Back to Journals » Open Access Rheumatology: Research and Reviews » Volume 17
Acute Clinical Features and Persistence of Joint Pain in Probable Cases of Chikungunya Fever in Eritrea
Authors Frezgi O , Berhane A, Ghebrewelde G, Tekie H, Kiflezgi T, Mohamedsied A, Tekie Y, Asrat MM, Gebrejesus T
Received 28 November 2024
Accepted for publication 29 January 2025
Published 1 February 2025 Volume 2025:17 Pages 13—24
DOI https://doi.org/10.2147/OARRR.S465082
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Chuan-Ju Liu
Okbu Frezgi,1,2 Araia Berhane,3 Ghide Ghebrewelde,4 Henok Tekie,4 Tsegezab Kiflezgi,4 Abdelaziz Mohamedsied,4 Yonas Tekie,4 Medhanie Medhin Asrat,5 Tewaldemedhine Gebrejesus6
1Orotta National Referral Maternity Hospital, Ministry of Health, Asmara, Eritrea; 2Gynecology and Obstetrics Department, Orotta College of Medicine and Health Sciences, Asmara, Eritrea; 3Communicable Disease Control program, Ministry of Health, Asmara, Eritrea; 4Tesseney Hospital, Ministry of Health, Tesseney, Eritrea; 5Statistical Data Processing, Eritrean national Statistics office, Asmara, Eritrea; 6National Tuberculosis and Leprosy Control Program of Eritrea Ministry of Health, Virginia, USA
Correspondence: Okbu Frezgi, Email [email protected]
Background: Chikungunya fever is a mosquito-borne viral illness that has re-emerged as an important global concern. Persistent arthralgia following chikungunya fever is common and requires advanced pharmacological interventions as pain does not respond well to analgesics.
Objective: The study aimed to describe the acute clinical features of probable cases of chikungunya fever and risk factors associated with the persistence of joint pain.
Methods: A prospective, descriptive cohort study was conducted on probable cases of chikungunya fever from October 2018 to March 2019 in the Tesseney subzone of Eritrea.
Results: A total of 203 probable cases of chikungunya fever were enrolled, majority being males (68%) with a mean age of 39.2 years. The acute phase symptoms include the triad of polyarthralgia (97%), fever (96.1%), and skin rash (56.7%). Commonly affected joint sites were the wrist (59.4%) and interphalangeal joints of the hands (56.9%). Fever had a mean duration of 4.1 ± 3 days, while headache had a mean duration of 3.8 ± 3 days. Skin rash was maculopapular, which was pruritic in (85.2%) and the common involved sites were the hands (71%) and trunk (46.5%). Complete blood count during acute phase includes lymphocytosis (64.5%) and granulocytopenia (43.3%). Joint pain persisted at three months in 52.1% of cases and at six months in 21.7%. Age > 41 (p = 0.001, OR: 1.588; 95% CI: 0.935– 2.695) and having the O-type blood group (p = 0.033, OR: 0.704; 95% CI: 0.448– 1.105) were found to be associated with the persistence of joint pain.
Conclusion: Our study indicates polyarthralgia, fever, and skin rash as a triad of symptoms during the acute phase. Persistent arthralgia was a frequent long-term complication of chikungunya fever in which increasing age was identified to be a significant risk factor.
Keywords: chikungunya fever, clinical features, persistent joint pain, Eritrea
Introduction
Chikungunya fever (CHIKF) is a crippling mosquito-borne viral disease that has become a major public health concern in recent years. The name “chikungunya” is derived from the Makonde word meaning ‘he, who walks bends up” in reference to the stooped posture developed due to the arthritic symptoms of the disease.1–3 The disease is caused by the chikungunya virus (CHIKV) which is transmitted to humans through the bite of infected Aedes aegypti and Aedes albopictus mosquitoes.1,2 The etiologic agent is a single-stranded positive sense RNA virus identified as an arbovirus of the Alphavirus genus.2,4 Humans and other vertebrate hosts (ie, monkeys, rodents, birds, etc) serve as reservoirs during CHIKF epidemics.2 The virus has two distinct transmission cycles based on the geographical location and human settlement density: enzootic and sylvatic (urban).2 The enzootic cycles mainly occurs on African tropical regions where arboreal mosquitoes transmit the virus to nonhuman primates which serve as the main reservoir host.2 The sylvatic cycle is concentrated in urban centers where the virus is transmitted via the Ae. aegypti and Ae. albopictus mosquitos from human-to-human.2 The enzootic cycle allows interhuman transmission during outbreaks as well as reducing the probability of eliminating the virus circulation in an environment.2,5
CHIKV was first isolated in Tanzania in 1953, later spreading across sub-Saharan Africa.6 Three distinct strains of CHIKV have been identified based on phylogenetic analysis: West African, East-Central-South African (ECSA), and the Asian lineage.7 Before 2000, CHIKF was largely restricted within the sub-Saharan African region, but later the ECSA strain re-emerged within the Kenyan coast and spread across the Indian Ocean islands, simultaneously evolving into a new strain called Indian Ocean lineage (IOL).7 Major CHIKF outbreaks emerged across the Indian Ocean islands between 2004 and 2007 and infected more than 272,000 people, most notably on Reunion island.8,9 The Reunion island epidemic of 2005–2006 reported 270,000 infected cases, approximately a third of the island’s population.8
Major epidemics, such as those found on Reunion Island, can cause significant productivity loss and immense economic cost, especially for developing countries.10 After 2004, CHIKF outbreaks were later documented in Italy, Bangladesh, Cameroon, and France, likely due to international travelers who, during the Reunion epidemic, likely became infected and, when returning home, dispersed the CHIKF to other countries.6 Presently, CHIKF has a wide geographical distribution, including North and South America, Europe, Asia, and the Pacific Islands, with an estimated global incidence of more than 6 million confirmed cases worldwide.8 Ecological factors such as temperature, availability of breeding sites, rainfall, vegetation, and globalization contribute to CHIKF dissemination, which impacts human migration and the range of mosquito prone areas. Human demographic changes (migration, international travel, tourism, global trade, etc) linked to population movements has largely been affected by the CHIKFFungunya virus.2,10–12
CHIKVs, like the dengue and Zika viruses, are commonly classified as arthritogenic viruses as these viruses cause musculoskeletal inflammatory disease in humans.13 Upon infection, CHIKF has an incubation period of 3–7 days, but may last as long as fourteen days.6,14 Seroprevalence studies have demonstrated that 30–40% of CHIKF infected individuals can be asymptomatic, but the majority (60–80%) of infected individuals are symptomatic.14,15 After the incubation period, sudden onset of high-grade fever, polyarthralgia, headache, myalgia, and transient maculopapular skin rash commonly develop.13,14 In addition, swollen joints, tenosynovitis, vomiting, and nausea have also been observed.10 CHIKF is rarely fatal with an acute crippling phase that lasts 1–2 weeks followed by convalescence. However, in a subset of people, joint pain and swelling can last for months to years and often fluctuating, leading to long-term persistent polyarthralgia.3,8,9,15 CHIKF pathogenesis of arthropathy is likely attributed to CHIKV residing and replicating within muscle and joint tissue. Although recent advances have shed light on the CHIKF infection, the immunopathogenic mechanism of CHIKF resulting in arthralgia still remains unclear.6 Chronic polyarthralgia is described to possess both neuropathic and nociceptive characteristics, requiring advanced pharmacological interventions as pain does not respond well with analgesics.3
Diagnosis of a CHIKF infection is often performed via molecular detection of a viral genome and/or identification of a virus-specific antibody in a laboratory setting.10,15 Reverse transcription–polymerase chain reaction (RT-PCR) is often used for molecular detection from a blood sample; ELISA, immunofluorescence assay, and rapid immunochromatographic tests are performed for serologic analysis to capture virus-specific antibodies from a patient’s serum (IgM antibody or demonstrating rising titer of IgG antibody).6,10,16,17 Differential diagnosis may be required as CHIKFFV manifestations may co-exist with other similar alphaviruses, such as dengue.15 Differential diagnosis from dengue infection is often based on the presence of hemoconcentration, while symptoms of high-grade fever and joint pain are known only to be exhibited in CHIKF infection.10
No specific antiviral drug has yet been introduced to prevent or treat CHIKF, but individuals previously infected are believed to incur life-long immunity.7,15,18 New studies, however, have reported several novel preclinical vaccines are in development with limited number of clinical trials, but more time is required before these vaccines are approved for the global market.6 Thus, treatment of CHIKF is largely focused on symptomatic relief with the use of anti-inflammatory drugs as the viral disease has a relatively low-fatality rate. Nevertheless, little is known about the viral–host interactions, cellular factors involved in viral pathogenesis, and the role of immune system during the course of CHIKF, which hinders the development of effective vaccines and management strategies for the disease. The reemergence of CHIKF epidemics in different parts of the world and their related economic burden incited the need to study the clinical features of this disease. Eritrea’s subtropical climate is suitable for the transmission of mosquito-borne diseases, such as CHIKF. The first cases of CHIKF was reported in Tesseney subzone during the October 2018 outbreak.19 Our aim in carrying out this study is to describe the acute clinical features of probable cases of CHIKF and the risk factors associated with the persistence of polyarthralgia.
Objective
General Objective
The primary objective of this study was to describe acute clinical features of probable cases of CHIKFFungunya fever and the risk factors for persistence of arthralgia.
Specific Objective
- To describe the acute clinical features of probable cases of CHIKF during the acute prodromal phase.
- To identify risk factors associated with the persistent arthralgia.
Materials and Methods
Study Design
This was a prospective, descriptive cohort hospital-based study at the Tesseney Community Hospital.
Study Area
Tesseney hospital is a community hospital in the Gash-Barka region of Eritrea, which serves the catchment population of 87,992 individuals distributed in an area of 1,096.83 km2. The hospital provides inpatient and outpatient services, delivery service, laboratory services, imaging unit, physiotherapy unit, and possesses a 115-bed capacity.
Study Population
Probable CHIKF cases that met the clinical and epidemiological criteria during the October 2018 CHIKF outbreak were included as the study population.
Inclusion Criteria
All probable cases of CHIKF who had signs and symptoms of acute febrile illness during the outbreak and tested negative for malaria and dengue fever were included in the study.
Exclusion Criteria
All probable cases of CHIKF who had signs and symptoms of acute febrile illness during the outbreak and returning with positive results for malaria and dengue fever were excluded from the study.
Sampling Procedure
Non-probability convenience sampling method was used with inclusion of all probable cases of CHIKF based on clinical symptoms and epidemiological data.
Data Collection
Method of Data Collection
Data collection was conducted by interviewing and examining probable cases of CHIKF by pre-designed questionnaire. All cases were assessed for regional and systemic manifestations by general practitioners. The follow-up of the patients was conducted monthly for six months with each manifestation documented during their visit.
Laboratory Investigations
Serologic analysis for each case of CHIKF was not feasible. However, during the outbreak, a sample of 30 patients was randomly collected and sent to a regional WHO virology laboratory in Kenya for CHIKFV analysis. All samples reported positive for CHIKF and the criteria for CHIKF outbreak were met. Five cc of venous blood was drawn from each enrolled patient to investigate malaria and dengue fever using rapid tests. A complete blood count was also performed only on the initial visit with blood group and respective Rh factor being identified for each patient.
Data Analysis and Interpretation
The collected data was tabulated and analyzed using Epi-info software and further analyzed via SPSS software version 26. Data were presented as frequencies and percentages. The chi-square test was used as a significance test with p-value <0.05 considered as statistically significant. Further logistical regression analysis was also performed.
Case Definitions
Chikungunya infection criteria definitions and case definition (See Tables 1 and 2) used during our study based on the European Centre for Disease Control.15
 |
Table 1 Chikungunya Infection Criteria Definitions15 |
 |
Table 2 Chikungunya Infection Case Definitions15 |
Ethical Clearance
Ethical approval was obtained from the zonal branch of the Ministry of Health, Research and Ethics Review Committee (reference number 15/10/2018) and written informed consent was sought from each patient. Data confidentiality was assured by coding the personal identifiers and removing identifiers from the final analysis, and the study complies with the Declaration of Helsinki.
Results
Based on an epidemiological data and clinical criteria, a total of 203 probable cases of CHIKF were included in the study. The study observed a high male-to-female sex ratio of 2.1:1 (males = 68%; females = 32%) with a mean age of 39.2 years old. Most of patients were 25–35 years old (40.9%) and 20.7% above 45 years old. Regarding occupational frequencies, healthcare workers (47.8%) and civil servants (30.5%) working in the town (teachers, immigration staff, commercial bank staff, telecommunication staff) ranked the most common profession, followed by subzone administration staff (21.7%). Majority of patients reached either college-level (51.7%) or secondary education (32.5%) with only 5.4% of cases being illiterate working as cleaners and gatekeepers. From our study, 51.2% identified as O-type blood group with a large portion of cases possessing the positive Rh antigen factor (96.4%) (Table 3).
 |
Table 3 Socio-Demographic Data, Including Blood Group and Rh Factor, of Probable Cases of CHIKFFungunya Fever |
During the acute phase (first visit), common reported symptoms were polyarthralgia (97%), fever (96.1%), gastrointestinal symptoms (64.5%), headache (62%) and skin rash (56.7%). Joint pain was the main symptom with the most frequent affected joints being the wrist (59.4%), interphalangeal joints of the hands (56.9%), and knee (53.8%). In terms of dermatological features, 56.7% of cases experienced transient maculopapular rash, which was largely pruritic (85.2% of skin rash cases) with the most involved anatomical sites being the hands (71%), trunk (46.5%), and face (45.6%) (Table 4). Arthralgia gradually reduced over the course of the study period, persisting at the sixth month in only 21.7% of the cases (Figure 1). Arthralgia was largely symmetrical (84.3%) during the acute phase, but over time, the symmetry of joint pain gradually reduced to 78% reported during the last visit. Fever was the second leading symptom with the mean duration of 4.1 ± 3 days and was commonly responded with antipyretics in 95.9% of cases. Fever was accompanied by epistaxis in a minority of the patients (0.6%). Headache was also a common symptom with a mean duration of 3.8 ± 3 days (Table 5).
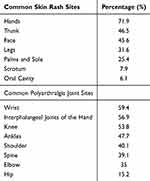 |
Table 4 Common Skin Rash Sites and Joint Sites Involved with Transient Maculopapular Rash and Polyarthralgia, Respectively |
 |
Table 5 Clinical Presentation of Probable Cases of CHIKFFungunya Fever with Related Duration for Fever and Headache During the Acute Phase |
 |
Figure 1 Persistence of CHIKFFungunya-induced joint pain based on monthly follow-up reports across the entire study period. |
Gastrointestinal symptoms were experienced by 64.5% of cases with anorexia (80.9%), nausea (41.2%), and vomiting (32.2%) as the highest ranked symptoms. Lymphadenopathy was found in 44.3% of cases with greatest affliction amongst inguinal lymph node (67.8%), followed by cervical lymph nodes (51.1%). Ocular symptoms were seen in 32.5% of cases in which retro-orbital pain (27.1%) was the main presented eye symptom followed by conjunctival hyperemia (24%) (Table 6). CBC showed lymphocytosis (64.5%), granulocytopenia (43.3%), and mild anemia (31%) were the most common abnormal hematological findings, followed by leukopenia (16.7%) and granulocytosis (14.3%) (Table 7).
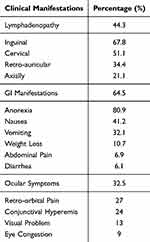 |
Table 6 Lymphadenopathic Features, GI Manifestations, and Ocular Symptoms of Probable Cases of CHIKFFungunya Fever During the Acute Phase |
 |
Table 7 Abnormal Hematological Findings from CBC Profile |
At the end of the acute phase, patients continued to be monitored via monthly follow-up visits for the remainder of the 6-month study period. The main clinical features that continued to be monitored were joint pain and joint swelling. Across the entire study period, case ages 25–35 and greater 45 years old expressed higher and consistent frequencies of joint pain and joint swelling compared to other age groups, with the exception of joint swelling reported higher among 36–40 age group. Health workers followed by administration workers continued to experience higher levels of CHIKF symptoms compared to other professional groups. The same pattern was found with educational status as individuals possessing college or secondary level education had higher levels of CHIKF symptoms compared to groups of lower educational status (Table 8). Logistical regression was performed to further analyze significant factors associated with persistence of joint pain and joint swelling. Patient’s age >41 years (p = 0.001, OR: 1.588; 95% CI: 0.935–2.695) and occupation (p = 0.003; OR: 0.370 95% CI: 0.194–0.707), O blood type (p = 0.033, OR: 1.153; 95% CI: 0.843–1.579) showed significant association with the likelihood of persistent joint pain (Table 8). Joint pain was often accompanied by joint swelling (42.1% of joint pain cases) with being female (p = 0.001, OR: 0.355; 95% CI: 0.216–0.583) and possessing O-type blood group (p = 0.02; OR: 1.836; 95% CI: 0.820–4.110) as significant predisposing factors to experiencing joint swelling (Table 8).
 |
Table 8 Socio-Demographic Data and Their Association for Persistence of Joint Pain and Joint Swelling During Monthly Follow-up |
Discussion
CHIKV is responsible for the recent explosive outbreaks of debilitating disease in humans, and this study has public health implications such as knowing the acute clinical presentation and to take preventive measures early. This arthritogenic virus has re-emerged in many tropical and subtropical regions due to its genomic polymorphism, which increases the vector susceptibility.20 Global warming/climate change, globalization with significant increase in international travels, and adaptation of virus to new vectors has also increased the vector susceptibility and transmission capacity.13,17 CHIKV most probably first emerged as a human pathogen in the 18th century, but has currently been identified in nearly 80 countries across 5 continents.10 Brazil is the epicenter of the disease today, accounts for 99% of CHIKF cases in the Americas this year, 50% of cases in the past 10 years, and over 90% of CHIKF cases globally this year.21 Most epidemics of CHIKF occurred in tropical or subtropical areas; Eritrea’s subtropical climate is suitable for Aedes mosquitoes and the transmission of mosquito-borne diseases, such as CHIKF. The first confirmed CHIKF outbreak in Eritrea was found within the Tesseney subzone in October 2018. This study aims to describe the clinical features of probable cases of CHIKF during and following the October 2018 outbreak as well as the risk factors associated with the persistence of polyarthralgia within the Tesseney subzone of Eritrea.
The clinical manifestations of CHIKF depend on the host–viral interactions, which determine the course of infection and key to understanding viral pathogenesis and treatment.22,23 During the acute phase, our patients mainly presented with sudden onset of high-grade fever (96.1%) and polyarthralgia (97%), follow by headache (62%) and skin rash (56.7%). Our results align with previous literature, which reports the typical triad of symptoms during the acute stage of CHIKF: high-grade fever, skin rash, and polyarthralgia.2,8,15,20 Prospective studies performed in the Philippines and Maldives also exhibited similar frequencies of high-grade fever (94.3% in Philippines; 100% in Maldives) and arthralgia (98.6% in Philippines; 82% in Maldives) with only a difference in skin rash incidence (87.1% in Philippines; 54% in Maldives).8,20 Cross-sectional analyses done in Columbia and Bangladesh after CHIKF outbreaks reported arthralgia (91.2% in Columbia; 99.2% in Bangladesh) and skin rash (44.7% in Columbia; 50.2% in Bangladesh) in similar frequency, but high-grade fever in different frequency (50% in Columbia; 100% in Bangladesh).24,25 These differences in occurrence to commonly reported symptoms could be attributed to host–viral interactions.23 A case study in Brazil illustrates this idea as a patient with pain from a previous finger joint injury was reignited upon CHIKF infection and was hypothesized that the CHIKF targeted and exacerbated the latent injury.13 Interestingly, a longitudinal cohort study in Sri Lanka found a unusually high expression of acute polyarthritis (45%) that later progressed to chronic polyarthritis (99% of acute polyarthritis cases) with a reduced frequency of skin rash symptoms (20%) commonly reported in the literature, showcasing the uncertainties of CHIKF manifestations.5
Acute arthralgia (97%) was highly expressed amongst our patients, which aligns with the results of previous studies and general pathology of CHIKF manifestation.4,5,8,15,20 Within our study, the most involved joint sites were the wrist (59.4%), interphalangeal joints of the hands (56.9%), knee (53.8%), and ankles (47.7%). Joint sites involvement for arthralgia in a Bangladesh study was similar with the wrist (54.1%) and small joints in the hand (46.8%) as primary joint sites.24 Contrarily, arthralgia was more expressed among weight-bearing joints (ie, ankles and knees) within previous Sri Lanka (74% for ankles), Philippines (60% for ankles), and Columbia (74.1% for ankles) studies.5,8,25 Joint site frequency was relatively high among all joint groups in our findings, thus minor differences amongst sites are likely not significant in understanding CHIKF infection patterns in joint site involvement. Transient maculopapular rash, stomatitis, and oral ulcers are often seen in adults, while retro-orbital pain, vomiting, and diarrhea commonly exhibited in children.15 56.7% of our cases experienced transient maculopapular skin rash with 0.6% experiencing epistaxis most likely associated to the hemorrhagic complication of the virus. Bangladesh (50.2%) and Maldives (50%) found roughly half of their patients develop maculopapular skin rash similar to our study.20,23 Skin rashes were frequently exhibited on the hands (71%) and trunk (46.5%) which corresponds with a study in Columbia that exhibited the hands and limbs as frequent skin rash sites.25 However, a seroprevalence study done in the rural areas of Chandrapur, Maharashtra, India, reported frequent rash sites on the knees (71%), feet (56%), and fingers and palms (54%) as the most common locations, emphasizing the importance of viral–host interactions imposing irregularities in symptom manifestations.18,26
The literature often reports that, following the acute phase (7–10 days), CHIKF infection can develop into a chronic phase with persistent rheumatoid-like symptoms that can persist for months to years.15,18,25,27 The majority of CHIKF-infected individuals become symptom-free four months after initial symptoms with only a minority developing persistent, debilitating arthralgia.2,22 CHIKF induced persistent arthralgia is hypothesized to derive from prolongation of the acute inflammatory course of viral infection within joint and muscle tissue, however the mechanism in which CHIKV RNA persists within joint and muscle tissue still remains unknown.7 Within our study, only 21.6% of cases continued with persistent joint pain at six months past the initial infection. The association of chronic joint pain and CHIKF has been assessed in only a few studies, but generally infers that only a minority of cases return with CHIKF induced chronic arthralgia.2,3,22 A cohort study following a 2014–2015 CHIKF epidemic in Columbia found roughly 1/4th of the serologically confirmed study cases developed persistent polyarthralgia.27 A seroprevalence study performed in the US Virgin Islands showed 12% of the islanders continue to report polyarthralgia one year after the initial CHIKF outbreak, likely attributing the finding to the CHIKV infection. Forms of persistent arthralgia have also been described in a 1980 retrospective study performed in South Africa, which exhibited episodic stiffness and pain in 3.7% of cases, persistent stiffness without pain in 2.8%, and persistent painful restriction of joint movements in 5.6%.15
In our study, age above 41 years old (p = 0.001, OR: 1.588; 95% CI: 0.935–2.695), working in health or administrative job (p = 0.003; OR: 0.370 95% CI: 0.194–0.707), and having O-type blood group (p = 0.033, OR: 1.153; 95% CI: 0.843–1.579) were factors significantly associated with increased risk of persistent joint pain upon CHIKF infection. In terms of age, CHIKF induced arthralgia is more commonly found in adults compared to children, likely due to the nature of the immune system.20 Younger persons possess a more robust immune system able to clear viremia more effectively compared to an adult’s immune system, thus reducing the probability of CHIKF affecting musculoskeletal tissue attributed to joints.20 Higher frequencies of joint pain among health and administrative workers could be explained as most of these workers were older in age with jobs that immobilize them for long working hours. Additionally, during the initial phases of the outbreak at the Tesseney subzone, the hospital crowded wards of CHIKF infected patients fostered an environment where the Aedes mosquito may spread the virus from infected to noninfected individuals. Thus, healthcare staff may have contracted the viral disease as a result of treating patients, contributing to higher number of health workers being affected with CHIKF during the study. However, there is no clear explanation for O-type blood group association with increased risk of joint pain, but the high proportion of cases with O-type blood group compared to other blood types may have a bias on this finding. Joint swelling often accompanied arthralgia with being female (p = 0.001, OR: 0.355; 95% CI: 0.216–0.583) possessing significant protective association for joint swelling, while O-type blood group (p = 0.02; OR: 1.836; 95% CI: 0.820–4.110) having significant risk association with joint swelling. Though our study is novel in raising female protective association in joint swelling, the question of female predisposition to arthralgia has already been raised in some studies, and there is yet a clear answer for the cause of the result.4,5,20 Currently, there is effective FDA vaccine for prevention; but, mosquito-based surveillance and control is the appropriate strategy to control and contain the infection.28
Our study was subject to a number of limitations. First, confirmation of CHIKF infection on all probable cases via serological and/or molecular analysis was neither economical nor feasible within the study. Ruling out other febrile disease (dengue and malaria fever) cannot confirm the detection of CHIKF. Therefore, a probable case definition based on the European Centre for Disease Control was used as an alternative diagnostic criterion. Second, though nomadic people likely initiated the outbreak, the study design was restricted to non-probability convenience patient sampling as the nomadic lifestyle was not suitable to follow for long periods of time. Third, patients with persistent polyarthralgia at 6 months were not followed then after, therefore the complete long-term clinical picture for persistence of joint pain was not obtained in our study.
Conclusion
Our finding indicated that polyarthralgia, fever, and skin rash are a triad of symptoms during acute prodromal phase of CHIKF, accompanied by bowel habit alteration, lymphadenopathy, and ocular pain. Knowing these clinical features can help us for prompt community health education about the preventive measures. Persistent joint pain was a frequent long-term complication of CHIKF found in a subset of cases. Individuals above the age of 41 were found to be risk factor for persistence of joint pain. Further studies are needed to determine the association CHIKF infection and chronic arthralgia as well as chronic CHIKF -induced complications and associated risk factors.
Recommendations
Currently, there is effective FDA vaccine for prevention; however, mosquito-based surveillance and control are the appropriate strategy to control and contain the infection. Vector control methods should be implemented and mobilized in endemic areas with municipalities and communities being aware of the preventive measures. At an individual level, wearing long-sleeved shirts, using mosquito repellent, and sleeping under a mosquito bed net may reduce the chances of being bitten by an infected mosquito. Since an immunologic etiology is suspected in chronic cases of CHIKF, a short course of steroids may be useful, but care must be taken to monitor all adverse events of the drug and should not be continued indefinitely. Additionally, cold compresses have been reported to reduce joint pain and swelling.
Abbreviations
WHO, World Health Organization; CHIKF, chikungunya fever; CHIKV, chikungunya virus; ELISA, Enzyme-linked immunosorbent assay; RT-PCR, Reverse-transcription polymerase chain reaction; ESCA, East-Central-South African; IOL,Indian Ocean lineage; GI, Gastrointestinal; CI, confidence interval.
Data Sharing Statement
All available information is included in the manuscript.
Ethical Approval
Ethical approval was obtained from zonal branch of the Ministry of Health Research and Ethical Approval Committee and a written informed consent was obtained from the patient to participate in this report and publish.
Consent
A written informed consent was taken from the patients and data confidentiality was secured.
Acknowledgment
Authors acknowledge the support of Tesseney hospital staff: Kibrom T, Abraham T, Teckle T, Abel Alem, Samuel W, Hussein M, and Awet Mebrahtu. This paper has been uploaded to preprint server: https://www.researchgate.net/publication/377839978. Acute clinical features and persistence of joint pain in probable cases of chikungunya fever Fever in Eritrea, as a preprint.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The research did not have any source of funding.
Disclosure
The authors do not have any conflict of interest to disclose for this work.
References
1. Khongwichit S, Chansaenroj J, Thongmee T, et al. Large-scale outbreak of CHIKFFungunya virus infection in Thailand, 2018-2019. PLoS One. 2021;16(3):e0247314. doi:10.1371/journal.pone.0247314
2. Fini R, Dos S MIC, Carvalho DO, et al. CHIKFFungunya Fever: Biology and Epidemiological Aspects. Vector-Borne Diseases & Treatment:p1–22.
3. De ADC, Jean S, Clavelou P, Dallel R, Bouhassira D. Chronic pain associated with the CHIKFFungunya fever: long lasting burden of an acute illness. BMC Infect Dis. 2010;10(31):1–6.
4. Lakshmi V, Neeraja M, Subbalaxmi MVS, et al. Clinical features and molecular diagnosis of CHIKFFungunya fever from south India. Clin Infect Dis. 2008;46(9):1436–1442. doi:10.1086/529444
5. Kularatne SAM, Weerasinghe SC, Gihan C, et al. Epidemiology, clinical manifestations, and long-term outcomes of a major outbreak of CHIKFFungunya in a hamlet in Sri Lanka, in 2007: a longitudinal cohort study. J Trop Med. 2012;2012:1–6. doi:10.1155/2012/639178
6. de Lima Cavalcanti TYV, Pereira MR, de Paula SO, Franca RFDO. A review on CHIKFFungunya virus epidemiology, pathogenesis and current vaccine development. Viruses. 2022;14:969. doi:10.3390/v14050969
7. Kril V, Aïqui-reboul-paviet O, Briant L, Amara A. New insights into CHIKFFungunya virus infection and pathogenesis. Annu Rev Virol. 2021;8:327–347. doi:10.1146/annurev-virology-091919-102021
8. Gutierrez-rubio AK, Magbitang AD, Penserga EG. A three-month follow up of musculoskeletal manifestions in CHIKFFungunya fever. Philipp J Intern Med. 2014;52(1):1–5.
9. Virology H. CHIKFFungunya association with different presentation at tertiary care centre. J Hum Virol Retrovirology. 2017;6(1):6–9.
10. Atalay T, Kaygusuz S, Azkur AK. A study of the CHIKFFungunya virus in humans in Turkey. Turkish J Med Sci. 2017;47:1161–1164. doi:10.3906/sag-1604-36
11. Sissoko D, Moendandze A, Malvy D, et al. Seroprevalence and risk factors of CHIKFFungunya Virus Infection in Mayotte, Indian Ocean, 2005-2006: a population-based survey. PLoS One. 2008;3(8):2005–2006. doi:10.1371/journal.pone.0003066
12. Tanay A. CHIKFFungunya fever presenting as a systemic disease with fever, arthritis and rash: our experience in Israel. Isr Med Assoc J. 2016;18(3–4):162–163.
13. Eyer-Silva WD, Pinto HD, Silva GA, Ferry FR, A case of CHIKFFungunya virus disease presenting with remarkable acute arthritis of a previously damaged finger joint. J Brazilian Soc Trop Med. 2016;49790–2.
14. Faisal A. Clinical management of CHIKFFungunya fever. Guideline Health Facilities Maldives. 2019.
15. World Health Organization Regional Office for South-East Asia. Guidelines on Clinical Management of CHIKFFungunya Fever. New Delhi; 2008.
16. Larrieu S, Pouderoux N, Pistone T, et al. Factors associated with persistence of arthralgia among CHIKFFungunya virus-infected travellers: report of 42 French cases. J Clin Virol. 2010;47(1):85–88. doi:10.1016/j.jcv.2009.11.014
17. Pouriayevali MH, Rezaei F, Jalali T, et al. Imported cases of CHIKFFungunya virus in Iran. BMC Infect Dis. 2019;19. doi:10.1186/s12879-019-4637-4
18. Hennessey MJ, Ellis EM, Delorey MJ, et al. Seroprevalence and symptomatic attack rate of CHIKFFungunya virus infection, United States Virgin Islands, 2014–2015. Am J Trop Med Hyg. 2018;99(5):1321–1326. doi:10.4269/ajtmh.18-0437
19. Johnson BW, Russell BJ, Goodman CH. Laboratory diagnosis of CHIKFFungunya virus infections and commercial sources for diagnostic assays. J Infect Dis. 2016;214(Suppl 5):S471–4. doi:10.1093/infdis/jiw274
20. Imad HA, Phadungsombat JN, Suzuki EE, et al. Clinical features of acute CHIKFFungunya virus infection in children and adults during an outbreak in the Maldives. Am J Top Med Hyg. 2021;105(4):946–954. doi:10.4269/ajtmh.21-0189
21. De Souza WM, Ribeiro GS, de Lima STS, et al. Chikungunya: a decade of burden in the Americas.Lancet. Reg Health Am. 2024;30:100673. doi:10.1016/j.lana.2023.100673
22. Hawman DW, Stoermer KA, Montgomery SA, et al. Chronic joint disease caused by persistent CHIKFFungunya virus infection is controlled by the adaptive immune response. J Virol. 2013;87(24):13878–13888. doi:10.1128/JVI.02666-13
23. Long KM, Whitmore AC, Ferris MT, et al. Dendritic cell immunoreceptor regulates CHIKFFungunya virus pathogenesis in mice. J Virol. 2013;87(10):5697–5706. doi:10.1128/JVI.01611-12
24. Rahman M, Jakaria SK, Sayed B, et al. Clinical and laboratory characteristics of an acute CHIKFFungunya outbreak in Bangladesh in 2017. Am J Top Med Hyg. 2019;100(2):405–410. doi:10.4269/ajtmh.18-0636
25. Rueda JC, Santos AM, Angarita J, et al. Demographic and clinical characteristics of CHIKFFungunya patients from six Colombian cities, 2014 – 2015. Emerg Microbes Infect. 2019;8(1):1490–1500. doi:10.1080/22221751.2019.1678366
26. Kawle AP, Nayak AR, Bhullar SS, et al. Seroprevalence and clinical manifestations of CHIKFFungunya virus infection in rural areas of Chandrapur, Maharashtra, India. J Vector Borne Dis. 2017;54(1):35–43. doi:10.4103/0972-9062.203167
27. Chang A, Encinales L, Porras A, et al. Frequency of chronic joint pain following CHIKFFungunya virus infection: a Colombian cohort study. Arthritis Rheumatol. 2018;70(4):578–584. doi:10.1002/art.40384
28. Ly H. Ixchiq (VLA1553): the first FDA-approved vaccine to prevent disease caused by Chikungunya virus infection. Virulence. 2024;15(1):2301573. doi:10.1080/21505594.2023.2301573
29. Division of vector-borne diseases. CHIKFFungunya: vector surveillance and control in the United States
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

