T, Kumazawa R, et al. Treatments and outcomes of generalized pustular psoriasis: a cohort of 1516 patients in a nationwide inpatient database in Japan. J Am Acad Dermatol. 2022;86(6):1266–1274. doi:10.1016/j.jaad.2021.06.008
6. Kharawala S, Golembesky AK, Bohn RL, Esser D. The clinical, humanistic, and economic burden of generalized pustular psoriasis: a structured review. Expert Rev Clin Immunol. 2020;16(3):239–252. doi:10.1080/1744666X.2019.1708193
7. Prinz JC, Choon SE, Griffiths CEM, et al. Prevalence, comorbidities and mortality of generalized pustular psoriasis: a literature review. J Eur Acad Dermatol Venereol. 2023;37(2):256–273. doi:10.1111/jdv.18720
8. Navarini AA, Burden AD, Capon F, et al. European consensus statement on phenotypes of pustular psoriasis. J Eur Acad Dermatology Venereol. 2017;31:1792–1799. doi:10.1111/jdv.14386
9. Choon SE, van de Kerkhof P, Gudjonsson JE, et al. International consensus definition and diagnostic criteria for generalized pustular psoriasis from the international psoriasis council. JAMA Dermatol. 2024;160(7):758–768. doi:10.1001/jamadermatol.2024.0915
10. Kardaun SH, Kuiper H, Fidler V, Jonkman MF. The histopathological spectrum of acute generalized exanthematous pustulosis (AGEP) and its differentiation from generalized pustular psoriasis. J Cutan Pathol. 2010;37(12):1220–1229. doi:10.1111/j.1600-0560.2010.01612.x
11. NavariniAA, Valeyrie-AllanoreL, Setta-KaffetziN, et al.Rare variations in IL36RN in severe adverse drug reactions manifesting as acute generalized exanthematous pustulosis. J Invest Dermatol. 2013;133(7):1904–1907. doi:10.1038/jid.2013.44
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Successful Treatment of Pediatric Generalized Pustular Psoriasis (GPP) with Spesolimab: 5 Case Reports and Evaluations of Circulating IL-36 Levels
Chen Y, Wang Z, Liang Y, Shen C, Jiao L, Xiang X, Miao C, Xu Z
Journal of Inflammation Research 2024, 17:8199-8206
Published Date: 4 November 2024
Generalized Pustular Psoriasis with Cushing’s Syndrome: A Case of Effective Spesolimab Treatment
Peng G, Zhang Y, Zhang S, Li Y, Luo L, Luo J, Nie X, Zhang H, Liao C
Biologics: Targets and Therapy 2025, 19:321-329
Published Date: 10 May 2025