Back to Journals » Journal of Inflammation Research » Volume 17
Advancing Polyphenol-Based Nanomedicine for Inflammatory Bowel Disease: Challenges and Opportunities
Authors Wang L, Chen G, Yang Y, Xu C, Zhu L, Yang H, Yu M
Received 12 August 2024
Accepted for publication 14 November 2024
Published 27 November 2024 Volume 2024:17 Pages 9889—9904
DOI https://doi.org/10.2147/JIR.S487942
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Tara Strutt
Liucan Wang,* Guoqing Chen,* Yang Yang, Chao Xu, Li Zhu, Hua Yang, Min Yu
Department of General Surgery, Chongqing General Hospital, Chongqing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hua Yang; Min Yu, Email [email protected]; [email protected]
Abstract: Oxidative stress, characterized by excessive production of reactive oxygen species (ROS), is a critical factor in the progression of inflammatory bowel disease (IBD) and presents a potential therapeutic target. Anti-oxidant therapy, aimed at mitigating excessive ROS, is emerging as a cornerstone in IBD treatment. Nanomaterials with robust anti-oxidant properties offer promise by inhibiting inflammation through ROS scavenging, enhancing IBD therapeutic efficacy. Recent focus in ROS scavenging has centered on metal oxide nanoenzymes and polyphenol-based nanomaterials. The primary challenges are the catalytic efficiency of nanoenzymes and the functional integration of these nanomaterials with therapeutic agents. Polyphenols, natural plant extracts, have garnered significant interest due to their potent anti-oxidant properties and unique catechol groups that interact with biomolecules such as proteins and nucleic acids. The strong metal ion chelating ability of catechols enriches the structure and functionality of nanomaterials, improving the physicochemical properties of nanocarriers and enabling innovative designs of multifunctional drug delivery systems (DDSs). Research on polyphenol-based DDSs has expanded to include agents like epigallocatechin gallate, curcumin, resveratrol, tannic acid, and polydopamine. These nanocarriers and anti-oxidants, which incorporate polyphenols, have demonstrated potential anti-oxidant properties in novel DDSs as therapeutic agents to reduce inflammation and as essential components of drug carriers. This review focuses on the design and application of natural polyphenol-based anti-oxidant nanomaterials for IBD treatment, offering a comprehensive discussion on the use of polyphenols in DDSs and the potential challenges posed by their diverse roles in innovative drug delivery strategies, including their impact on the physical and chemical properties of DDSs.
Keywords: anti-oxidant therapy, polyphenols nanomaterial, ROS scavenging, inflammation inhibition
Introduction
Inflammatory bowel diseases (IBD), including ulcerative colitis (UC) and Crohn’s disease (CD), are chronic conditions that affect the gastrointestinal tract. These diseases are progressive and destructive, with an increasing incidence and prevalence.1,2 Although the exact pathogenesis of IBD remains unclear, substantial evidence supports the dysregulation of inflammatory cytokines, such as IL-6, IL-10, TNF-α, and IL-1β, along with a significant increase in oxidative stress among patients. These factors are critical in triggering the development of IBD.3–5 Current clinical therapies for IBD aim to induce remission using low molecular-weight pharmaceuticals, including amino-salicylates, corticosteroids, immunomodulators, and biologics. However, these treatments often have limited efficacy in clinical practice, facing challenges such as susceptibility to opportunistic infections, rapid clearance, limited functionality, and potential liver toxicity.6–8 Notably, oxidative stress is known to drive the infiltration of neutrophils and macrophages into the intestinal mucosa at the site of IBD, resulting in the release of large quantities of reactive oxygen species (ROS) and inflammatory cytokines.9 There is increasing evidence that the overproduction of ROS plays a critical role in the pathogenesis and progression of IBD.10 Strategies that reduce inflammatory cytokines and scavenge ROS could alleviate IBD symptoms.11–13 In this context, there is substantial potential for developing innovative therapies that effectively mitigate ROS-induced oxidative stress while exhibiting anti-inflammatory properties. Recently, nanomaterial-based interventions have emerged as promising strategies for mitigating inflammatory and oxidative damage in IBD. Among these, polyphenol-containing nanomaterials have gained extensive attention from researchers.
Natural polyphenols are secondary metabolites with a polyphenolic structure, representing the predominant bioactive constituents derived from commonly consumed plants. The therapeutic potential of polyphenols in preventing or alleviating various metabolic disorders in humans has been convincingly demonstrated.14 This naturally occurring group of substances includes a variety of compounds such as epigallocatechin gallate (EGCG), epigallocatechin (EGC), epicatechin gallate (ECG), catechin, tannic acid (TA), quercetin, gallic acid, gossypol, ellagic acid, resveratrol, caffeic acid, curcumin (CUR), rosmarinic acid (RA), and many others. These compounds have attracted increasing attention from researchers due to their potential therapeutic benefits and health-promoting effects, primarily attributed to their anti-oxidant and anti-inflammatory properties.15,16 Specifically, the anti-oxidant capabilities of polyphenols provide cellular protection against damage from ultraviolet light and help scavenge ROS in stressed cells. However, the water-insolubility of many natural polyphenols, such as CUR and RA, somewhat limits their oral administration. The low absorption and poor bioavailability of these compounds pose significant challenges to their clinical and industrial applications.17,18
With the advancement of nanotechnology in biomedicine, extensive research has been conducted to enhance the stability and bioavailability of polyphenol-loaded drug delivery systems (DDSs) both in vitro and in vivo.19–21 From an application perspective, these polyphenol compounds can be categorized into therapeutic and functional polyphenols. They can function independently or be integrated with other materials. Notably, these compounds exhibit strong affinities for various biomolecules like proteins and nucleic acids through combinations of hydrogen bonding and hydrophobic interactions, making them promising candidates as constituents of nanomaterials.15 For instance, the preparation of functional nanoparticles by polymerization and self-assembly of catechins and macromolecules was also reported, which exhibited good bioactivity in vitro and in vivo.22 In addition, Zeng et al developed a universal nanotechnology for preparing polyphenol nanoparticles with diverse functions by the polyphenolic condensation of epigallocatechin gallate with small molecules.23 Compared to the strategies of molecular assembly or combination with other molecules, Yi et al designed and fabricated various polyphenol nanoparticles through amino acid-triggered Mannish condensation reactions. This simple and rapid method offers a new way to utilize naturally reproducible polyphenols.24
Additionally, a functional polyphenolic polymer known as mussel-inspired polydopamine (PDA), which contains abundant reductive groups such as phenol and catechol, possesses exceptional redox capabilities that may mitigate oxidative stress through electron liberation, thereby serving as a potential scavenger of ROS.25,26 Thus, this review will elaborate on some design strategies for PDA-based anti-oxidant materials, especially in IBD therapy.
This review focuses on the delivery of polyphenols and their application as components in the construction of DDSs, as well as the comprehensive exploration of polyphenols’ effectiveness in IBD therapy (Figure 1). The inherent functional groups of polyphenols enable them to form hydrogen bonds, metal chelate interactions, covalent bonds, hydrophobic interactions, π-π interactions, etc., which opens the way for their application in the field of nanomaterials. Various intermolecular interactions between polyphenols and other macromolecules not only promote the morphology engineering of polyphenol nanomaterials, but also enhance the loading and stimulus-response release capabilities of drugs (including small molecule drugs, proteins and nucleic acids). Unloaded polyphenol nanoparticles can treat diseases similar to polyphenol compounds because they still retain many of the good properties of polyphenols, such as their ability to clear ROS and inhibit inflammation. For future perspectives, we will also discuss the potential limitations and risks associated with the diverse roles of polyphenols in innovative drug delivery strategies, including their impacts on the physical and chemical properties of nano-DDSs and their influence on normal physiological functions within the organism.
 |
Figure 1 Natural polyphenols as therapeutic agents/components in nanomaterials and the application of natural polyphenol-based nanomaterials for efficient IBD therapy. |
Natural Polyphenol as Therapeutic Agents
Recently, there has been a substantial increase in the use of therapies based on naturally active compounds for treating IBD, primarily due to their broad range of beneficial biological activities and excellent biocompatibility.27 The therapeutic efficacy of natural polyphenols, known for their ROS scavenging activity, is particularly effective in treating IBD. Notably, it has been reported that the ortho phenolic hydroxyl group found in the phenolic hydroxyl structure of plant polyphenols (such as catechol or pyrogallol) can readily oxidize to form quinones. These quinones deplete oxygen from the environment and exhibit potent scavenging activity against ROS and other free radicals,28,29 endowing polyphenols with robust anti-oxidant capacities and multiple free radical scavenging abilities.
For patients diagnosed with clinical IBD, the oral route of drug administration is widely recognized as optimal due to its superior patient adherence, convenient self-administration, exceptional safety profile, and cost-effective production. Consequently, oral administration of naturally active compounds is highly desirable.30,31 However, the harsh gastrointestinal microenvironment poses a significant challenge that must be addressed. Additionally, issues of water insolubility and poor bioavailability also need consideration. Therefore, developing a therapeutic polyphenols delivery system that effectively protects against gastrointestinal degradation, ensures specific adherence, and enables sustained retention in the inflamed colon is crucial. Such advancements would significantly enhance the efficacy of colitis treatment.
According to the literature, plant-derived polyphenols such as CUR, RA, coffee cherry husk extracts, and grape seed extracts, among others, have been explored in nanomaterial-mediated DDSs for managing conditions characterized by inflammation, particularly IBD. Below, we discuss some research works involving therapeutic polyphenols as agents or guest molecules in DDSs.
Curcumin
Curcumin is a polyphenolic compound extracted from turmeric. It exhibits free radical scavenging, anti-cancer, antiviral, antiarthritic, anti-oxidant, and anti-inflammatory properties. Recognized by the Food and Drug Administration as a biocompatible, low-cost, and safe food additive, curcumin is derived from the perennial herb Curcuma longa. Despite its diverse molecular targets, curcumin faces significant challenges, including low solubility (<1 μg mL−1), limited tissue targeting ability, and rapid oxidative degradation, contributing to its poor bioavailability and stability in inflammation treatment. Its anti-oxidant and anti-inflammatory properties have been extensively studied in both in vivo and in vitro models, making it a promising candidate for IBD therapy.32,33 Curcumin also modulates various inflammatory cytokines by suppressing the activity of NF-κB, an inflammatory transcription factor closely associated with high oxidative stress.34 However, its spontaneous autoxidation at physiological pH and rapid degradation in oxidizing environments result in inadequate oxidative stability, which hampers its therapeutic efficacy.35 Moreover, the oral administration of curcumin for IBD may encounter the strongly acidic conditions of the gastrointestinal environment and lack specific targeting to the inflamed colon.
To enhance its clinical utility, various research teams have focused on developing DDSs that address these limitations and improve the targeted treatment of inflammation. For instance, to improve bioavailability and the efficacy of colon inflammation inhibition, some self-assembled nanoparticles with curcumin as the core therapeutic agent have partially solved the problems of effective transport and utilization. Liang et al (Figure 2a) fabricated a poly (diselenide-oxalate-curcumin) nanoparticle (SeOC-NP) with dual-ROS sensitive chemical moieties.36 The nanosystem was synthesized via a one-step assembly strategy, incorporating diselenide and peroxalate ester bonds, which endowed the structure of SeOC-NP with ROS-responsive abilities, significantly improving the solubility and oxidation stability of curcumin. This ROS-responsive nanosystem significantly enhances the targeted delivery and oxidative stability of curcumin for high-efficiency inflammatory therapy. Similarly, Xu et al (Figure 2b) developed polymeric micelle nanoparticles, referred to as DHC NPs, formed through the self-assembly of hydroxyethyl starch (HES) and curcumin-conjugated polymers.37 These nanoparticles encapsulate the anti-inflammatory drug dexamethasone (DEX), further enhancing the therapeutic effect.
 |
Figure 2 (a) Schematic illustration of orally deliverable ROS-responsive linkage-bridged nanoparticle SeOC-NP for colitis alleviation in mice model. Reproduced from Liang D, Shen X, Han L, et al. Dual-ROS sensitive moieties conjugate inhibits curcumin oxidative degradation for colitis precise therapy. Adv Healthcare Mater. 2024;13(13):2303016. © 2024 Wiley-VCH GmbH.36 (b) Schematic representation of the fabrication process and oral drug delivery mechanism for dual drug-loaded DHC NPs. Reprinted from Int J Pharm. Volume: 623. Xu C, Chen S, Chen C, et al. Colon-targeted oral nanoparticles based on ROS-scavenging hydroxyethyl starch-curcumin conjugates for efficient inflammatory bowel disease therapy. 121884, Copyright 2022, with permission from Elsevier.37 (c) Schematic representation of the preparation and mechanism of action for CUR@Chs-PNC NPs in an IBD model. Using CUR@Chs-PNC NPs enables prolonged intestinal residence time for CUR, facilitating enhanced interaction between CUR and gut microbiota (GM), thereby restoring gut homeostasis. Reprinted from Mater Today Bio. Volume 20. Xie Y, Xu W, Jin Z, Zhao K. Chondroitin sulfate functionalized palmitic acid and cysteine cografted-quaternized chitosan for CD44 and gut microbiota dual-targeted delivery of curcumin. 100617, Copyright 2023, with permission from Elsevier.38 (d) Design and synthesis of oral nano-antioxidant for treating inflammatory bowel disease (IBD). The preparation of oral nano-antioxidant and therapeutic effects of nano-antioxidant on IBD by macrophage polarization and ferroptosis inhibition. Reprinted from Chem Eng J. Volume: 465. Yang J, Bai Y, Shen S, et al. An oral nano-antioxidant for targeted treatment of inflammatory bowel disease by regulating macrophage polarization and inhibiting ferroptosis of intestinal cells. 142940, Copyright 2023, with permission from Elsevier.39 |
Results from in vitro and in vivo experiments have demonstrated that the simultaneous delivery of anti-inflammatory drugs and reactive ROS scavengers yields a synergistic therapeutic effect, combining the benefits of anti-inflammatory therapy and ROS scavenging. The self-assembly strategy utilizing HES-CUR conjugates ensures stable drug delivery and targeted release, with the natural polyphenol CUR significantly improving the biocompatibility of the DDS. This approach is particularly promising, considering its potential to modulate gut microbiota dysbiosis and enhance short-chain fatty acid (SCFA) production in IBD. Xie et al synthesized CD44 and gut microbiota dual-targeting nanoparticles (NPs) and loaded them with CUR (CUR@Chs-PNC NPs) (Figure 2c)38 that exhibited prolonged intestinal residence time and excellent on-demand drug release behavior in the inflamed colon. This nanoplatform promoted the production of SCFAs, maintained intestinal microbiome homeostasis, and alleviated inflammation symptoms in vivo.
Moreover, curcumin has been shown to protect against ferroptosis in IBD by inducing GPX4, suppressing inflammation by inhibiting macrophage infiltration, and modulating macrophage polarization to the M2 phenotype.40,41 Thus, curcumin holds promise as both a ferroptosis inhibitor and an M2-activating drug in treating IBD.42 In a recent study, Yang et al (Figure 2d) synthesized a mannose-chitosan (M-CS) based drug delivery vehicle with ROS scavenging and ferroptosis inhibition properties, known as Cur-Ce@MCS, for targeted delivery of the nano-antioxidant CeO2 nano-enzyme and natural curcumin. Both in vitro and in vivo results demonstrated that this oral nano-antioxidant was effective in inhibiting inflammation and ferroptosis, as well as in regulating intestinal microbial composition and maintaining intestinal microecological balance.39
The aforementioned polymeric nanoparticles have effectively encapsulated the hydrophobic drug curcumin. By utilizing these functional nanoparticles, the bioavailability of polyphenolic drugs and the efficiency of targeted delivery to disease sites are significantly improved, thus regulating the high oxidative stress environment of IBD. With the rapid progression of research on plant polyphenols, an increasing number of dual drug-loaded systems based on curcumin are being developed for the treatment of IBD.43
Rosmarinic Acid
RA, another polyphenol-based anti-oxidant, has garnered increasing attention due to its broad range of bioactive properties, including anti-inflammatory, immune-modulatory, anti-cancer, and antibacterial activities.44 Despite RA’s significant therapeutic potential, its limited water solubility and poor bioavailability have hindered its advancement toward clinical use. A recent study reported the synthesis of RA based nanoparticles through the self-assembly of PEGylated RA to enhance its water solubility and bioavailability.45 These water-dispersible PEGylated nanoparticles (RANPs) demonstrated a dose-dependent reduction of colonic inflammation in in vivo experiments, surpassing the effects of free RA. Moreover, the therapeutic efficacy of RANPs was further enhanced when combined with DEX, indicating a potential synergistic effect with conventional medications. Utilizing a mouse model of acute colitis, the study showcased the effective application of RA-derived nanoparticles as a promising nanomedicine for treating IBD. The RA-based DDS efficiently scavenged ROS, protected cells from ROS-induced damage, and selectively targeted the inflamed colon to mitigate oxidative damage.
Natural Polyphenol as Components of Nanomaterials
In recent years, with the continuous advancement of materials science, scientists’ research on phenolic compounds has turned to the field of nanotechnology. Polyphenols can exert their effects individually or in combination with other metal ions, proteins, and various components to form composite materials, thereby exhibiting enhanced performance. These distinctive chemical and functional characteristics have led to extensive applications of natural polyphenols in DDSs, including mesoporous nanoparticles, nanocapsules, and hydrogels. Material engineering has recently experienced a surge in interest, and research has focused on DDSs derived from natural polyphenols.46,47
The field of nanomedicine has consistently been at the forefront of scientific research, with ongoing discoveries of new methodologies and substances aimed at enhancing human health. Numerous DDSs such as hydrogels,48 metal ion-based composite nano-enzyme systems,49 and mesoporous silica nanoparticles,50 are employed in treating IBD. However, these materials often face challenges related to metal ion toxicity and degradation within the body. Remarkably, the expansion of research possibilities for treating IBD with nanomaterials has been facilitated by the emergence of innovative DDSs that leverage the inherent physical and chemical characteristics of naturally derived active components.
Among the various substances studied, natural polyphenols have been recognized for their significant medicinal value, facilitating the fabrication and assembly of DDSs utilizing natural products known for their minimal toxicity and widespread availability. Polyphenols such as TA, EGCG, and catechin (CAT) are extensively employed as key components in DDSs for treating diverse diseases. These systems effectively encapsulate polyphenols to optimize therapeutic outcomes. Ongoing investigations into these significant phenolic compounds reveal their promising prospects across various biomedical applications, including therapeutic delivery.
A key feature of polyphenols used in drug delivery lies in their abundant o-hydroxyl and m-hydroxyl groups found in constituents such as dihydroxyphenyl (catechol) and trihydroxyphenyl (galloyl), which exhibit significant biological activities and excellent therapeutic effects in IBD treatment.
The FDA has recognized TA, a naturally occurring polyphenolic compound, as “generally recognized as safe”. Numerous studies have utilized TA to create diverse drug delivery platforms, including hydrogels, nanoparticles, and microcapsules. These systems are developed based on hydrogen bonding, electrostatic interactions, or metal-coordination interactions.51–56 The binding of TA to numerous proteins involves phenolic hydroxyl-rich components, specifically comprising five gallol groups (with three -OH groups attached to an aromatic ring) and five catechol groups (consisting of two -OH groups covalently bonded to an aromatic ring). These components establish multiple hydrogen bonds and hydrophobic interactions with target proteins.57 In addition, the therapeutic capacity of TA has attracted considerable interest owing to its exceptional biological properties that include antioxidative effects, anti-inflammatory characteristics, and antineoplastic impacts.58–60 Moreover, Wang et al’s findings suggest that TA may inhibit colitis through the suppression of the IL-17-NF-κB pathway and the enhancement of microbiota-mediated methylation pathways, further contributing to its therapeutic potential in IBD management.61 Based on the study of the biological activity of TA, integrating nanomaterials with TA has enhanced its efficacy through improved bioavailability and targeted delivery, improving therapeutic effects and minimizing potential side effects.
TA-Armored DDSs for IBD Treatment
As is widely recognized, the primary challenge in administering anti-inflammatory medications orally is ensuring swift absorption within the small intestine while achieving targeted release, specifically within the inflamed colon. To address this issue, He et al (Figure 3a) reported an oral nanomedicine comprising a core of small interfering RNA (siRNA) targeting tumor necrosis factor-α (TNF-α) encapsulated in gallic acid-mediated graphene quantum dot and bovine serum albumin nanoparticles (siRNA-GBSA NPs), surrounded by a chitosan and tannic acid (CHI/TA) multilayer armor.62 In this nanoplatform, TA provides antioxidative stress and prebiotic properties that help regulate the GM and offer protection against harsh gastrointestinal conditions while facilitating targeted delivery to inflamed colon sites.
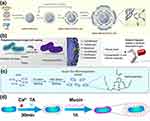 |
Figure 3 TA-armored nanoparticles and probiotic delivery systems. (a) Synthesis scheme for siRNA-GBSA(CHI/TA)n NPs. Reproduced from He H, Qin Q, Xu F, et al. Oral polyphenol-armored nanomedicine for targeted modulation of gut microbiota–brain interactions in colitis. Sci Adv. 2023;9(21):eadf3887. Creative Commons.62 (b) The nanoarmor facilitates rapid and highly biocompatible encapsulation of individual cells, protecting against a diverse range of antibiotics with varying molecular structures and properties. Reproduced with permission from ref.63 (c) Diagram illustrates the SGM preparation process. Reprinted from Chem Eng J. Volume 446. Yang J, Zhang G, Yang X, et al. An oral “Super probiotics” with versatile self-assembly adventitia for enhanced intestinal colonization by autonomous regulating the pathological microenvironment. 137204, Copyright 2022, with permission from Elsevier.64 (d) The therapeutic bacteria are decorated with TA and mucin using layer-by-layer coating technology. Reproduced from Yang X, Yang J, Ye Z, et al. Physiologically inspired mucin coated Escherichia coli nissle 1917 enhances biotherapy by regulating the pathological microenvironment to improve intestinal colonization. ACS Nano. 2022;16(3):4041–4058. Copyright 2022. American Chemical Society.65 |
In another approach, Khorshid et al devised and produced reservoir microdevices coated with bio-adhesive TA, which can establish physical/chemical connections with the amino acid residues in zein while facilitating a mussel-inspired attachment to the intestinal mucosa.66 The ex vivo experiments reveal that the incorporation of TA significantly enhances the adhesive properties of the coating within the intestinal tract, an enhancement further confirmed by in vivo studies on intestinal retention. Similarly, Zha’s group design and fabricated TA capped hafnium disulfide (HfS2@TA) nanosheets for IBD therapy. Benefiting from the transformation of the S2–/S6+ valence state and the modification of TA, the obtained HfS2@TA nanosheets were not only capable of eliminating ROS/reactive nitrogen species (RNS) and downregulating pro-inflammatory factors but also exhibit excellent targeting capability to an inflamed colon.67
Maintaining the balance and stability of GM is crucial for immune regulation in managing IBD. Consequently, the oral administration of living bacteria is gaining increasing attention as a potential therapeutic approach for gastrointestinal disorders. Nanomaterial-armored probiotic delivery strategies have been developed to enhance the efficacy of therapeutic bacteria. For instance, Pan et al demonstrated that a protective layer called “nanoarmor” (Figure 3b), consisting of TAs and ferric ions, can effectively shield bacteria from the effects of antibiotics.63 The interaction between catechol groups and Fe3+ allows the hydroxyl groups in TA to form stable metal-acid complexes, which engage in diverse interactions-including hydrogen bonding, hydrophobic interactions, and electrostatic interactions-with antibiotic molecules on the bacterial surface. These interactions impede the entry of antibiotic molecules into bacterial cells, thus safeguarding the bacteria. With its good biocompatibility, the plant extracts of TA nanoarmor serve as an ideal candidate for probiotic engineering.
Furthermore, Zhang’s research team illustrated the construction of a “Super Gut Microorganism” (Figure 3c) where cells are encapsulated with plant polyphenols TA and poloxamer 188 (F68, intravenous excipients) to form a functional surface “armor”.64 They also employed a layer-by-layer technology to develop a tannic acid and mucin-coated super probiotic (EcN@TA-Ca2+@Mucin) as an alternative strategy (Figure 3d).65 All the synthetic coating facilitates robust adhesion to the intestinal mucosa, thereby promoting the colonization of probiotics in diseased conditions and exhibiting remarkable abilities to regulate GM and reduce inflammation through TA.
TA-Mediated Supramolecular Self-Assembly DDSs for IBD Treatment
As previously mentioned, TA possesses abundant galloyl and catechol groups, which can establish hydrogen bonds and hydrophobic interactions with macromolecules like polymers and proteins, facilitating the creation of particles suitable for biomedical purposes.51,68,69 Due to its distinctive structural characteristics, TA has garnered considerable attention across various biological activities. Fortunately, using polyphenols as building blocks for particle assembly has improved bioavailability and targeted delivery capabilities. Due to its multiple hydroxyl groups and phenolic structure, TA is an excellent candidate for nanoparticle engineering.
Additionally, they serve as a secure and efficient nanoplatform containing TA for preventing and treating acute lung injury and colon diseases.70,71 Through aqueous self-assembly, poly (ethylene glycol) (PEG) containing polymers DSPE-PEG2k can be captured by TA through hydrogen bonding, and the supramolecular nanoparticles constructed are used for the protective delivery of infliximab. Substantial improvements in the effectiveness of IBD treatment can be attained by administering these nanoparticles orally, leading to enhanced therapeutic outcomes compared to free infliximab. As depicted in Figure 4a, the combination of TA and D-α-tocopherol polyethylene glycol 1000 succinate (TPGS) can generate condensed nanoparticles via hydrophobic interactions and hydrogen bonding.72 These nanoparticles can scavenge ROS, exhibiting anti-oxidant and anti-inflammatory properties.
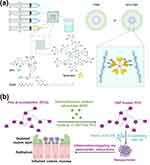 |
Figure 4 TA-mediated supramolecular assembly nanoparticles. (a) Schematic representation of the scalable synthesis of TTNP and DTX-TTNP using flash nanotechnology based on a turbulent-mixing device. The red dashed lines indicate the formation of intermolecular hydrogen bonds. Reproduced with permission from Royal Society of Chemistry. Le Z, He Z, Liu H, et al. Orally administrable polyphenol-based nanoparticles achieve anti-inflammation and antitumor treatment of colon diseases. Biomater Sci. 2022;10(15):4156–4169.72 (b) Schematic depiction of the incorporation of DSP into PCD and subsequent generation of nanoparticles loaded with DSP through the crosslinking process between TA and DSP-loaded PCD, aiming at addressing colon-related ailments. Reprinted from Acta Biomater. Volume169. Zhang C, Zeng F, Fan Z, et al. An oral polyphenol host-guest nanoparticle for targeted therapy of inflammatory bowel disease. 422–433, Copyright 2023, with permission from Elsevier.73 |
In addition, it has been reported that the interaction between the pyrogallol groups of TA and the CD cavities of PCD can lead to the assembly of TA with poly-β-cyclodextrin (PCD) through host-guest interactions.72 Unlike the process of oxidation self-polymerization74 or the mechanism of metal ion chelation,75 the host-guest assembly effectively maintains the exceptional pyrogallol group activity of TA, showcasing its remarkable ability to combat oxidative stress.72 Inspired by a unique assembly technique, Zhang et al developed an orally administered nanoparticle formulation for treating IBD.73 This formulation is based on the rapid host-guest assembly (within 10s) between dexamethasone sodium phosphate (DSP)-loaded PCD and TA, resulting in effective drug delivery (Figure 4b). And the oral nanoparticle containing polyphenols not only exhibits a selective affinity towards the inflamed colonic mucosa due to electrostatic attraction, but also demonstrates a potent ability to scavenge ROS, which is attributed to its TA constituent.
In another study, Hu et al designed polyphenolic nanoparticles pBDT-TA through the controlled self-assembly of TA and a self-polymerizable aromatic dithiol (benzene-1,4-dithiol, BDT). The fabricated pBDT-TA demonstrated improved biocompatibility, significant anti-oxidant, and anti-inflammatory activity. Alginate, an FDA-approved material for food additives, has been frequently applied as a substrate for bacterial encapsulation due to its resistance to acidic environments. To protect probiotics from the harsh oral environment, sodium alginate and pBDT-TA were coated onto the surface of EcN, and the resulting collaborative probiotics platform protected EcN from the adverse environment of the gastrointestinal tract and achieved efficient intestinal retention in dextran sulfate sodium (DSS)-induced colitis model mice.
In conclusion, the medicinal value of the natural polyphenol TA and its application in conjunction with nanomaterials offer a promising approach to treating IBD. The initial results are encouraging, but further research is necessary to fully understand and optimize this treatment strategy. Combining traditional medicinal substances with modern nanotechnology could potentially revolutionize how we treat complex diseases like IBD.
Other Functional Polyphenols Mediated DDSs for IBD Treatment
The natural compound (-)-epigallocatechin-3-O-gallate (EGCG), found in green tea, exhibits a high affinity for DNA, RNA, and proteins due to its ability to form hydrogen bonds with these molecules.76 Moreover, EGCG has been reported to have therapeutic effects in relieving colitis; this bioactive compound, rich in polyphenols, combats inflammation and oxidative stress and improves colonic inflammation, partly depending on the gut microbiota.77–79 Inspired by these benefits, using polyphenols and proteins to create hydrogels for IBD drug intervention has become feasible. In related research, scientists successfully formed functional hydrogels composed of amyloid fibrils through surface coverage with EGCG, facilitating self-assembly and encapsulation of EGCG into nanofilaments (up to 4.0 wt%).80 After oral administration, these hydrogels significantly improved colitis in vivo, enhancing intestinal barrier function, suppressing the expression of pro-inflammatory mRNA, and effectively regulating dysbiosis in GM.
Additionally, due to its strong binding affinity, EGCG can serve as a building blocks for siRNA delivery. Researchers have demonstrated that the EGCG/siRNA complex, carrying a negative charge, is further coated with cationic polymers through electrostatic interactions, facilitating the condensation of siRNA into uniform nanoparticles by polymers. These nanoparticles exhibit specific down-regulation of target genes in vitro and in vivo, effectively attenuating chronic intestinal inflammation for treating IBD.76 Inspired by the structural and functional characteristics of EGCG, Shen et al conducted a subsequent investigation where they introduced polymers with catechol grafting to enhance siRNA binding, cellular uptake, and gene silencing. The synthesized polycatechols/siRNA complex exhibited superior efficacy in delivering siRNA and effectively alleviated TNF-α induced inflammation in an intestinal injury model. This system has undergone extensive optimization by developing a robust and efficient strategy for facilitating polymer-mediated RNA interference (RNAi) using natural polyphenols containing multiple phenol groups.81
It is also worth mentioning that metal-polyphenol networks (MPNs), the supramolecular amorphous networks formed by the coordination interactions between multivalent metal ions and polyphenols, have gained wide attention for various biomedical applications, including drug delivery and surface coating due to their remarkable physicochemical properties.82 MPNs (Fe3+ and EGCG) possess ROS-responsive properties and can specifically release EGCG at inflammatory lesions. Deng et al developed a delivery nanosystem based on metal-polyphenol network/cerium oxide artificial enzymes (MPN@CeOx) to manage UC and evaluate the severity of inflammation via MRI/CT imaging. The nanoplatform was engineered with Fe3+ and EGCG wrapped around CeOx nanozymes. Fe3+ and Ce endowed MPN@CeOx with MRI/CT imaging capabilities for UC. Oral administration of enteric-coated MPN@CeOx successfully attenuated intestinal inflammation and dysfunction in protective and therapeutic colitis models. RNA sequencing analysis revealed that MPN@CeOx ameliorated UC symptoms primarily through inflammation-related signaling pathways, representing an innovative diagnostic and treatment approach for UC.
Although budesonide (BUD) possesses potent local anti-inflammatory activity, its therapeutic efficacy is limited due to first-pass metabolism and limited bioavailability. Addressing these limitations, Kumar Mishra et al developed micellar nanomaterials using stearic acid and the phenolic compound caffeic acid (CA).83 The presence of CA promotes the formation of micelles for efficient delivery of BUD and demonstrates anti-inflammatory effects by inhibiting NF-kB, COX-2, and several pro-inflammatory cytokines.
Natural Polyphenols Inspired Bioactive Polydopamine for IBD Treatment
In addition to the TA mentioned above, phenolic compounds represented by mussel-inspired PDA has also aroused great interest and the incorporation of PDA will provide additional advantages: PDA contains a similar chemical structure to polyphenols which provides extra antioxidant activity.84 Besides, due to the strong affinity of natural polyphenols towards biomolecules like proteins and nucleic acids,53,54,85 various phenolic structures in PDA, including phenol, catechol, and pyrogallol moieties, have been utilized to develop highly effective polymers.
Intestinal immune homeostasis plays a crucial role in the onset and progression of IBD. Following an abnormal immune response, the aberrant expression of pro-inflammatory and anti-inflammatory cytokines, along with the accumulation of ROS, is considered a cause of tissue damage. Therefore, immunoregulation and ROS scavenging are essential for treating intestinal inflammation.86 Nature phenolic compounds, polydopamine nanoparticles (PDA NPs), derived from the self-polymerization of dopamine, have garnered significant attention due to their excellent biocompatibility, biodegradability, and anti-inflammatory and antioxidative properties.87,88 Moreover, due to its ability to adhere to both inorganic and organic surfaces, DA finds extensive applications in surface modification, protein immobilization, and cell adhesion.82 Furthermore, the amine groups of DA facilitate convenient conjugation, thereby enabling further customization of surface properties tailored to specific applications.89
Consequently, harnessing the distinctive characteristics of PDA to modulate gut immunity and mitigate oxidative stress emerges as an appealing therapeutic approach for intestinal disorders, particularly IBD.
Immune Regulation Properties of PDA
Despite the importance of immunosuppression in mitigating excessive immune activation, the clinical use of immunosuppressive agents is significantly limited due to their inadequate inhibition efficacy and unpredictable off-target toxicities. In this context, nanoparticles with immunomodulatory capabilities, particularly phenolic structure-containing polymer nanoparticles like PDA NPs, offer new strategies. These nanoparticles can impede intestinal inflammation by facilitating the activation of regulatory T (Treg) cells and directly impeding the differentiation of naïve T cells into effector T helper (Th) cells, such as Th1, Th2, and Th17. Furthermore, they also demonstrate suppressive effects on dendritic cell stimulation.90 Some of the recent research illustrated the excellent immune regulation properties of PDA. As an illustration, Liu et al developed biomimetic nanomedicine using PDA (PDA@mCRAMP@MM, PCM NPs) to regulate mucosal immune homeostasis for managing IBD. As anticipated, the nanoplatform significantly alleviates cellular inflammation by acting as an immunosuppressive modulator, attributed to its capacity to combat oxidative stress induced by PDA. Liu et al also elucidated the role of dopamine in dopaminergic immunoregulation for mitigating excessive immune activation within inflamed tissues. The PDNI, a multifunctional immunosuppressant composed of polydopamine nanoparticles, is synthesized through the self-polymerization of DA in an alkaline environment. PDNI can induce regulatory T (Treg) cells and directly inhibit T helper (Th) cells. Moreover, PDNI can hinder the activation of dendritic cells (DCs), leading to an increase in the ratio of Treg/Th17. This beneficially reduces inflammatory responses in pathological tissues,84 providing a universal approach for developing novel immunotherapies targeting IBD.
This discussion highlights that intestinal macrophages exhibit heterogeneity and can be classified into two distinct subsets, M1 and M2,91 which are implicated in disrupting the intestinal epithelial barrier. It is crucial to maintain intestinal immune homeostasis by achieving a meticulous and delicate equilibrium in the polarization of macrophages into M1/M2 phenotypes. Several studies have identified PDA as a potential modulator of macrophage polarization, directing macrophages towards the M2 phenotype to reduce the inflammatory response.92,93 Therefore, regulating macrophage polarization via PDA represents a promising therapeutic strategy for IBD treatment. Meng et al observed that Thali nanocrystals with a core-shell structure (Thali@PDA) exhibited multifunctional properties. These nanocrystals, consisting of thalidomide known for its anti-inflammatory effects and coated with PDA, significantly increased the polarization of macrophages towards the M2 phenotype during radiotherapy, thereby enhancing the therapeutic effects of Thali@PDA on IBD.94
Anti-Oxidant Properties of PDA
Additionally, PDA NPs possess potent redox capabilities for scavenging ROS in the inflamed colon, owing to their abundant reductive groups such as phenol and catechol.95,96 Specifically, PDA can transfer electrons to different oxidants, providing a favorable anti-oxidant effect by neutralizing oxidative free radicals.25 This characteristic makes it a promising candidate for scavenging ROS and broadens its applications in biomedical anti-oxidants.
Recent research has explored using PDA-based nanomedicine for ROS scavenging in IBD therapy. For example, Yan et al provided a representative example of PDA-based nanoparticles that met anti-oxidant activity for ROS scavenging and oxidative stress regulation.97 The nanoplatform (LS@PDA NPs) was synthesized via a simple one-step method by encapsulating loxoprofen sodium (LS) within PDA during the polymerization of PDA NPs. When administered orally, LS@PDA NPs effectively scavenged excessive ROS, alleviating inflammation and improving acute inflammatory bowel conditions compared to free LS. Moreover, the negatively charged LS@PDA NPs achieved targeted delivery due to the positively charged surface of the inflamed colon, reducing undesirable drug toxicity. Additionally, mesoporous polydopamine nanoparticles (MPDA NPs), distinguished by their regularly structured pores, have garnered significant attention in surface modification and drug delivery due to their enhanced accessibility to active sites compared to their nonporous counterparts. A previous study presented a combined therapy approach (PAA@MPDA-SAP NPs), where MPDA acted as both the carrier and scavenger of ROS, whereas polyacrylic acid (PAA) served as a “molecular switch” to prevent premature drug degradation and release in the stomach following oral administration. As anticipated, the PAA@MPDA-SAP NPs demonstrated enhanced therapeutic efficacy compared to free SAP and PAA@MPDA NPs, evidenced by their synergistic reduction of inflammatory cytokines and ROS expression facilitated by MPDA.98
Conclusions and Future Perspectives
For decades, the health benefits of polyphenols have been primarily associated with their ability of anti-oxidants and ROS scavenging (Table 1), which has predominantly been demonstrated. For decades, the commercial development of polyphenols for clinical use has been hindered by inherent shortcomings such as water insolubility, low absorption and bioavailability, and rapid elimination and metabolism from the system. Moreover, the harsh gastrointestinal microenvironment of IBD presents a significant challenge for polyphenol-based therapeutic agents, necessitating innovative solutions. With the advancement of nanomedicine, naturally derived polyphenols play a crucial role in creating and applying anti-oxidant nanomaterials, serving both as therapeutic agents and components in DDSs. The ortho phenolic hydroxyl group in polyphenols such as catechol or pyrogallol endows them with potent anti-oxidant properties and scavenging activity against ROS and other free radicals. Additionally, the presence of dense o-hydroxyl and m-hydroxyl groups in the catechol and galloyl segments of polyphenols enables their binding with diverse materials, including metals, proteins, polymers, nucleic acids, and even small molecular compounds through mechanisms like hydrogen bonding, covalent interactions, metal coordination, and π-π stacking. This organized intermolecular binding confers novel functional orientations to polyphenols within innovative DDSs. However, with ongoing research, developing further DDSs suitable for more therapeutic polyphenols presents a significant challenge, and exploring more clinical treatment possibilities remains a critical need.
 |
Table 1 Representative Advances of the Biomedical Studies of Polyphenol-Containing Nanomaterials |
Although bioactive polyphenols have advantages in the treatment of inflammatory bowel disease, and many new methods for the treatment of intestinal diseases have been proposed in combination with the development of nanomaterials, there are still many problems and challenges in practice. The purpose of this review is to describe the current development of polyphenol-based treatment strategies and analyze their advantages and disadvantages, with a view to paving the way for the treatment of IBD.
The main problem is targeting and adhesion. The bio-adhesion of polyphenols is inspired by the marine adhesion of mussels, which exhibit strong adhesion to various substrates in seawater through the secreted mussel adhesive protein, attributed to their abundant catechol groups. Compounds like tannic acid and polydopamine can interact with colonic tissues through electrostatic adsorption, hydrogen bonding, cation-π interactions, and covalent bonds. However, whether this interaction can truly provide strong wet adhesion remains a question, especially in the complex pathological environment of IBD. Therefore, developing novel DDSs with efficient wet adhesion properties could significantly enhance the accumulation efficiency of polyphenols at the sites of intestinal inflammation, thereby improving therapeutic effects.
Optimizing and developing new probiotic dosage forms based on natural polyphenols. In this review, the design of polyphenol-armored probiotics, based on the antioxidant and targeting properties of natural polyphenols in inflamed intestinal sites, opens up a new direction for clinical oral probiotics therapy. However, the complex synthesis process will greatly limit its large-scale application. In addition, optimizing the coating of natural polyphenols on the surface of probiotics and reducing synthesis complexity and the effect of armor shell on the activity of probiotics will contribute to its therapeutic application.
To develop polyphenol-based hydrogels for the in-situ repair of intestinal mucosal barrier damage. The most prominent pathological feature of IBD is the destruction of the intestinal mucus barrier, which facilitates the transfer of pathogenic microorganisms and disrupts microbial homeostasis. Polyphenols offer significant advantages in the development of polyphenol-based hydrogels due to their antioxidant properties, excellent biocompatibility, and ease of modification. Creating a hydrogel system that can be administered orally and cross-link in situ at the site of inflamed colon to seal the damaged area and alleviate oxidative stress will fundamentally alleviate IBD.
Polyphenol-based polymeric nanoparticles are not only useful for drug delivery but also for labeling functional metal ions and encapsulating fluorescent dyes for various bioimaging techniques. These techniques include positron emission tomography (PET), magnetic resonance (MR), and near-infrared fluorescence (NIRF). Their primary advantage lies in their excellent biocompatibility, along with the ease of chelating of metal ions.
Although there have been many studies based on polyphenols in IBD treatment, the clinical transformation of polyphenol-based nanomedicines still remains great challenges. Investigating polyphenol-based DDSs for IBD treatment represents a promising and rapidly expanding area. However, there is a stark contrast between the abundant basic research results and the limited investigations into the clinical applications of these systems. Additionally, the interaction between polyphenols and proteins in the gastrointestinal environment threatens the effectiveness and safety of polyphenol-based nano-drugs. We believe these obstacles will soon be overcome, significantly promoting the application research of polyphenol-based DDSs in clinical settings.
Abbreviations
IBD, inflammatory bowel disease; CD, Crohn’s disease; UC, ulcerative colitis; DDSs, drug delivery systems; NPs, nanoparticles; ROS, reactive oxygen species; EGCG, epigallocatechin gallate; EGC, epigallocatechin; ECG, epicatechin gallate; TA, tannic acid; CUR, curcumin; RA, rosmarinic acid; PDA, polydopamine.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (NSFC82300634 (Liucan Wang), NFSC82370567 (Hua Yang)) and Natural Science Foundation of Chongqing (CSTB2023NSCQ-MSX0027 (Min Yu), CSTB2022NSCQ-MSX0485 (Guoqing Chen)). This paper has been uploaded to Authorea as a preprint: https://advance.sagepub.com/doi/full/10.22541/au.169956081.14133273/v1.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kudelka MR, Stowell SR, Cummings RD, et al. Intestinal epithelial glycosylation in homeostasis and gut microbiota interactions in IBD. Nat Rev Gastroenterol Hepatol. 2020;17(10):597–617. doi:10.1038/s41575-020-0331-7
2. Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol. 2015;12(12):720–727. doi:10.1038/nrgastro.2015.150
3. Cheng Y, Cheng C, Yao J, et al. Mn3O4 nanozyme for inflammatory bowel disease therapy. Adv Ther. 2021;4(9):2100081. doi:10.1002/adtp.202100081
4. Zhao J, Cai X, Gao W, et al. Prussian blue nanozyme with multienzyme activity reduces colitis in mice. ACS Appl Mater Interfaces. 2018;10(31):26108–26117. doi:10.1021/acsami.8b10345
5. Zhou X, Li W, Wang S, et al. YAP aggravates inflammatory bowel disease by regulating M1/M2 macrophage polarization and gut microbial homeostasis. Cell Rep. 2019;27(4):1176–1189.e5. doi:10.1016/j.celrep.2019.03.028
6. Geert RD, van Deventer S. 25 years of anti-TNF treatment for inflammatory bowel disease: lessons from the past and a look to the future. Gut. 2021;70(7):1396. doi:10.1136/gutjnl-2019-320022
7. Lee Y, Sugihara K, Gillilland MG, et al. Hyaluronic acid–bilirubin nanomedicine for targeted modulation of dysregulated intestinal barrier, microbiome and immune responses in colitis. Nat Mater. 2020;19(1):118–126. doi:10.1038/s41563-019-0462-9
8. Villablanca EJ, Selin K, Hedin CRH. Mechanisms of mucosal healing: treating inflammatory bowel disease without immunosuppression? Nat Rev Gastroenterol Hepatol. 2022;19(8):493–507. doi:10.1038/s41575-022-00604-y
9. Zhang C, Wang H, Yang X, et al. Oral zero-valent-molybdenum nanodots for inflammatory bowel disease therapy. Sci Adv. 2022;8(37):eabp9882. doi:10.1126/sciadv.abp9882
10. Xu J, Chu T, Yu T, et al. Design of diselenide-bridged hyaluronic acid nano-antioxidant for efficient ROS scavenging to relieve colitis. ACS Nano. 2022;16(8):13037–13048. doi:10.1021/acsnano.2c05558
11. Zhao J, Wang Y, Wang W, et al. In situ growth of nano-antioxidants on cellular vesicles for efficient reactive oxygen species elimination in acute inflammatory diseases. Nano Today. 2021;40:101282. doi:10.1016/j.nantod.2021.101282
12. Ma N, Guo P, Chen J, et al. Poly-β-hydroxybutyrate alleviated diarrhea and colitis via Lactobacillus johnsonii biofilm-mediated maturation of sulfomucin. Sci China Life Sci. 2023;66(7):1569–1588. doi:10.1007/s11427-022-2213-6
13. Zhou J, Li M, Chen Q, et al. Programmable probiotics modulate inflammation and gut microbiota for inflammatory bowel disease treatment after effective oral delivery. Nat Commun. 2022;13(1):3432. doi:10.1038/s41467-022-31171-0
14. Tomas-Barberan FA, González-Sarrías A, García-Villalba R. Dietary Polyphenols: Metabolism and Health Effects. John Wiley & Sons; 2020.
15. Chen Z, Farag MA, Zhong Z, et al. Multifaceted role of phyto-derived polyphenols in nanodrug delivery systems. Adv Drug Deliv Rev. 2021;176:113870.
16. Saw PE, Lee S, Jon S. Naturally occurring bioactive compound-derived nanoparticles for biomedical applications. Adv Ther. 2019;2(5):1800146. doi:10.1002/adtp.201800146
17. Li Z, Jiang H, Xu C, et al. A review: using nanoparticles to enhance absorption and bioavailability of phenolic phytochemicals. Food Hydrocoll. 2015;43:153–164. doi:10.1016/j.foodhyd.2014.05.010
18. Zhang L, McClements DJ, Wei Z, Wang G, Liu X, Liu F. Delivery of synergistic polyphenol combinations using biopolymer-based systems: advances in physicochemical properties, stability and bioavailability. Crit Rev Food Sci Nutr. 2020;60(12):2083–2097. doi:10.1080/10408398.2019.1630358
19. Pimentel-Moral S, Teixeira MC, Fernandes AR, et al. Lipid nanocarriers for the loading of polyphenols – a comprehensive review. Adv Colloid Interface Sci. 2018;260:85–94. doi:10.1016/j.cis.2018.08.007
20. Wang Y, Byrne JD, Napier ME, DeSimone JM. Engineering nanomedicines using stimuli-responsive biomaterials. Adv Drug Delivery Rev. 2012;64(11):1021–1030. doi:10.1016/j.addr.2012.01.003
21. Han Y, Zhou J, Hu Y, et al. Polyphenol-based nanoparticles for intracellular protein delivery via competing supramolecular interactions. ACS Nano. 2020;14(10):12972–12981. doi:10.1021/acsnano.0c04197
22. Chen X, Yi Z, Chen G, et al. DOX-assisted functionalization of green tea polyphenol nanoparticles for effective chemo-photothermal cancer therapy. J Mater Chem B. 2019;7(25):4066–4078. doi:10.1039/C9TB00751B
23. Yi Z, Chen G, Chen X, et al. Modular assembly of versatile nanoparticles with epigallocatechin gallate. ACS Sustainable Chem Eng. 2020;8(26):9833–9845. doi:10.1021/acssuschemeng.0c02538
24. Yi Z, Chen G, Chen X, et al. Preparation of strong antioxidative, therapeutic nanoparticles based on amino acid-induced ultrafast assembly of tea polyphenols. ACS Appl Mater Interfaces. 2020;12(30):33550–33563. doi:10.1021/acsami.0c10282
25. Liu H, Qu X, Tan H, et al. Role of polydopamine’s redox-activity on its pro-oxidant, radical-scavenging, and antimicrobial activities. Acta Biomater. 2019;88:181–196. doi:10.1016/j.actbio.2019.02.032
26. Hu J, Yang L, Yang P, et al. Polydopamine free radical scavengers. Biomater Sci. 2020;8(18):4940–4950. doi:10.1039/D0BM01070G
27. Debnath T, Kim DH, Lim BO. Natural products as a source of anti-inflammatory agents associated with inflammatory bowel disease. Molecules. 2013;18(6):7253–7270. doi:10.3390/molecules18067253
28. Hou M, Zhang Y, Jiao X, et al. Polyphenol-modified zero-valent iron prepared using ball milling technology for hexavalent chromium removal: kinetics and mechanisms. Sep Purif Technol. 2023;326:124874. doi:10.1016/j.seppur.2023.124874
29. Guo Y, Sun Q, Wu F-G, Dai Y, Chen X. Polyphenol-containing nanoparticles: synthesis, properties, and therapeutic delivery. Adv Mater. 2021;33(22):2007356. doi:10.1002/adma.202007356
30. Zhou Y, Chen Z, Zhao D, Li D, He C, Chen X. A pH-triggered self-unpacking capsule containing zwitterionic hydrogel-coated MOF nanoparticles for efficient oral exendin-4 delivery. Adv Mater. 2021;33(32):2102044. doi:10.1002/adma.202102044
31. Zhong D, Zhang D, Chen W, et al. Orally deliverable strategy based on microalgal biomass for intestinal disease treatment. Sci Adv. 2021;7(48):eabi9265. doi:10.1126/sciadv.abi9265
32. Vecchi Brumatti L, Marcuzzi A, Tricarico PM, et al. Curcumin and inflammatory bowel disease: potential and limits of innovative treatments. Molecules. 2014;19(12):21127–21153. doi:10.3390/molecules191221127
33. Gao X, Xu Z, Liu G, et al. Polyphenols as a versatile component in tissue engineering. Acta Biomater. 2021;119:57–74. doi:10.1016/j.actbio.2020.11.004
34. Kunnumakkara AB, Anand P, Aggarwal BB. Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer Lett. 2008;269(2):199–225. doi:10.1016/j.canlet.2008.03.009
35. Thangavel S, Yoshitomi T, Sakharkar MK, et al. Redox nanoparticles inhibit curcumin oxidative degradation and enhance its therapeutic effect on prostate cancer. J Control Release. 2015;209:110–119. doi:10.1016/j.jconrel.2015.04.025
36. Liang D, Shen X, Han L, et al. Dual-ROS sensitive moieties conjugate inhibits curcumin oxidative degradation for colitis precise therapy. Adv Healthcare Mater. 2024;13(13):2303016. doi:10.1002/adhm.202303016
37. Xu C, Chen S, Chen C, et al. Colon-targeted oral nanoparticles based on ROS-scavenging hydroxyethyl starch-curcumin conjugates for efficient inflammatory bowel disease therapy. Int J Pharm. 2022;623:121884. doi:10.1016/j.ijpharm.2022.121884
38. Xie Y, Xu W, Jin Z, Zhao K. Chondroitin sulfate functionalized palmitic acid and cysteine cografted-quaternized chitosan for CD44 and gut microbiota dual-targeted delivery of curcumin. Mater Today Bio. 2023;20:100617. doi:10.1016/j.mtbio.2023.100617
39. Yang J, Bai Y, Shen S, et al. An oral nano-antioxidant for targeted treatment of inflammatory bowel disease by regulating macrophage polarization and inhibiting ferroptosis of intestinal cells. Chem Eng J. 2023;465:142940. doi:10.1016/j.cej.2023.142940
40. Liu C, Yan X, Zhang Y, et al. Oral administration of turmeric-derived exosome-like nanovesicles with anti-inflammatory and pro-resolving bioactions for murine colitis therapy. J Nanobiotechnol. 2022;20(1):206. doi:10.1186/s12951-022-01421-w
41. Mouzaoui S, Banerjee S, Djerdjouri B. Low-dose curcumin reduced TNBS-associated mucin depleted foci in mice by scavenging superoxide anion and lipid peroxides, rebalancing matrix NO synthase and aconitase activities, and recoupling mitochondria. Inflammopharmacology. 2020;28(4):949–965. doi:10.1007/s10787-019-00684-4
42. Zhang L, Ren X, Zhou P, et al. Sequential self-assembly and disassembly of curcumin hydrogel effectively alleviates inflammatory bowel disease. Biomater Sci. 2022;10(22):6517–6524. doi:10.1039/D2BM01120D
43. Han W, Xie B, Li Y, et al. Orally deliverable nanotherapeutics for the synergistic treatment of colitis-associated colorectal cancer. Theranostics. 2019;9(24):7458–7473. doi:10.7150/thno.38081
44. Zhu F, Asada T, Sato A, et al. Rosmarinic acid extract for antioxidant, antiallergic, and α-glucosidase inhibitory activities, isolated by supramolecular technique and solvent extraction from perilla leaves. J Agric Food Chem. 2014;62(4):885–892. doi:10.1021/jf404318j
45. Chung CH, Jung W, Keum H, et al. Nanoparticles derived from the natural antioxidant rosmarinic acid ameliorate acute inflammatory bowel disease. ACS Nano. 2020;14(6):6887–6896. doi:10.1021/acsnano.0c01018
46. Cheng W, Wen J. Now and future: development and perspectives of using polyphenol nanomaterials in environmental pollution control. Coord Chem Rev. 2022;473:214825. doi:10.1016/j.ccr.2022.214825
47. Shan L, Gao G, Wang W, et al. Self-assembled green tea polyphenol-based coordination nanomaterials to improve chemotherapy efficacy by inhibition of carbonyl reductase 1. Biomaterials. 2019;210:62–69. doi:10.1016/j.biomaterials.2019.04.032
48. Yang X, He S, Wang J, et al. Hyaluronic acid-based injectable nanocomposite hydrogels with photo-thermal antibacterial properties for infected chronic diabetic wound healing. Int J Biol Macromol. 2023;242:124872. doi:10.1016/j.ijbiomac.2023.124872
49. Cao Y, Cheng K, Yang M, et al. Orally administration of cerium oxide nanozyme for computed tomography imaging and anti-inflammatory/anti-fibrotic therapy of inflammatory bowel disease. J Nanobiotechnol. 2023;21(1):21. doi:10.1186/s12951-023-01770-0
50. Shi C, Dawulieti J, Shi F, et al. A nanoparticulate dual scavenger for targeted therapy of inflammatory bowel disease. Sci Adv. 2022;8(4):eabj2372. doi:10.1126/sciadv.abj2372
51. Rahim MA, Kristufek SL, Pan S, et al. Phenolic building blocks for the assembly of functional materials. Angew Chem Int Ed. 2019;58(7):1904–1927. doi:10.1002/anie.201807804
52. Ejima H, Richardson JJ, Liang K, et al. One-step assembly of coordination complexes for versatile film and particle engineering. Science. 2013;341(6142):154–157. doi:10.1126/science.1237265
53. Shin M, Lee H-A, Lee M, et al. Targeting protein and peptide therapeutics to the heart via tannic acid modification. Nat Biomed Eng. 2018;2(5):304–317. doi:10.1038/s41551-018-0227-9
54. Wang H, Wang C, Zou Y, et al. Natural polyphenols in drug delivery systems: current status and future challenges. Giant. 2020;3:100022. doi:10.1016/j.giant.2020.100022
55. Shin M, Park E, Lee H. Plant-inspired pyrogallol-containing functional materials. Adv Funct Mater. 2019;29(43):1903022. doi:10.1002/adfm.201903022
56. Lin S, Cheng Y, Zhang H, et al. Copper tannic acid coordination nanosheet: a potent nanozyme for scavenging ROS from cigarette smoke. Small. 2020;16(27):1902123. doi:10.1002/smll.201902123
57. Shukla A, Fang JC, Puranam S, et al. Hemostatic multilayer coatings. Adv Mater. 2012;24(4):492–496. doi:10.1002/adma.201103794
58. Baer-Dubowska W, Szaefer H, Majchrzak-Celińska A, et al. Tannic acid: specific form of tannins in cancer chemoprevention and therapy-old and new applications. Curr Pharmacol Rep. 2020;6(2):28–37. doi:10.1007/s40495-020-00211-y
59. Liu F, Sheng S, Shao D, et al. Targeting multiple mediators of sepsis using multifunctional tannic acid-Zn2+-gentamicin nanoparticles. Matter. 2021;4(11):3677–3695. doi:10.1016/j.matt.2021.09.001
60. Zhang J, Fu Y, Yang P, et al. ROS scavenging biopolymers for anti-inflammatory diseases: classification and formulation. Adv Mater Interfaces. 2020;7(16):2000632. doi:10.1002/admi.202000632
61. Wang M, Xu X, Sheng M, et al. Tannic acid protects against colitis by regulating the IL17 – NF-κB and microbiota - methylation pathways. Int J Biol Macromol. 2024;274:133334. doi:10.1016/j.ijbiomac.2024.133334
62. He H, Qin Q, Xu F, et al. Oral polyphenol-armored nanomedicine for targeted modulation of gut microbiota–brain interactions in colitis. Sci Adv. 2023;9(21):eadf3887. doi:10.1126/sciadv.adf3887
63. Pan J, Gong G, Wang Q, et al. A single-cell nanocoating of probiotics for enhanced amelioration of antibiotic-associated diarrhea. Nat Commun. 2022;13(1):2117. doi:10.1038/s41467-022-29672-z
64. Yang J, Zhang G, Yang X, et al. An oral “Super probiotics” with versatile self-assembly adventitia for enhanced intestinal colonization by autonomous regulating the pathological microenvironment. Chem Eng J. 2022;446:137204. doi:10.1016/j.cej.2022.137204
65. Yang X, Yang J, Ye Z, et al. Physiologically inspired mucin coated Escherichia coli nissle 1917 enhances biotherapy by regulating the pathological microenvironment to improve intestinal colonization. ACS Nano. 2022;16(3):4041–4058. doi:10.1021/acsnano.1c09681
66. Kamguyan K, Kjeldsen RB, Moghaddam SZ, et al. Bioadhesive tannic-acid-functionalized zein coating achieves engineered colonic delivery of IBD therapeutics via reservoir microdevices. Pharmaceutics. 2022;14(11):2536. doi:10.3390/pharmaceutics14112536
67. Li R, Fan Y, Liu L, et al. Ultrathin hafnium disulfide atomic crystals with ROS-scavenging and colon-targeting capabilities for inflammatory bowel disease treatment. ACS Nano. 2022;16(9):15026–15041. doi:10.1021/acsnano.2c06151
68. Le Z, Chen Y, Han H, et al. Hydrogen-bonded tannic acid-based anticancer nanoparticle for enhancement of oral chemotherapy. ACS Appl Mater Interfaces. 2018;10(49):42186–42197. doi:10.1021/acsami.8b18979
69. Fan H, Wang L, Feng X, et al. Supramolecular Hydrogel Formation Based on Tannic Acid. Macromolecules. 2017;50(2):666–676. doi:10.1021/acs.macromol.6b02106
70. Wang X, Yan J, Wang L, et al. Oral delivery of anti-TNF antibody shielded by natural polyphenol-mediated supramolecular assembly for inflammatory bowel disease therapy. Research Paper. Theranostics. 2020;10(23):10808–10822. doi:10.7150/thno.47601
71. Le Z, Liu Z, Sun L, et al. Augmenting therapeutic potential of polyphenols by hydrogen-bonding complexation for the treatment of acute lung inflammation. ACS Appl Bio Mater. 2020;3(8):5202–5212. doi:10.1021/acsabm.0c00616
72. Le Z, He Z, Liu H, et al. Orally administrable polyphenol-based nanoparticles achieve anti-inflammation and antitumor treatment of colon diseases. Biomater Sci. 2022;10(15):4156–4169. doi:10.1039/D2BM00540A
73. Zhang C, Zeng F, Fan Z, et al. An oral polyphenol host-guest nanoparticle for targeted therapy of inflammatory bowel disease. Acta Biomater. 2023;169:422–433. doi:10.1016/j.actbio.2023.08.020
74. Han L, Ji J, Zhang C, et al. One-step assembly of versatile multifunctional coatings based on host-guest and polyphenol chemistry. Small. 2023;19(22):2206943. doi:10.1002/smll.202206943
75. Dhand C, Harini S, Venkatesh M, et al. Multifunctional polyphenols- and catecholamines-based self-defensive films for health care applications. ACS Appl Mater Interfaces. 2016;8(2):1220–1232. doi:10.1021/acsami.5b09633
76. Shen W, Wang Q, Shen Y, et al. Green tea catechin dramatically promotes RNAi mediated by low-molecular-weight polymers. ACS Cent Sci. 2018;4(10):1326–1333. doi:10.1021/acscentsci.8b00363
77. Gan R-Y, Li H-B, Sui Z-Q, et al. Absorption, metabolism, anti-cancer effect and molecular targets of epigallocatechin gallate (EGCG): an updated review. Crit Rev Food Sci Nutr. 2018;58(6):924–941. doi:10.1080/10408398.2016.1231168
78. Chikara S, Nagaprashantha LD, Singhal J, et al. Oxidative stress and dietary phytochemicals: role in cancer chemoprevention and treatment. Cancer Lett. 2018;413:122–134. doi:10.1016/j.canlet.2017.11.002
79. Wu Z, Huang S, Li T, et al. Gut microbiota from green tea polyphenol-dosed mice improves intestinal epithelial homeostasis and ameliorates experimental colitis. Microbiome. 2021;9(1):184. doi:10.1186/s40168-021-01115-9
80. Hu B, Yu S, Shi C, et al. Amyloid–polyphenol hybrid nanofilaments mitigate colitis and regulate gut microbial dysbiosis. ACS Nano. 2020;14(3):2760–2776. doi:10.1021/acsnano.9b09125
81. Shen W, Wang R, Fan Q, et al. Natural polyphenol inspired polycatechols for efficient siRNA delivery. CCS Chem. 2020;2(3):146–157. doi:10.31635/ccschem.020.201900084
82. Ejima H, Richardson JJ, Caruso F. Metal-phenolic networks as a versatile platform to engineer nanomaterials and biointerfaces. Nano Today. 2017;12:136–148. doi:10.1016/j.nantod.2016.12.012
83. Mishra RK, Ahmad A, Kanika K, et al. Caffeic acid-conjugated budesonide-loaded nanomicelle attenuates inflammation in experimental colitis. Mol Pharm. 2023;20(1):172–182. doi:10.1021/acs.molpharmaceut.2c00558
84. Wang Q, Zhang R, Lu M, et al. Bioinspired polydopamine-coated hemoglobin as potential oxygen carrier with antioxidant properties. Biomacromolecules. 2017;18(4):1333–1341. doi:10.1021/acs.biomac.7b00077
85. Chung JE, Tan S, Gao SJ, et al. Self-assembled micellar nanocomplexes comprising green tea catechin derivatives and protein drugs for cancer therapy. Nat Nanotechnol. 2014;9(11):907–912. doi:10.1038/nnano.2014.208
86. Liu Y, Ai K, Lu L. Polydopamine and its derivative materials: synthesis and promising applications in energy, environmental, and biomedical fields. Chem Rev. 2014;114(9):5057–5115.
87. Zhou L, Du C, Zhang R, et al. Stimuli-responsive dual drugs-conjugated polydopamine nanoparticles for the combination photothermal-cocktail chemotherapy. Chin Chem Lett. 2021;32(1):561–564. doi:10.1016/j.cclet.2020.02.043
88. Song Y, Jiang H, Wang B, et al. Silver-incorporated mussel-inspired polydopamine coatings on mesoporous silica as an efficient nanocatalyst and antimicrobial agent. ACS Appl Mater Interfaces. 2018;10(2):1792–1801. doi:10.1021/acsami.7b18136
89. Friedrich M, Pohin M, Powrie F. Cytokine networks in the pathophysiology of inflammatory bowel disease. Immunity. 2019;50(4):992–1006. doi:10.1016/j.immuni.2019.03.017
90. Zhong Y, Li T, Zhu Y, et al. Targeting proinflammatory molecules using multifunctional mno nanoparticles to inhibit breast cancer recurrence and metastasis. ACS Nano. 2022;16(12):20430–20444. doi:10.1021/acsnano.2c06713
91. Okabe Y, Medzhitov R. Tissue biology perspective on macrophages. Nat Immunol. 2016;17(1):9–17.
92. Bai B, Gu C, Lu X, et al. Polydopamine functionalized mesoporous silica as ROS-sensitive drug delivery vehicles for periodontitis treatment by modulating macrophage polarization. Nano Res. 2021;14(12):4577–4583. doi:10.1007/s12274-021-3376-1
93. Liu X, Chen W, Shao B, et al. Mussel patterned with 4D biodegrading elastomer durably recruits regenerative macrophages to promote regeneration of craniofacial bone. Biomaterials. 2021;276:120998. doi:10.1016/j.biomaterials.2021.120998
94. Meng Z, Fu B, Yang Z, et al. Polydopamine-coated thalidomide nanocrystals promote DSS-induced murine colitis recovery through macrophage M2 polarization together with the synergistic anti-inflammatory and anti-angiogenic effects. Int J Pharm. 2023;630:122376. doi:10.1016/j.ijpharm.2022.122376
95. Bao X, Zhao J, Sun J, et al. Polydopamine nanoparticles as efficient scavengers for reactive oxygen species in periodontal disease. ACS Nano. 2018;12(9):8882–8892. doi:10.1021/acsnano.8b04022
96. Li J, Qiu H, Gong H, et al. Broad-spectrum reactive oxygen species scavenging and activated macrophage-targeting microparticles ameliorate inflammatory bowel disease. Biomacromolecules. 2021;22(7):3107–3118. doi:10.1021/acs.biomac.1c00551
97. Yan M, Zhu L, Wu S, et al. ROS responsive polydopamine nanoparticles to relieve oxidative stress and inflammation for ameliorating acute inflammatory bowel. Biomater Adv. 2022;142:213126. doi:10.1016/j.bioadv.2022.213126
98. Wang Z, Zhang C, Meng J, et al. A targeted exosome therapeutic confers both cfdna scavenging and macrophage polarization for ameliorating rheumatoid arthritis. Adv Mater. 2023;35(48):2302503. doi:10.1002/adma.202302503
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

