Back to Journals » Clinical Ophthalmology » Volume 18
Alleviation of Allergic Rhinoconjunctivitis Symptoms in Participants Treated with a 0.005% Tacrolimus Eye-Drop Solution
Authors Sladek S, Unger-Manhart N, Siegl C, Dellago H, Zieglmayer PU , Lemell P, Savli M, Zieglmayer R, Geitzenauer W, Längauer M , Prieschl-Grassauer E
Received 27 June 2024
Accepted for publication 27 August 2024
Published 5 October 2024 Volume 2024:18 Pages 2797—2811
DOI https://doi.org/10.2147/OPTH.S476163
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Svenja Sladek,1 Nicole Unger-Manhart,1 Cornelia Siegl,1 Hanna Dellago,1 Petra U Zieglmayer,2 Patrick Lemell,3 Markus Savli,4 René Zieglmayer,3 Wolfgang Geitzenauer,5 Matthias Längauer,5 Eva Prieschl-Grassauer1
1Marinomed Biotech AG, Korneuburg, Austria; 2Competence Center for Allergology and Immunology, Department of General Health Studies, Karl Landsteiner Private University for Health Sciences, Krems, Austria; 3Power Project GmbH, Vienna Challenge Chamber, Vienna, Austria; 4Biostatistik & Consulting GmbH, Zuerich, Switzerland; 5Krankenhaus der Barmherzigen Brüder, St. John Hospital, Department of Ophthalmology, Vienna, Austria
Correspondence: Eva Prieschl-Grassauer, Marinomed Biotech AG, Hovengasse 25, Korneuburg, 2100, Austria, Tel +43 226292300, Email [email protected]
Purpose: This randomized, placebo-controlled, crossover, double‐blind trial aimed to evaluate the efficacy and safety of Tacrosolv, a novel 0.005% tacrolimus eye-drop solution, in adults with grass pollen–induced allergic conjunctivitis.
Methods: A total of 64 adult participants were randomized to receive 2.5 μg or 5 μg tacrolimus/eye/day or placebo treatment for 8 days, with grass pollen exposure on day 1 and day 8. After a 2-week washout period, placebo participants crossed over to Tacrosolv treatment and vice versa, with repeated treatment and exposure. During exposure, participants recorded ocular, nasal, and respiratory allergy symptoms every 15 minutes. The primary endpoint was the mean total ocular symptom score (TOSS) on day 8. Objective ocular safety parameters were assessed before, during, and after exposure. Adverse events were recorded throughout the study.
Results: On day 8, high-dose Tacrosolv reduced the TOSS compared to placebo towards the end of exposure (p< 0.05 at time points 3 hours, 45 minutes and 4 hours). A 26% reduction in baseline adjusted TOSS from day 1 to day 8 was observed in participants treated with high-dose Tacrosolv, whereas placebo-treated participants showed no difference in TOSS between day 1 and day 8. Nasal symptoms were reduced on both day 1 and day 8 in participants treated with high-dose Tacrosolv (p< 0.05). No safety concerns were raised. All adverse events were resolved within the study period.
Conclusion: High-dose Tacrosolv is safe and effective for alleviating symptoms of allergic rhinoconjunctivitis.
Trial Registration: NCT04532710; EudraCT No. 2019‐002847‐62.
Keywords: allergic rhinoconjunctivitis, tacrolimus, ocular redness, ocular itching, topical administration, clinical trial
Introduction
Tacrolimus is a macrolide lactone that acts as an immunosuppressant by inhibiting T-lymphocyte signal transduction and mast-cell function1,2 suppressing cytokine and histamine release, and impairing prostaglandin synthesis.3,4 Tacrolimus is widely used to prevent organ rejection after transplantation and in chronic inflammatory conditions of the skin.5 Recently, clinical trials have been conducted applying tacrolimus in ocular diseases like corneal graft rejection, herpetic stromal keratitis, inflammatory conjunctival and corneal diseases, and uveitis.6,7 There is a long‐term established off‐label use of systemically applied tacrolimus for the treatment of noninfectious uveitis posterior.8–10 Topical application of tacrolimus has also been documented for the treatment of allergic eye diseases, such as refractory vernal keratoconjunctivitis or refractory atopic keratoconjunctivitis, and anterior-segment inflammatory disorders, such as anterior uveitis, graft-versus-host-disease, viral keratitis, or corneal transplantation, as summarized in a comprehensive review.11 Tacrolimus is currently available only as a suspension or emulsion, since the substance’s highly hydrophobic character and high molecular weight (804.02 g/mol) have so far precluded development of a tacrolimus solution. According to the literature, solubility of tacrolimus in water is 4–12 μg/mL.12 Other sources like the NIH’s PubChem database even classify tacrolimus as insoluble in water.5 The currently available dosage forms do not allow the substance to effectively penetrate the cornea or conjunctiva and reach effective therapeutic intraocular concentrations. This has so far hindered delivery of its full therapeutic potential for use in immunologic eye diseases. Currently, there is only one ophthalmic formulation of tacrolimus, marketed as Talymus in Japan and South Korea for the treatment of vernal keratoconjunctivitis (Senju Pharmaceutical, Osaka, Japan). Talymus is a suspension with a tacrolimus concentration of 0.1% (1 mg/mL).
Allergic conjunctivitis is one of the most common comorbidities of allergic diseases, especially of allergic rhinitis. Rhinoconjunctivitis is an allergic condition of the nasal mucosa and the eyes. Allergic conjunctivitis is triggered by hypersensitivity to certain pollens and other airborne allergens, and causes several symptoms, such as red eyes, itchy eyes, watery eyes, and a scratchy feeling in the eye. It is clinically defined as a symptomatic disorder induced by IgE‐mediated inflammation after exposure of the conjunctiva to an allergen. Allergen‐bound IgE on the surface of mast cells induces mast-cell degranulation and release of allergic and inflammatory mediators, such as histamines, leukotrienes, prostaglandin D2, tryptase, and kinins, and proinflammatory cytokines, such as TNFα.13
Environmental challenge chambers (ECC) allow for the exposure of allergy-prone study participants to a physiological allergen challenge akin to real-life allergen exposure, in contrast to intranasal and intraocular challenge by direct application of challenge solutions. The Vienna Challenge Chamber (VCC) has offered the opportunity to obtain assessments of allergic responses within a few hours with high reproducibility, resulting in a rather small number of participants necessary to obtain significant results.
Tacrolimus eye drops (Tacrosolv) contain 50 µg/mL (0.005%) tacrolimus dissolved in our proprietary Marinosolv formulation. Our formulation increases solubility by five- to tenfold compared to the published solubility in water of 4–12 μg/mL.12 We have previously demonstrated the bioavailability and permeability of solubilized tacrolimus when applying Tacrosolv topically in an ex vivo and in vivo animal model.14 For the intended clinical purpose, a concentration of 50 µg/mL (0.005%, with a maximum recommended dose of maximal two drops per eye [maximal daily dose of 5 µg tacrolimus per eye]) was chosen based on ex vivo experiments with porcine eyes, where a similar volume (50 µL) of Tacrosolv (5 µg tacrolimus/eye) and of Talymus (50 µg tacrolimus/eye) resulted in similar conjunctival drug concentrations (data not shown).
The goal of the study presented here was to establish proof of concept for the efficacy and safety of Tacrosolv for the treatment of inflammation-driven ophthalmic diseases using an allergen-exposure challenge as a simple, quick, and controllable model system. This is the first clinical study to use the proprietary Tacrosolv formulation.
Methods
Study Design
This was a randomized, placebo-controlled, crossover, double-blind, single-site trial in adult participants (18–65 years of age) with documented grass-specific IgE reactivity and a history of grass pollen–induced rhinoconjunctivitis with or without controlled asthma. Two dose groups, low dose (2.5 µg tacrolimus/eye/day) and high dose (5 µg tacrolimus/eye/day), were evaluated during two treatment periods of 8 days each. The crossover design ensured that individual participants received either the low dose or the high dose of Tacrosolv in one treatment period and placebo in the other treatment period.
At screening (visit 1), medical and allergic history and inclusion and exclusion criteria were assessed, and all safety assessments were conducted. At least 1 week prior to the first treatment period (visit 2), participants were screened for appropriate allergic response during a grass pollen VCC session.
At visit 3 (day 1 of treatment period [TMP] 1), eligible participants were randomly assigned to one of the four treatment arms in a fully blinded fashion. See graphical abstract in Figure 1 for treatment arms. After completion of all study-relevant assessments, baseline values for symptom scores were assessed and participants were administered their first treatment 30 minutes before entering the VCC. Total ocular symptom score (TOSS), total nasal symptom score (TNSS), total respiratory symptom score (TRSS), nasal airflow (active anterior rhinomanometry [AAR]), and lung function were assessed at defined time points during exposure, as shown in Figure 1B. Objective ocular assessments were performed before, 2 hours after start, and after the end of the provocation session. After the allergen exposure, participants received Tacrosolv for the home treatment phase (days 2 to 7), with administration continued until day 7.
 |
Figure 1 Graphical abstract of the clinical study. (A) Study overview. (B) Assessments carried out on day 1 and day 8 of each treatment period. |
At visit 4 (day 8 of TMP1), participants returned the study medication kit to the study-site staff for compliance evaluation. Baseline symptom scores were assessed, participants received the last dose of their assigned treatment, and 30 minutes after the final treatment, they entered the VCC for another 4-hour allergen exposure. Again, subjective and objective symptom assessments were carried out as already described. After completion of TMP1, a washout period of at least 13 days had to be adhered to, allowing complete dissipation of the previous treatment. Subsequently, participants crossed over to their next treatment period. Visits 5 and 6 were conducted in an analogous manner to visits 3 and 4. A follow-up visit (visit 7, end-of-trial visit) was scheduled for 1–2 weeks after the final allergen exposure session (visit 6).
Participants were asked to record adverse events (AEs) and use of concomitant medications on a form (provided) during the entire study.
Participants
Participants were female and male adults aged 18–65 years of any ethnicity with a documented history of clinically relevant moderate-to-severe seasonal allergic rhinitis with rhinoconjunctivitis for the previous 2 years. Participants were selected from the VCC database and had to satisfy study inclusion and exclusion criteria in order to be enrolled in the study.
The key inclusion criterion was a moderate-to-severe response to approximately 1800 grains/m3 of standard grass pollen in the VCC, defined as a TOSS of at least 4 (of a maximum 12) within the first 2 hours in the VCC, with at least one single ocular symptom scoring ≥2 (moderate) at least twice during the first 2 hours. In addition, participants had to fulfill inclusion criteria of a positive skin-prick test (SPT) response (wheal diameter at least 3 mm larger than diluent control) to a grass pollen skin-prick test solution (standard Allergopharma), positive serum-specific IgE against recombinant major allergen components of the grass pollen, e.g. g6 (specific CAP IgE ≥0.70 kU/L), and a forced expiratory volume in 1 second (FEV1) of at least 80% of predicted value. Key exclusion criteria were uncontrolled or moderate to severe asthma, pregnancy or lactation, smoking, use of contact lenses, previous successful or ongoing treatment with any allergen-specific immunotherapy, and symptoms of or treatment for any clinically relevant chronic, systemic or ocular disease affecting the immune response. Female participants of childbearing potential were required to use birth control.
Randomization and Blinding
In total, 64 eligible participants were planned to be enrolled into the study. Randomization numbers were allocated to the study participants in ascending order of their screening numbers at visit 3 (TMP1). Treatment allocation was based on crossover randomization with balanced blocks. All personnel involved in the study, including investigators, site personnel, and sponsor staff, were blinded to the medication codes. Unblinding at study end was done after database lock.
Interventions and Procedures
Tacrosolv is an aqueous solution of 50 µg/mL tacrolimus monohydrate (0.005%). All other components of Tacrosolv except for tacrolimus are classified as excipients and suitable for both ocular and nasal applications, since they have either already been used as excipients in ophthalmic market products and/or been assigned “generally recognised as safe” status.14 In this study, sterile buffered saline solution with propylene glycol was used as placebo.
Participants received their first treatment (high- or low-dose Tacrosolv or placebo) approximately 30 minutes before start of the allergen provocation session on day 1 of each TMP. Participants received study medication for the home-treatment phase (days 2–7 of both TMP1 and TMP2) and continued treatment at home into each conjunctival sac once a day in the morning until day 7. On day 8 of each TMP, participants applied Tacrosolv or placebo approximately 30 minutes before the start of the allergen provocation session.
At the inclusion visit (visit 2) and on day 1 and day 8 of both treatment periods, participants were exposed to a standard grass pollen allergen mixture (1800 grass pollen grains/m3) in the VCC for 4 hours using a validated method.15 The challenge agent was a qualitatively and quantitatively defined mixture of four grass pollen species (timothy, orchard, perennial rye, and sweet vernal grass; Allergon SB, Sweden). Air temperature (24°C), humidity (40%), and allergen load were constantly monitored and maintained. During the 4-hour exposure, subjective ocular, nasal, and respiratory symptoms (TOSS, TNSS, and TRSS) were recorded every 15 minutes. The TOSS is the sum of “ocular redness”, “ocular itching”, “watery eyes”, and “gritty feeling”. The TNSS is the sum of “nasal congestion”, “rhinorrhea”, “itchy nose”, and “sneezing”. TRSS is the sum of “cough”, “wheeze”, and “dyspnea”. Each individual symptom was scored on a 4-point categorical scale from 0 to 3, with 0 being complete absence of symptom, 1 mild, 2 moderate, and 3 severe.
Lung function was assessed using a Piston spirometer for FEV1 and forced vital capacity before, every 60 minutes during, and at the end of the 4-hour allergen exposure. Nasal airflow was measured by AAR at a pressure difference of 150 Pa across the nasal passages (sum of the right and left nostril values) at baseline (45 minutes before exposure start), every 60 minutes during and at the end of the exposure.
Objective ocular assessments carried out before, 2 hours after start, and after the end of the allergen challenge session included tear-film breakup time (TBUT) measurement, staining of the conjunctiva with lissamine green and of the cornea with fluorescein to evaluate epithelial and corneal damage, evaluation of conjunctival chemosis, lid-parallel conjunctival folds (LIPCOFs), conjunctival redness, eyelid edema, and conjunctival papillae with slit-lamp biomicroscopy, and assessment of intraocular pressure with a tonometer.
Female participants of childbearing potential in addition had a urine pregnancy test done at screening and on day 1 of each treatment period.
Endpoints
The primary efficacy endpoint was the mean TOSS on day 8, calculated as the mean TOSS measured every 15 minutes during the pollen allergen exposure.
The key secondary endpoint was the onset of action of either dose of Tacrosolv during the first allergen exposure, defined as first time point when the TOSS difference between active treatment and placebo was p<0.05. Additional secondary efficacy endpoints were changes in ocular redness image score assessed by the investigator, TNSS, TRSS, and nasal airflow assessed by AAR.
Safety endpoints were frequency, severity, seriousness, and causality of AEs, lung function (FEV1), vital signs, and findings of ocular examinations at screening (V1) and throughout the study (V2–V7), as well as findings of physical examinations, laboratory blood analysis, and electrocardiograms at screening (V1) and at the follow-up visit (V7).
Objective ophthalmic assessments (eyelid edema, noninvasive first (NIF) TBUT, chemosis, conjunctival papillae, LIPCOFs) served as readouts for both efficacy (comparison placebo vs. Tacrosolv treatment) and safety (comparison screening vs. follow-up).
Sample-Size Calculation
Sample-size calculation was based on the minimum clinically relevant TOSS difference, which was estimated at about 1 point based on a previous study on solubilized budesonide.16 Expecting a mean difference of 1.2 points with a standard deviation of 2.2 (untreated 8, test 6.8, effect size d=0.55, and power 80%) for each dose group, a total of n=54 participants were needed at α=0.05. Considering the dropout rate of 10%–15% and 30%–40% screening failures, up to 107 participants needed to be screened to randomize about 64 participants and to obtain evaluable data from at least 54 participants at the end of the trial.
Statistical Analysis
The final analysis, including unblinding, was performed on data that had been documented as meeting the cleaning and approval requirements defined in the SAP and after the finalization and approval of the SAP document.
The following three analysis populations were defined for this study.
- Full analysis set (FAS), comprising all participants to whom the study drug was assigned by randomization, analyzed following the intent‐to‐treat principle, i.e. according to the treatment assigned at randomization.
- Per-protocol set (PPS), comprising all participants in the FAS who did not have any clinically important protocol deviations.
- Safety set, comprising all participants who received the investigational product: used for all safety analyses, including vital signs, laboratory data, and AEs.
All attempts were made to collect all data as per protocol. Missing or invalid data were not replaced or extrapolated. Outliers were not excluded from the primary analysis.
For the primary efficacy analysis, a 95% CI was calculated for the mean difference between the active treatment and placebo from a two-sided paired t-test. Superiority of Tacrosolv versus placebo was to be assumed if the lower limit of the CI did not fall below 0. The FAS was the primary analysis population for the primary efficacy variable.
Secondary efficacy variables were analyzed in an explorative sense. Statistical tests and corresponding p-values were regarded as descriptive and not as tests of hypotheses.
The analysis of baseline and demographic characteristics was subject to descriptive analyses. Safety endpoints were analyzed in the safety set. AEs were summarized descriptively.
Phase effects were tested using Wilcoxon tests for both placebo and Tacrosolv. Carryover effects were tested using ANOVA. Normal distribution was checked using the Shapiro–Wilk test. If normal distribution was assumed, the paired t-test was used for the group comparison. Otherwise, the paired Wilcoxon test was used. CIs are based on t-distributions. Significance was set to α=5%. R version 4.0.3 was used for all statistical analyses.
Results
Participant Disposition and Baseline Characteristics
Figure 1 shows the graphical abstract of the clinical study, outlining the timeline of visits and treatments and the assessments carried out on day 1 and day 8. The study was conducted between December 2020 and April 2021. A total of 93 participants with grass pollen allergy were screened after giving informed consent. Of these, 64 participants complied with all inclusion and exclusion criteria and were randomized to one of four treatment groups, thus constituting the safety set and the FAS. One participant in the high-dose group was lost to follow-up after day 1, one participant in the low dose group missed both visits of treatment period 2 due to an AE not related to the study treatment, and one participant was classified as a nonresponder after not developing any significant ocular symptoms during the first 2 hours of the allergy exposure on day 1. Hence, 61 subjects completed the study as per protocol and comprised the PPS. participantFigure 2 provides a CONSORT flowchart detailing participant allocation and analysis sets.
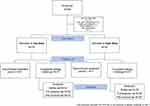 |
Figure 2 CONSORT flowchart. |
Demographic characteristics are summarized in Table 1. Overall, 59% of the participants were female and 41% were male. Participants were aged between 19 and 57 years, with a mean of 32.4 years. The mean BMI was 23.6 kg/m2. All participants had a documented history of moderate-to-severe seasonal allergic rhinitis with rhinoconjunctivitis to grass pollen with a prior duration of between 3 and 43 years (average 20.5 years).
 |
Table 1 Demographic characteristics (safety set) |
Efficacy
All efficacy results are shown for the FAS. Results for the PPS were similar to those for the FAS.
The primary efficacy endpoint was the mean TOSS, calculated as the mean of all TOSS assessments carried out at 15 minutes intervals during the 4-hour grass pollen allergen exposure on day 8. As shown in Figure 3, there was no statistically significant difference in mean TOSS between the active treatment group and the placebo group for either high-dose or low-dose Tacrosolv on day 8. With a mean difference of placebo vs. Tacrosolv of 0.31 (95% CI −0.32 to 0.94, p=0.328; paired t-test) in the high-dose group and −0.24 (95% CI −1.04 to 0.56, p=0.54; paired t-test) in the low-dose group, superiority of Tacrosolv over placebo in terms of TOSS on day 8 could not be stated for either dose group.
On day 1, the mean TOSS difference between active treatment and placebo was similar for the low-dose and high-dose groups. However, in the high-dose group, the mean TOSS difference between Tacrosolv and placebo rose by 1.25 symptom points from −0.94 on day 1 to 0.31 on day 8. Even though the difference between active treatment and placebo was not statistically significant on either day, it showed a trend towards improvement over time.
Since the low dose of Tacrosolv failed to show any beneficial effect in any of the measured parameters, in the following, we focus on the results for high-dose Tacrosolv treatment.
The key secondary endpoint was the onset of action of Tacrosolv during the first allergen exposure on day 1. As shown in Figure 4, the mean TOSS was higher in the high-dose Tacrosolv group than in the placebo group at all time points of day 1, reaching a peak value of 6 at 3 hours, 30 min and plateauing around 6 for the remaining time. Hence, no onset of action could be determined on day 1. However, on day 8, the mean TOSS in the high-dose Tacrosolv group had already reached a plateau of only 4 at 1 hour, 45 minutes and showed no further increase and only a small range of fluctuation for the remaining duration of the allergen exposure, with the between-group difference in mean TOSS becoming significant (p≤0.05) at time points 3 hours, 45 minutes and 4 hours (Figure 4, right panel). The mean differences at these time points exceeded the minimum clinically relevant TOSS difference that was defined as 1 point before study start. The time course of TOSS in the placebo group was the same on day 1 and day 8, indicating a high reproducibility of subjective ocular symptoms and no effect of the 8 days’ placebo treatment.
When expressing day 8 mean TOSS as a percentage of day 1 mean TOSS, it became obvious that only high-dose Tacrosolv treatment led to a significantly reduced TOSS on day 8 compared to day 1 (Figure 5). In contrast, there was no difference in mean TOSS between day 1 and day 8 in the placebo group (Figure 5).
Time courses of individual TOSS symptoms (itching, redness, watery eyes, gritty feeling) on day 8 were analyzed post hoc. As shown in Figure 6, treatment with Tacrosolv impacted the three main ocular symptoms associated with allergic conjunctivitis: itchy eyes, redness, and watery eyes. The difference in redness and watery eyes in favor of Tacrosolv became statistically significant towards the end of the allergen exposure. “Gritty feeling” did not contribute to the effect of Tacrosolv on cumulative TOSS.
 |
Figure 6 Time course of mean individual baseline-adjusted TOSS symptoms on day 8 for FAS, high-dose group. n=31 for both groups. Error bars indicate SEM. *p≤0.05. |
Interestingly, Tacrosolv treatment benefited not only ocular but also nasal symptoms of allergic rhinoconjunctivitis. Figure 7 shows differences in mean TNSS between high-dose Tacrosolv and placebo on day 1 and day 8. On both days, the difference was in favor of Tacrosolv, with p=0.061 on day 1 and p=0.034 on day 8.
Time courses of mean TNSS over the 4-hour allergen exposure on day 1 and day 8 show that the difference in mean TNSS between high-dose Tacrosolv and placebo became statistically significant at later time points, with 2 hours, 30 minutes and 1 hour, 45 minutes being the earliest time points with significant TNSS difference between treatment groups on day 1 and day 8, respectively.
No marked differences between Tacrosolv and placebo were observed for ocular redness image score, TRSS, or nasal airflow on day 1 and day 8. Objective ophthalmic assessments did not reveal any clinically significant findings (data not shown). No phase effect was found for placebo (p>0.05) or Tacrosolv (p>0.05). No carryover effect was observed either (p>0.05).
Safety and Tolerability
AEs reported throughout the study are summarized for the safety set in Table 2. No serious AE, no life-threatening (grade IV) AE, and no death occurred throughout the study. A total of 57 (89%) participants reported any AE, of which 29 were in the high-dose group and 28 in the low-dose group.
In sum, 20 (31%) participants reported at least one AE during the placebo treatment, 55 (86%) during the active treatment phase with Tacrosolv, and 18 (28%) in both study phases. Severe (grade III) AEs occurred in 12 (19%) participants during the study (seven were in the high-dose group and five in the low-dose group), and six participants required medication in the form of artificial tears for treatment of their severe AE(s). Overall, 11 (17%) participants reported severe AEs during the active treatment phase and one during the placebo phase.
 |
Table 2 Overview of adverse events recorded during the study (safety set) |
A total of 174 AEs were reported during the entire study, 30 of which (17%) occurred during the placebo phase and 144 (83%) during the active treatment phase, and 158 (91%) were eye disorders. Supplementary Table S1 shows AEs by system organ class and preferred term. Overall, 141 AEs were classified by the investigator as probably or possibly treatment-related, a majority of which (129, 91%) occurred during the active treatment phase.
All AEs had been resolved by study end.
Lung-function measurements, blood pressure, electrocardiograms, and laboratory blood analyses all showed a stable course during the study. No substantial deviations or clinically significant abnormalities were reported. No clinically relevant changes from baseline or relevant differences between treatment groups were observed for any of the analyzed safety parameters.
For ophthalmic parameters (staining of the conjunctiva and cornea, slit-lamp microscopy [eyelid edema], fundoscopy, intraocular pressure, nasal and temporal chemosis, conjunctival papillae, temporal LIPCOFs, NIF TBUT), similar results were observed in the treatment and placebo groups, and minor abnormalities were resolved at follow-up.
Discussion
In this proof-of-concept phase II study, we have demonstrated that ophthalmic administration of Tacrosolv, an aqueous solution of 0.005% tacrolimus, applied at a dose of 5 µg/eye/day (“high dose”) over a course of 8 days significantly alleviated ocular and nasal symptoms of pollen allergy in adults with a history of allergic rhinoconjunctivitis.
The high-dose Tacrosolv failed to reduce the TOSS on day 1, presumably based on a short-term adverse reaction of a stinging or burning sensation that is well known for tacrolimus17–19 and that obscures the beneficial, immunosuppressive effect at the start of treatment. Such instillation-site discomfort is also frequently reported for other immunomodulatory ocular medications like cyclosporine or lifitegrast.20
In contrast to ocular symptoms, nasal allergy symptoms (like nasal congestion, rhinorrhea, itching, and sneezing) were alleviated immediately after the first dose of Tacrosolv. This substantiates the hypothesis that the observed lack of efficacy of Tacrosolv on ocular symptoms on day 1 may most probably have been due to an initial, transient adverse effect of tacrolimus that was limited to the site of administration. This transient adverse reaction is known and commonly reported for topical ophthalmic tacrolimus application.17–19,21
It is known that nasal allergen exposure can lead to ocular symptoms,22,23 and that treatment of nasal allergy symptoms, e.g. using intranasal corticosteroids, can diminish ocular symptoms.24–26 We have previously demonstrated this effect for our Budesolv nasal spray, an aqueous formulation containing solubilized budesonide.16
This nasal–ocular relationship is thought to be mediated via a direct and an indirect route.27 The direct relationship occurs via the nasolacrimal duct that connects the lacrimal sac of the eye with the inferior meatus of the nasal cavity. Along this duct, allergens and allergy mediators drain along with tears into the nasal cavity. As the flow of secretion is from the eye to the nose, it is plausible that ocular drugs travel through the nasolacrimal duct and take effect in the nasal cavity. In addition, an indirect mutual connection between nasal and ocular allergic symptoms may occur through a lymphatic and/or neurogenic pathway.28,29 Although the nasal–ocular relationship has mostly been studied in the direction of nose to eye, there is also evidence for antiallergic treatment of the eye having an effect on the nose.30 Our study confirms the bidirectionality of the nasal–ocular relationship.
The lack of any differences in ophthalmic parameters (staining of the conjunctiva and cornea, slit-lamp microscopy [eyelid edema], fundoscopy, intraocular pressure, nasal and temporal chemosis, conjunctival papillae, temporal LIPCOFs, NIF TBUT) between screening and follow-up showed that both treatment with low- and high-dose Tacrosolv and exposure to the allergen in the VCC are safe and do not induce any lasting damage, as assessed by the applied methods.
Our data indicate an early onset of effect on nasal allergy symptoms and an attenuation of ocular allergy symptoms after 1 week of treatment. The beneficial effect of Tacrosolv became more pronounced the longer the duration of allergen exposure. We speculate that the effect may further increase with an extended treatment period of more than 1 week and/or when extending the observation period to more than 4 hours. In addition, it may be more meaningful to assess efficacy after reaching the symptom plateau, which occurs approximately 2 hours after start of exposure.
Others have defined the meaningful within-patient change and meaningful between-group difference for patient-reported ocular itching and redness to be approximately 0.5.31 It should be noted that in that study, ocular itching, redness, and tearing were scored on a 0–4 scale, in contrast to the 0–3 scale used in our study. Others have defined a threshold of 0.23 units as a minimum clinically important difference for the TNSS, i.e. the scoring system we have applied in our study.32 With a mean TNSS difference between Tacrosolv and placebo of 0.83 units (p=0.061) on day 1 and 0.76 units (p=0.034) on day 8, we see a clear trend towards a clinically important difference.
Hence, our approach defining 1 point as a relevant difference can be considered ambitious, and we conclude that the observed difference in TNSS overall and in TOSS towards the end of exposure on day 8 can be considered clinically meaningful.
The observed effect of Tacrosolv is remarkable, since the total dose of tacrolimus applied in this study was only 10 µg per day (i.e. 5 µg per eye per day) for the high dose. Talymus, a tacrolimus ocular suspension (Senju Pharmaceutical, Osaka, Japan) marketed in Japan and South Korea for treatment of vernal conjunctivitis, contains tacrolimus at a concentration of 0.1%. With a recommended dosing of two drops per eye per day and assuming a drop size of 50 µL, Talymus is used at a maximum daily dose of 100 µg per eye per day, which corresponds to 20-fold the high dose applied in our study. This means that with only 5% of the dose used for Talymus, we had achieved a significant, beneficial effect by the end of the 8-day treatment period. Talymus is formulated as a suspension of finely dispersed tacrolimus particles. For molecules with low solubility and high permeability, like tacrolimus, bioavailability is greatly influenced by the rate of particle dissolution and the concentration of molecules in the solution while in contact with the ocular tissue. In contrast, Tacrosolv enables the solubilization of tacrolimus, thus enhancing bioavailability and strongly reducing the administered effective dose. At the same time, no systematic immunosuppressive effects are to be expected when Tacrosolv is applied topically: a previous study in a porcine model showed that when Tacrosolv was applied as ophthalmic drops at concentrations of 50–140 µg/day/eye (compared to 5 µg/eye/day in the clinical study), no relevant systemic exposure to the drug could be detected.14
The safety and efficacy of tacrolimus has previously been proven for the treatment of severe vernal keratoconjunctivitis in children who were treated with 0.1% tacrolimus twice daily for up to 18 months.33 Moreover, a long-term study following patients with severe atopic keratoconjunctivitis and vernal keratoconjunctivitis who were treated with Talymus (0.1% tacrolimus suspension) for up to 10 years demonstrated safety and efficacy in long-term users.34
In general, all clinical studies with tacrolimus in allergic eye disease were using either a suspension or an ointment (reviewed in11). Hence, to the best of our knowledge, we present the first clinical trial using solubilized tacrolimus in an ophthalmic formulation.
In Europe and the US, where Talymus is not available, immunomodulating therapy of severe inflammatory ocular disease is limited to cyclosporine eye drops, while tacrolimus can currently only be used off-label for ophthalmic indications.35 Comparisons between cyclosporine and tacrolimus in terms of safety and efficacy have mainly been made in the context of immunosuppression after organ and tissue transplantation, where tacrolimus has been found to offer similar efficacy to cyclosporine at 20- to 50-fold-reduced concentrations.36 Studies comparing cyclosporine and tacrolimus eye ointment for the treatment of refractory vernal keratoconjunctivitis have found that tacrolimus demonstrated similar or superior efficacy in reduction of inflammatory symptoms as well as patient compliance,37,38 and propose tacrolimus as a safe alternative for treatment of cyclosporine-refractory vernal keratoconjunctivitis.39
An additional benefit of tacrolimus is that it helps in reducing corticosteroid use,40 which to this day is commonly used to treat inflammatory eye disease, despite its potential for severe side effects. Tacrolimus treatment efficacy in allergic ocular diseases enabled complete weaning in 50% of patients previously using topical steroids.34,41
Severe systemic adverse reactions like hyperglycemia, nephrotoxicity, neurotoxicity, weight loss, liver damage, and diarrhea, which have been reported after systemic use of tacrolimus after bone marrow transplantations,42,43 are unlikely to occur upon topical use of Tacrosolv and other tacrolimus-containing eye drops, because the percentage of tacrolimus reaching the bloodstream with twice-daily topical use is very low.44,45
In contrast to allergic rhinoconjunctivitis, which is caused by activation of mast cells, the most severe forms of allergic ocular disease, such as vernal and atopic keratoconjunctivitis, involve predominantly T lymphocytes.46 Tacrolimus acts on T cells by disrupting calcium-dependent signaling events and subsequently inhibiting T-cell activation, differentiation, and cytokine production,46,47 and it is thought that tacrolimus inhibits T cells even more effectively than mast cells (3,48–50 and our own unpublished data). Therefore, Tacrosolv may be even more effective in T cell–mediated ocular diseases than in allergic rhinoconjunctivitis.
One major upside of our study is the setting for eliciting allergy symptoms. Many studies on antiallergic eye drops apply the conjunctival allergen challenge, also called the conjunctival allergen provocation test or conjunctival provocation test.51,52 In that approach, the allergen is applied directly to the conjunctival mucosa to trigger an allergic response. It is used in clinical practice to determine which allergens trigger specific symptoms and in clinical research to investigate treatments. In contrast to the conjunctival allergen challenge, where the initially applied allergen concentration in the eye is quickly diluted and cleared from the mucosa by lacrimation and blinking, use of an ECC enables continuous exposure to the air-dispersed allergen over several hours. In fact, several validation studies have demonstrated the reproducibility and specificity of symptoms induced by ECCs53,54 and shown good correlations between ocular symptoms elicited by ECCs and those assessed during natural exposure.55–57 Hence, the use of the VCC comes very close to allergen exposure in the real world, but with the added benefit of consistent exposure conditions across participants.
In sum, our data demonstrate a beneficial effect on nasal and ocular symptoms of allergic conjunctivitis after 8 days of daily treatment with Tacrosolv 0.005% tacrolimus solution. Hence, our results confirm the therapeutic capacity of tacrolimus for the treatment of allergic eye diseases,46 and highlight the potential of Tacrosolv as a safe and effective treatment option for allergic or inflammatory eye diseases.
Conclusion
Anti-inflammatory activity of solubilized tacrolimus was observed in participants suffering from rhinoconjunctivitis at doses as low as 5 µg tacrolimus per eye per day.
No major safety concerns were raised during the study. AEs were comparable to marketed products containing calcineurin inhibitors.
Ethics Approval and Informed Consent
The study was conducted in Austria in accordance with the Declaration of Helsinki on Ethical Principles for Medical Research Involving Human Subjects, the International Council for Harmonisation Guideline on Good Clinical Practice, and all applicable local regulatory requirements and laws. The study was approved by the Ethics Committee of the City of Vienna (protocol code TCS_19_02, EK 19-275-1219). Informed consent was obtained from all study participants.
Acknowledgments
The Tacrosolv eye drops were developed by Marinomed Biotech AG. This paper has been uploaded to Medrxiv as a preprint: https://www.medrxiv.org/content/10.1101/2024.04.30.24306626v1.
Funding
This research was funded by Marinomed Biotech AG. The sponsor was involved in the study design, study oversight, manuscript writing, and submission for publication, and assumes full sponsor responsibilities as per ICH GCP.
Disclosure
NUM, SS, CS, HD, and EPG are employees of Marinomed Biotech AG. EPG reports grants from FFG outside the submitted work. In addition, CS and EPG report a patent (PCT/EP2016/066999) issued. MS has received consulting fees from Marinomed Biotech AG. WG and ML have received honoraria from Marinomed Biotech AG. The other authors have no competing interests in this work.
References
1. Lee H, Myoung H, Kim SM. Review of two immunosuppressants: Tacrolimus and cyclosporine. J Korean Assoc Oral Maxillofac Surg. 2023;49(6):311–323. doi:10.5125/jkaoms.2023.49.6.311
2. Burchett JR, Dailey JM, Kee SA, et al. Targeting mast cells in allergic disease: Current therapies and drug repurposing. Cells. 2022;11(19):3031. doi:10.3390/cells11193031
3. de PA, Stellato C, Cirillo R, Ciccarelli A, Oriente A, Marone G. Anti-inflammatory effect of FK-506 on human skin mast cells. J Invest Dermatol. 1992;99(6):723–728. doi:10.1111/1523-1747.ep12614216
4. Ruzicka T, Assmann T, Homey B. Tacrolimus: The drug for the turn of the millennium? Arch Dermatol. 1999;135(5):574–580. doi:10.1001/archderm.135.5.574
5. PubChem. PubChem compound summary for CID 445643, tacrolimus; 2024. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/445643.
6. Zhai J, Gu J, Yuan J, Chen J. Tacrolimus in the treatment of ocular diseases. BioDrugs. 2011;25(2):89–103. doi:10.2165/11587010-000000000-00000
7. Akbari M, Soltani Moghadam R, Elmi R, Nosrati A, Taghiabadi E, Aghdami N. Topical tacrolimus as an adjunct to conventional therapy for stromal herpetic keratitis: A randomized clinical trial. J Ophthalmic Vis Res. 2019;14(4):400–411. doi:10.18502/jovr.v14i4.5437
8. Luis J, Alsaedi A, Phatak S, Kapoor B, Rees A, Westcott M. Efficacy of tacrolimus in uveitis, and the usefulness of serum tacrolimus levels in predicting disease control. results from a single large center. Ocul Immunol Inflamm. 2022;30(7–8):1654–1658. doi:10.1080/09273948.2021.1930063
9. Pleyer U, Neri P, Deuter C. New pharmacotherapy options for noninfectious posterior uveitis. Int Ophthalmol. 2021;41(6):2265–2281. doi:10.1007/s10792-021-01763-8
10. Ishioka M, Ohno S, Nakamura S, et al. FK506 treatment of noninfectious uveitis. Am J Ophthalmol. 1994;118(6):723–729. doi:10.1016/s0002-9394(14)72551-6
11. Shoughy SS. Topical tacrolimus in anterior segment inflammatory disorders. Eye Vis. 2017;4:7. doi:10.1186/s40662-017-0072-z
12. Patel P, Patel H, Panchal S, Mehta T. Formulation strategies for drug delivery of tacrolimus: an overview. Int J Pharm Investig. 2012;2(4):169–175. doi:10.4103/2230-973X.106981
13. Bjermer L, Westman M, Holmström M, Wickman MC. The complex pathophysiology of allergic rhinitis: scientific rationale for the development of an alternative treatment option. Allergy Asthma Clin Immunol. 2019;15:24. doi:10.1186/s13223-018-0314-1
14. Siegl C, König-Schuster M, Nakowitsch S, et al. Pharmacokinetics of topically applied tacrolimus dissolved in Marinosolv, a novel aqueous eye drop formulation. Eur J Pharm Biopharm. 2019;134:88–95. doi:10.1016/j.ejpb.2018.11.015
15. Day JH, Briscoe MP, Rafeiro E, Ellis AK, Pettersson E, Akerlund A. Onset of action of intranasal budesonide (Rhinocort aqua) in seasonal allergic rhinitis studied in a controlled exposure model. J Allergy Clin Immunol. 2000;105(3):489–494. doi:10.1067/mai.2000.104550
16. Zieglmayer P, Schmutz R, Lemell P, et al. Fast effectiveness of a solubilized low-dose budesonide nasal spray in allergic rhinitis. Clin Exp Allergy. 2020;50(9):1065–1077. doi:10.1111/cea.13691
17. Müller EG, Santos MSD, Freitas D, Gomes JÁP, Belfort R. Tacrolimus eye drops as monotherapy for vernal keratoconjunctivitis: A randomized controlled trial. Arq Bras Oftalmol. 2017;80(3):154–158. doi:10.5935/0004-2749.20170038
18. Eltagoury M, Abou Samra W, Ghoneim E. Safety and efficacy of topical tacrolimus 0.03% in the management of vernal keratoconjunctivitis: A non-randomized controlled clinical trial. Med Hypothesis Discov Innov Ophthalmol. 2022;11(2):52–63. doi:10.51329/mehdiophthal1446
19. Hazarika AK, Singh PK. Efficacy of topical application of 0.03% tacrolimus eye ointment in the management of allergic conjunctivitis. J Nat Sci BiolMed. 2015;6(Suppl 1):S10–2. doi:10.4103/0976-9668.166051
20. White DE, Zhao Y, Jayapalan H, Machiraju P, Periyasamy R, Ogundele A. Treatment satisfaction among patients using anti-inflammatory topical medications for dry eye disease. Clin Ophthalmol. 2020;14:875–883. doi:10.2147/OPTH.S233194
21. Ohashi Y, Ebihara N, Fujishima H, et al. A randomized, placebo-controlled clinical trial of tacrolimus ophthalmic suspension 0.1% in severe allergic conjunctivitis. J Ocul Pharmacol Ther. 2010;26(2):165–174. doi:10.1089/jop.2009.0087
22. Lebel B, Bousquet J, Morel A, Chanal I, Godard P, Michel FB. Correlation between symptoms and the threshold for release of mediators in nasal secretions during nasal challenge with grass-pollen grains. J Allergy Clin Immunol. 1988;82(5Pt 1):869–877. doi:10.1016/0091-6749(88)90092-9
23. ZILSTORFF-PEDERSEN K. Quantitative measurements of the nasolacrimal reflex. Arch Otolaryngol. 1965;81:457–462. doi:10.1001/archotol.1965.00750050470003
24. Bernstein DI, Levy AL, Hampel FC, et al. Treatment with intranasal fluticasone propionate significantly improves ocular symptoms in patients with seasonal allergic rhinitis. Clin Exp Allergy. 2004;34(6):952–957. doi:10.1111/j.1365-2222.2004.01952.x
25. Martin BG, Ratner PH, Hampel FC, et al. Optimal dose selection of fluticasone furoate nasal spray for the treatment of seasonal allergic rhinitis in adults and adolescents. Allergy Asthma Proc. 2007;28(2):216–225. doi:10.2500/aap.2007.28.2983
26. Fokkens WJ, Jogi R, Reinartz S, et al. Once daily fluticasone furoate nasal spray is effective in seasonal allergic rhinitis caused by grass pollen. Allergy. 2007;62(9):1078–1084. doi:10.1111/j.1398-9995.2007.01522.x
27. Baroody FM, Naclerio RM. Nasal-ocular reflexes and their role in the management of allergic rhinoconjunctivitis with intranasal steroids. World Allergy Organ J. 2011;4(1 Suppl):S1–5. doi:10.1097/WOX.0b013e3181f32dcd
28. Hom MM, Bielory L. The anatomical and functional relationship between allergic conjunctivitis and allergic rhinitis. Allergy Rhinol. 2013;4(3):e110–9. doi:10.2500/ar.2013.4.0067
29. Weber RW. Ocular impact of intranasal corticosteroid therapy: All that surprising? Ann Allergy Asthma Immunol. 2008;100(3):193. doi:10.1016/S1081-1206(10)60441-3
30. Torkildsen GL, Williams JI, Gow JA, Gomes PJ, Abelson MB, McNamara TR. Bepotastine besilate ophthalmic solution for the relief of nonocular symptoms provoked by conjunctival allergen challenge. Ann Allergy Asthma Immunol. 2010;105(1):57–64. doi:10.1016/j.anai.2010.04.005
31. Cavanagh B, Gomes PJ, Starr CE, Nichols KK, Brady TC. Reproxalap activity and estimation of clinically relevant thresholds for ocular itching and redness in a randomized allergic conjunctivitis field trial. Ophthalmol Ther. 2022;11(4):1449–1461. doi:10.1007/s40123-022-00520-z
32. Barnes ML, Vaidyanathan S, Williamson PA, Lipworth BJ. The minimal clinically important difference in allergic rhinitis. Clin Exp Allergy. 2010;40(2):242–250. doi:10.1111/j.1365-2222.2009.03381.x
33. Caputo R, Marziali E, de LC, et al. Long-term safety and efficacy of tacrolimus 0.1% in severe pediatric vernal keratoconjunctivitis. Cornea. 2021;40(11):1395–1401. doi:10.1097/ICO.0000000000002751
34. Yazu H, Fukagawa K, Shimizu E, Sato Y, Fujishima H. Long-term outcomes of 0.1% tacrolimus eye drops in eyes with severe allergic conjunctival diseases. Allergy Asthma Clin Immunol. 2021;17(1):11. doi:10.1186/s13223-021-00513-w
35. Kassumeh S, Brunner BS, Priglinger SG, Messmer EM. Neue und zukünftige Therapieansätze in der Behandlung der allergischen Konjunktivitis [New and future treatment approaches for allergic conjunctivitis]. Ophthalmologie. 2024;121(3):180–186. doi:10.1007/s00347-024-01996-9
36. Kung L, Halloran PF. Immunophilins may limit calcineurin inhibition by cyclosporine and tacrolimus at high drug concentrations. Transplantation. 2000;70(2):327–335. doi:10.1097/00007890-200007270-00017
37. Labcharoenwongs P, Jirapongsananuruk O, Visitsunthorn N, Kosrirukvongs P, Saengin P, Vichyanond P. A double-masked comparison of 0.1% tacrolimus ointment and 2% cyclosporine eye drops in the treatment of vernal keratoconjunctivitis in children. Asian Pac J Allergy Immunol. 2012;30(3):177–184.
38. Heikal MA, Soliman TT, Abousaif WS, Shebl AA. A comparative study between ciclosporine A eye drop (2%) and tacrolimus eye ointment (0.03%) in management of children with refractory vernal keratoconjunctivitis. Graefes Arch Clin Exp Ophthalmol. 2022;260(1):353–361. doi:10.1007/s00417-021-05356-0
39. Pucci N, Caputo R, Di Grande L, et al. Tacrolimus vs. cyclosporine eyedrops in severe cyclosporine-resistant vernal keratoconjunctivitis: A randomized, comparative, double-blind, crossover study. Pediatr Allergy Immunol. 2015;26(3):256–261. doi:10.1111/pai.12360
40. Miyazaki D, Fukushima A, Ohashi Y, et al. Steroid-sparing effect of 0.1% tacrolimus eye drop for treatment of shield ulcer and corneal epitheliopathy in refractory allergic ocular diseases. Ophthalmology. 2017;124(3):287–294. doi:10.1016/j.ophtha.2016.11.002
41. Fukushima A, Ohashi Y, Ebihara N, et al. Therapeutic effects of 0.1% tacrolimus eye drops for refractory allergic ocular diseases with proliferative lesion or corneal involvement. Br J Ophthalmol. 2014;98(8):1023–1027. doi:10.1136/bjophthalmol-2013-304453
42. Przepiorka D, Nash RA, Wingard JR, et al. Relationship of tacrolimus whole blood levels to efficacy and safety outcomes after unrelated donor marrow transplantation. Biol Blood Marrow Transplant. 1999;5(2):94–97. doi:10.1053/bbmt.1999.v5.pm10371361
43. Shapiro R, Fung JJ, Jain AB, Parks P, Todo S, Starzl TE. The side effects of FK 506 in humans. Transplant Proc. 1990;22(1):35–36.
44. Ebihara N, Ohashi Y, Fujishima H, et al. Blood level of tacrolimus in patients with severe allergic conjunctivitis treated by 0.1% tacrolimus ophthalmic suspension. Allergol Int. 2012;61(2):275–282. doi:10.2332/allergolint.11-OA-0349
45. Fujita E, Teramura Y, Shiraga T, et al. Pharmacokinetics and tissue distribution of tacrolimus (FK506) after a single or repeated ocular instillation in rabbits. J Ocul Pharmacol Ther. 2008;24(3):309–319. doi:10.1089/jop.2007.0083
46. Erdinest N, Ben-Eli H, Solomon A. Topical tacrolimus for allergic eye diseases. Curr Opin Allergy Clin Immunol. 2019;19(5):535–543. doi:10.1097/ACI.0000000000000560
47. Thomson AW, Bonham CA, Zeevi A. Mode of action of tacrolimus (FK506): Molecular and cellular mechanisms. Ther Drug Monit. 1995;17(6):584–591. doi:10.1097/00007691-199512000-00007
48. Kino T, Hatanaka H, Miyata S, et al. FK-506, a novel immunosuppressant isolated from a Streptomyces. II. Immunosuppressive effect of FK-506 in vitro. J Antibiot. 1987;40(9):1256–1265. doi:10.7164/antibiotics.40.1256
49. Hatfield SM, Mynderse JS, Roehm NW. Rapamycin and FK506 differentially inhibit mast cell cytokine production and cytokine-induced proliferation and act as reciprocal antagonists. J Pharmacol Exp Ther. 1992;261(3):970–976.
50. Sengoku T, Morita K, Sakuma S, Motoyama Y, Goto T. Possible inhibitory mechanism of FK506 (tacrolimus hydrate) ointment for atopic dermatitis based on animal models. Eur J Pharmacol. 1999;379(2–3):183–189. doi:10.1016/S0014-2999(99)00500-2
51. Fauquert J-L, Jedrzejczak-Czechowicz M, Rondon C, et al. Conjunctival allergen provocation test: Guidelines for daily practice. Allergy. 2017;72(1):43–54. doi:10.1111/all.12986
52. Abelson MB, Chambers WA, Smith LM. Conjunctival allergen challenge. A clinical approach to studying allergic conjunctivitis. Arch Ophthalmol. 1990;108(1):84–88. doi:10.1001/ARCHOPHT.1990.01070030090035
53. Zuberbier T, Abelson MB, Akdis CA, et al. Validation of the global allergy and asthma European network (GA2LEN) chamber for trials in allergy: Innovation of a mobile allergen exposure chamber. J Allergy Clin Immunol. 2017;139(4):1158–1166. doi:10.1016/j.jaci.2016.08.025
54. Jacobs RL, Ramirez DA, Rather CG, et al. Redness response phenotypes of allergic conjunctivitis in an allergen challenge chamber. Ann Allergy Asthma Immunol. 2017;118(1):86–93. doi:10.1016/j.anai.2016.10.023
55. Gherasim A, Dietsch F, Beck M, Domis N, de BF. Birch-induced allergic rhinitis: results of exposure during nasal allergen challenge, environmental chamber, and pollen season. World Allergy Organ J. 2023;16(7):100801. doi:10.1016/j.waojou.2023.100801
56. Ellis AK, DeVeaux M, Steacy L, et al. Environmental exposure unit simulates natural seasonal birch pollen exposures while maximizing change in allergic symptoms. Ann Allergy Asthma Immunol. 2021;127(4):488–495.e5. doi:10.1016/j.anai.2021.06.015
57. Zieglmayer PU. Are results of environmental exposure units transferable to real-life exposure? Curr Opin Allergy Clin Immunol. 2013;13(3):244–248. doi:10.1097/ACI.0b013e328360c7b6
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





