Back to Journals » Patient Preference and Adherence » Volume 18
An Australian Community-Based Metabolic Dysfunction-Associated Steatotic Liver Disease Care Pathway for People with Type 2 Diabetes: Barriers and Considerations
Authors Gracen L, Aikebuse M, Sarraf B , McPhail SM , Russell AW , O’Beirne J, Irvine KM, Williams S, Valery PC , Powell EE
Received 13 March 2024
Accepted for publication 13 August 2024
Published 9 September 2024 Volume 2024:18 Pages 1845—1855
DOI https://doi.org/10.2147/PPA.S468705
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Lucy Gracen,1 Melanie Aikebuse,2,3 Babak Sarraf,2– 4 Steven M McPhail,5 Anthony W Russell,6 James O’Beirne,7 Katharine M Irvine,8 Suzanne Williams,9 Patricia C Valery,4,* Elizabeth E Powell2– 4,*
1Department of Gastroenterology and Hepatology, Royal Brisbane and Women’s Hospital, Herston, QLD, Australia; 2Department of Gastroenterology and Hepatology, Princess Alexandra Hospital, Woolloongabba, QLD, Australia; 3Centre for Liver Disease Research, Faculty of Medicine, Translational Research Institute, The University of Queensland, Woolloongabba, QLD, Australia; 4QIMR Berghofer Medical Research Institute, Brisbane, QLD, Australia; 5Australian Centre for Health Services Innovation School of Public Health, Institute of Health and Biomedical Innovation, Queensland University of Technology (QUT), Brisbane, QLD, Australia; 6Endocrinology and Diabetes, the Alfred Hospital, Melbourne, VIC, Australia; 7Department of Gastroenterology and Hepatology, Sunshine Coast University Hospital, Birtinya, QLD, Australia; 8Mater Research, Translational Research Institute, Brisbane, QLD, Australia; 9Inala Primary Care General Practice, Inala, QLD, Australia
*These authors contributed equally to this work
Correspondence: Elizabeth E Powell, Centre for Liver Disease Research, Faculty of Medicine, Translational, Research Institute, The University of Queensland, Level 5, West Wing, 37, Kent Street, Woolloongabba, Brisbane, QLD, 4102, Australia, Tel +61 412 014 337, Email [email protected]
Background: Although clinical guidelines endorse screening for metabolic dysfunction-associated steatotic liver disease (MASLD) with advanced fibrosis in people with type 2 diabetes (T2D), the feasibility of and barriers and considerations relevant to implementing this approach in the community remain unclear.
Methods: Sequential adults with T2D attending selected community clinics during 2021– 2023 were invited to receive a “liver health check” (n=543). A further 95 participants were referred directly from their general practitioner (GP) or self-referred to the study. A total of 302 participants underwent a point of care assessment of hepatic steatosis and stiffness (FibroScan) and were advised to see their GP to discuss the results. “Template” letters containing key results, their interpretation and advice about management of cardiometabolic risk, patient follow-up and referral criteria, were sent to participants’ GPs.
Results: Referral to a tertiary liver clinic was advised in GP letters for 45 (15%) participants with an increased risk of clinically significant fibrosis (liver stiffness measurement ≥ 8), 15 participants with ‘red flags’ (eg splenomegaly, thrombocytopenia) and 2 with unsuccessful FibroScan examinations. A referral from GPs to the liver clinic was received for 27 (44%) of these 62 participants. Approximately 90% of GPs rated the “template” letters favourably on a Likert rating scale.
Conclusion: The low rate of participation in the “liver health check” and liver clinic referral reflects a real-world scenario and may stem from societal under-recognition and engagement with MASLD, competing health priorities or under-appreciation of the link between liver fibrosis severity and mortality risk. Further studies need to address strategies to enhance participation in liver health assessments and determine their impact on liver-related morbidity/mortality and overall survival.
Keywords: NAFLD, primary care, chronic liver disease, FibroScan
Introduction
There is global concern from a public health perspective about the future burden of advanced liver disease due to metabolic dysfunction-associated steatotic liver disease (MASLD). Although somewhat limited, the data from Australia regarding its prevalence1,2 and liver-related outcomes3 is also cause for alarm, and has prompted interest in the development of clinical care pathways for MASLD. Without a systematic approach to screening and risk stratification, the diagnosis of MASLD often occurs incidentally, during investigation of other clinical concerns,4 and these individuals are more likely to have severe liver disease and hepatocellular carcinoma (HCC) at the time of diagnosis,5 which can restrict available therapeutic options. In contrast, many of the patients referred to liver clinics for evaluation of MASLD do not have advanced fibrosis and could be appropriately managed in primary care while addressing cardiometabolic risk factors.
Strategies to introduce routine assessment of liver fibrosis, the most compelling predictor of patient outcome,6 into primary care management of MASLD have been proposed, in order to provide earlier, targeted detection of advanced fibrosis, and reduce unnecessary referrals of people at low risk of adverse liver-related outcomes.7 Although clinical guidelines endorse the use of a stepwise Fibrosis-4 (FIB-4) – vibration-controlled transient elastography (VCTE) algorithm for noninvasive fibrosis risk stratification,8 for people with type 2 diabetes (T2D), the use of simple scores as a first step has low accuracy to classify individuals at low risk of advanced fibrosis.9,10 Among people with T2D, the prevalence of MASLD is 40–70% and around 15% have clinically significant fibrosis, with a >2-fold increased risk of developing cirrhosis-related complications and liver mortality.11,12
We and others have proposed that patients with T2D may benefit from an initial assessment with VCTE to identify at-risk patients who require specialized care. However, further work is needed to assess the feasibility of this approach and determine the barriers and considerations to implementing such a pathway in the community. Previous focus group discussions about MASLD with general practitioners (GPs), endocrinologists and diabetes educators, identified the need for an integrated approach to care, with better access to fibrosis tests, and guidance about management and referral criteria.13
In this study, we examined a strategy to offer a “liver health check” to people with T2D in the community, using FibroScan to assess liver stiffness and steatosis. The overall aims of the study were to determine whether this strategy increased the rate of detection of MASLD and the number of people with an increased risk of liver disease progression that require hepatology input. In addition, we examined the effect of the liver health check’s clinical findings and recommendations on GP referral to hepatology for further assessment.
Methods
The protocol for this prospective cohort study of a community care pathway for conducting a liver health check in people with T2D was published prospectively.14 Sequential adults with T2D attending community diabetes clinics, a community lifestyle intervention program or 43 GP practices within the Metro South Hospital and Health Service (HHS) district between June 2021 and December 2023 were invited to participate. Individuals were excluded if they had known chronic liver disease and were undergoing follow-up with a hepatology clinic or had a diagnosis of advanced cardiac disease or another terminal illness. The Metro South HHS district covers the south side of Brisbane and has a catchment area of 3860 km2, providing healthcare to 1.2 million people.
Liver Health Check in Primary Care
A trained liver specialist nurse (MA) or a clinician (LG, BS) recruited participants, performed the transient elastography, and collected clinical data. Participants were screened for MASLD using FibroScan to assess steatosis (Controlled Attenuation Parameter (CAP) score) and liver fibrosis (Liver Stiffness Measurement, LSM) as a point-of-care test following a 3 hour fast. At the FibroScan assessment, the study clinician assessed waist circumference and documented alcohol consumption using the short-form AUDIT15 to quantify alcohol misuse, based on 3 questions posed to patients about their consumption habits. A short medical history form was completed to obtain information about prior liver disease, presence of cardiometabolic risk factors, medications and sociodemographic factors. Pathology data to obtain liver enzymes and platelet count were reviewed, along with available prior liver imaging.
MASLD was diagnosed on the basis of the FibroScan CAP score (CAP score ≥248 dB considered as likely steatosis based on cut-offs determined in a large meta-analysis)16 and stratified to low or increased risk of clinically significant fibrosis on the basis of a FibroScan LSM < or ≥8 kPa.
Provision of a Letter to Each Participant and Their GP
Participants were informed of their FibroScan results during the liver health check, and provided with a standard letter,14 advising them to schedule an appointment with their GP to discuss the results of the FibroScan and further management. One of 4 “template” letters14 were sent to the participant’s GP, based on the outcome of the liver health check:
1) No MASLD: participants with CAP score < 248 dB and LSM < 8 kPa;
2) MASLD with low risk of clinically significant fibrosis: CAP score ≥ 248 dB and LSM < 8.0 kPa;
3) If liver enzymes were abnormal, the letter included guidance for further evaluation of abnormal liver enzymes;
4) Increased risk of clinically significant fibrosis: LSM ≥8.0 kPa.
The template letters were co-designed with GPs and all contained a brief summary of key results, their interpretation and advice about management of cardiometabolic risk, patient follow-up and referral criteria. If the participant had an increased risk of clinically significant fibrosis, the letter to the GP advised referral to a liver clinic for further evaluation, and included the Metro South HHS referral guidelines.
Referral of Participants with MASLD to Hepatology Clinics
The number of GP referrals to a hepatology clinic for study participants with an increased risk or low risk of clinically significant fibrosis was determined based on referral letters received by the centralised referral coordination unit. This is the single point of entry for referrals to specialist outpatients within the Metro South HHS district.
Collection of Data from GPs About the Utility of the “Template” Letters
An important component of this study was the provision of a letter to the participant’s GP that provided succinct guidance on the appropriate management and follow-up of their patient in primary care, as well as investigation of abnormal liver enzymes or referral to a liver clinic if required. Following the letter, a short questionnaire (Supplementary Figure 1) was sent to each participant’s GP to evaluate the letters for (1) their effectiveness in providing guidance for management of people with low-risk MASLD or (2) to ascertain the reasons for non-referral of a participant with elevated LSM to the hepatology clinic. Respondents indicated utility of different aspects of the letter on a 4-point Likert-type scale (“A lot”, “Some”, “A little” and “Not at all”) and commented (open-ended question) about each topic. A level of combined utility (“A lot” + “Some”) was used to summarise responses.
Data Analysis
Quantitative data were analysed using Stata/SE (Version 18; Stata Corporation, College Station, TX). Percentages, means (standard deviations (SD)), and medians (interquartile range (IQR)) were reported according to data distribution. Responses to the open-ended questions in the short questionnaire were transcribed into Microsoft excel. Thematic analysis was used to identify common themes using an inductive approach.17 Responses to the open-ended questions were first analysed by LG to assign codes and develop the initial themes. PCV and EEP independently reviewed the data, and these three team members then developed an overall interpretation of the data. Respondents were de-identified using a number “x” and grouped based on the letter they received including “low risk” (L), “low risk with abnormal liver enzymes” (LA) and “increased risk” (H). For example (LA4) refers to GP number 4 who received a “template” letter discussing “low risk of clinically significant fibrosis with abnormal liver enzymes”.
Results
Study Population
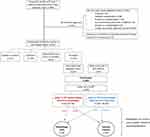
Between June 2021 and December 2023, 869 individuals with T2D who had been referred to a community diabetes clinic were ascertained and 543 were invited to have a liver health check (Figure 1). No text message was sent to 326 participants, of whom 253 did not meet study eligibility criteria (eg significant medical co-morbidities, inability to provide informed consent, pre-existing liver disease already known to a hepatologist) and 73 due to resource or technical constraints. Amongst those excluded, 64 had known pre-existing chronic liver disease and were currently seeing a hepatologist, including 49 (5.6%) with MASLD. Of the 543 individuals invited to participate in the study, 134 did not respond to a text or verbal invitation, 154 declined to participate, 24 requested more time to think about the study, and 231 accepted the invitation (207 attended the clinic and 24 failed to attend initial assessment). Ninety-five participants were referred directly from their general practitioner or self-referred to the study.
A total of 302 participant liver health assessments were completed. Overall, the mean age of subjects at assessment was 59.6 years (SD=10.9 years) and 61% were male. The majority (66%) were Caucasian, with a mean body mass index (BMI) of 32.7 (IQR 28.0–38.0) kg/m2 and mean waist circumference of 115 cm (IQR 103–126 cm). All participants had T2D and 16% of the cohort had Class 3 obesity (BMI ≥40 kg/ m2). Thirteen participants (4%) drank more than 20 g/day of alcohol.
Fibrosis Risk Assessment and Detection of Steatosis
Transient elastography met quality criteria in 298 of 302 (98.7%) participants. The median waist circumference of the 4 participants who did not meet quality criteria was 149 cm (IQR 128–167 cm) compared to 115cm (IQR 102–125 cm) for those who did; the prevalence of obesity and median BMI did not differ between successful and unsuccessful FibroScans. Median LSM was 5.8 kPa (IQR 4.5–7.1 kPa) and required use of the XL probe in 49%. LSM ≥8.0 kPa, consistent with an increased risk of clinically significant fibrosis, was present in 46 (15.4%) participants. A total of 254 (85%) participants had steatosis, based on a CAP score ≥248. Using a more stringent CAP score of ≥294 (M probe) and ≥297 (XL probe), steatosis was present in 130 (44%). Compared to participants without clinically significant fibrosis, those with LSM ≥8.0 kPa had a higher BMI (p=0.003), greater waist circumference (p=0.005), higher prevalence of metabolic comorbidities (hypertension, obstructive sleep apnoea, chronic kidney disease; p<0.05) and higher prevalence of abnormal liver enzymes (p=0.015) (Table 1). Overall, the median FIB-4 score was 1.01 (IQR 0.71–1.40). Indeterminate or high FIB-4 scores were present in 47 of the cohort, and would have led to referral for FibroScan assessment if GPs were following a 2-step pathway. Of concern, 29 of 45 people with LSM ≥8kPa (64%) had a low FIB-4 score and would not have been referred for FibroScan assessment (FIB-4 was missing for one person with LSM ≥8kPa as this participant was younger than age 35 and therefore the FIB-4 was not calculated). The FIB-4 risk stratification scores (low, indeterminate and high) according to LSM < or ≥8kPa are illustrated in Figure 2.
 |
Table 1 Selected Demographic and Clinical Data for Participants According to Liver Stiffness Measurement < or ≥8.0 kPa |
Communication with Participants’ GPs and Rate of Referral of Study Participants
Following the liver health check, participants were provided with a standard letter advising them to schedule an appointment with their GP to discuss the results of the FibroScan, and one of 4 template letters was sent to each participant’s GP. Referral to the liver clinic was advised in 62 patient letters to GPs (n=45 with LSM ≥8 kPa (1 patient with LSM ≥8 kPa declined to provide a GP), 15 with “red flags” including splenomegaly, thrombocytopenia, and 2 where FibroScan examination was unsuccessful).
A referral to the liver clinic was received for 27 of the 62 participants with a letter to the GP advising referral, and for 3 of 237 participants with a letter to the GP advising management in primary care. Seven of 35 GPs who did not refer following receipt of a letter advising referral, commented about their rationale. The referral was considered unnecessary by one GP (n=1) or the patient (n=3), and a lack of knowledge of care pathways within the healthcare system and the role/ability of specialists to initiate referrals to other specialists (n=3) were reported as reasons for not referring patients.
Utility of the “Template” Letters
Approximately 90% of GPs rated the “template” letters favourably on a Likert rating scale. A total of 56 of 62 GPs who responded to the question “Did you find the letter recommendations helpful?” rated helpfulness of the letters as “A lot” (37 responses) and “Some” (19 responses). Five participants rated the letter’s helpfulness as “A little” and only one as “Not at all”.
The letters were generally sent without the need for additional administrative time investment, as only 50 (16.7%) of the 299 GP letters required modifications. These were largely to advise reducing the follow-up FibroScan interval (from 3 years to 2 years) or to provide specific additional advice on alcohol intake.
Forty-seven questionnaires were completed. Comments from general practitioners on the utility of the “template” letters were analysed using qualitative thematic analysis. Four key themes stemmed from the open-ended question (Table 2): “communication”, “elements of the healthcare system”, “patient engagement” and “knowledge of assessment and management”.
 |
Table 2 Main Themes Emerging from the GP Responses to the Open-Ended Question about the Utility of the “Template” Letters |
Communication
Most GPs commented about the positive aspects of the “template” letter that facilitated communication with specialists. A few GPs found the letter somewhat confusing and were unclear of its relevance to their patient.
Elements of the Healthcare System
The majority of GPs commented on the limitations of the healthcare system in providing adequate services to meet the needs of patients with MASLD (eg access to a dietician or psychologist).
Patient Engagement
Patient engagement in care was varied. Some patients were actively engaged in lifestyle changes and weight loss. Other patients lacked motivation to engage in healthcare and interventions to make healthy lifestyle improvements.
Knowledge of Assessment and Management
The usefulness of the “template” letter was acknowledged by many GPs, particularly about some aspects of patient management (eg the role of FibroScan). Some GPs highlighted areas of patient management about which they were less confident (eg follow-up interval for FibroScans in the future, when to refer patients).
Discussion
Our findings confirm the high prevalence of steatosis (85%) and risk of clinically significant fibrosis (15.4%) in people with T2D evaluated for MASLD, with a higher BMI, girth and prevalence of metabolic comorbidities among the subgroup at risk of progressive liver disease.18 These findings confirm that T2D should trigger consideration for MASLD assessment in the community. Of note, the current screening recommendations using the low-risk FIB-4 score would have failed to exclude a high LSM result in 29 people and concerningly, more than half of patients with a high-risk LSM were not referred to hepatology.
In this cohort of people with T2D attending community diabetes clinics, 64 of 869 sequentially evaluated participants had a prior diagnosis of chronic liver disease, 49 (5.6%) with MASLD, and were known to a hepatology clinic. Our study demonstrated that offering a “liver health check” using FibroScan to people with T2D in the community expanded the detection of at-risk MASLD by a further 60 (6.9%), excluding the two patients referred for unsuccessful FibroScan examination. For these at-risk individuals, referral to a liver clinic was advised to investigate an elevated liver stiffness measurement or other findings that raised concern about progressive liver disease. These patients will benefit from closer management of metabolic comorbidities and lifestyle interventions, and in the presence of advanced fibrosis, surveillance for liver cancer and liver decompensation.18
Of concern, at least 50% of the participants offered a “liver health check” did not respond or declined to participate, reinforcing global concern about societal under-recognition and engagement with MASLD.19 Although determining the reasons for not participating were outside the scope of the current program, previous studies have shown a very low awareness of MASLD (19%) among people at metabolic risk for the condition20 and little knowledge of its role as a risk factor for the development of cirrhosis.21 The low rate of participation in our program may be influenced by lack of awareness of the health implications of steatotic liver disease. This was reinforced by the GPs’ comments about patients’ lack of engagement in healthcare and lifestyle improvements. Assessment of the impact of upskilling diabetes educators to include MASLD education in T2D self-management programs will be important in future studies.
Various MASLD models of care have been described, largely with the goal of providing earlier, targeted detection of liver disease in the community, in people with known risk factors for MASLD or abnormal liver enzymes.7 Most current pathways advocate a two-step risk stratification process, using FIB-4 for initial assessment of fibrosis in the community and second-line assessment with transient elastography or a patented serum fibrosis test such as the Enhanced Liver Fibrosis (ELF) test following referral to secondary care. In this model of care, we elected to use FibroScan as the initial tool in the community, in order to provide a well-validated, real-time assessment of liver fibrosis as well as steatosis, and to reduce unnecessary referrals to hepatology outpatients for second-line fibrosis risk assessment. The use of FIB-4 as a first step in people with T2D has low cost but also has low accuracy to classify individuals at low risk of advanced fibrosis. In this cohort, 45 out of 290 patients with available data to calculate a FIB-4 score had LSM≥8.0, and 29 (64%) of those would not have been referred for FibroScan assessment if GPs were following a 2-step pathway. In contrast, FibroScan has one of the highest negative predictive values (84%) of all noninvasive tests currently available for clinical use in Australia to exclude advanced fibrosis/cirrhosis in people with T2D.22 Relatively few patients in our cohort (5, 1.7%) had a high FIB-4 score (>2.67), supporting previous reports of the low diagnostic performance of the FIB-4 test to detect advanced fibrosis in people with T2D.23–25
In our study, use of FibroScan in the community allowed for timely delivery of the diagnosis and fibrosis risk category (low or increased risk) to the patient and their GP, along with recommendations for further management and follow-up. Somewhat surprisingly, a referral to the liver clinic was received for less than half of the participants with a letter to their GP advising referral following risk stratification. This is a concern, since an earlier study has shown that people with T2D and high index of suspicion for advanced fibrosis (based on FIB4 > 3.25), had better overall survival after referral to a hepatology clinic, possibly due to increased recognition of cirrhosis and cirrhosis complications in the referred populations.26 A similar rate of referral (56%) was seen in a recent study using clinical calculators (FIB-4 and NAFLD Fibrosis Score) and a case-finding algorithm to identify MASLD with advanced fibrosis in primary care patients with T2D and increase their access to specialty care.27 In a follow-up survey to elicit reasons for non-referral, 34% of primary care providers reported that the patient had other competing health priorities and 20% reported that the patient was offered referral, but declined or could not be reached.27 Although some information was available regarding GPs’ rationale for non-referral in our study, in most instances the reasons remain unclear.
Overall, the GP template letters were well received, with virtually all respondents reporting the letter content as helpful. Primary care providers report that receiving notification about a high-risk patient is very useful, and helps them to recognise cases of MASLD of which they were not aware.27 However, in view of the relatively low number of clinic referrals, future template letters should highlight the key relationship between advanced fibrosis and increased risk of liver-related complications, and recommendations for surveillance strategies for portal hypertension and liver cancer.28 The significant link between severity of liver fibrosis and higher risk of overall and liver‐related mortality may not be widely appreciated by non-hepatologists.28 In addition, the inability to use liver enzymes as a surrogate marker of progressive liver disease may not be clear.28
Limitations
Strengths of this study include the prospective design, comprehensive assessment of liver disease risk factors, and transient elastography performed by trained clinicians. Nevertheless, the study was conducted solely in an HHS district that provides hepatology services to a large geographical region, limiting its generalizability and applicability to other settings and locations. The relatively low rate of participation in the “liver health check” may be a sign of under-recognition of MASLD in the community. While face-to-face interviews may have provided opportunity for discussion of each topic in more depth, the use of open-ended questions means that social desirability bias, where participants answer questions in a certain way in the presence of an interviewer, was not an issue.
Conclusions
Although we have shown that a “liver health check” using FibroScan can be successfully undertaken in community diabetes clinics, our approach is relatively resource intensive and not readily scalable without expanding community FibroScan capacity. Point-of-care FibroScan use is limited by the requirement for operator training, maintenance of equipment, space within a busy diabetes clinic or general practice and the lack of a Medicare Benefits Schedule rebate for the test. Determining the utility and cost-effectiveness of the program will require further studies to assess how the pathway affects liver clinic referrals more broadly, the impact on liver-related morbidity/mortality and overall survival, and patient needs and lifestyle changes after referral.
Abbreviations
CAP, Controlled Attenuation Parameter; FIB-4, Fibrosis-4; GPs, General practitioners; HHS, Hospital and Health Service; HCC, Hepatocellular carcinoma; IQR, Interquartile range; LSM, Liver stiffness measurement; MASLD, Metabolic dysfunction-associated steatotic liver disease; SD, Standard deviation; T2D, Type 2 diabetes; VCTE, Vibration-controlled transient elastography.
Data Sharing Statement
The data that support the study findings may contain potentially identifying information that could compromise the privacy of the participants. Therefore, the data are not publicly available. Data may, however, be available from the corresponding author upon reasonable request with approval from relevant ethics committees.
Ethics Approval and Informed Consent
The study was conducted in accordance with both the Declarations of Helsinki and Istanbul. Informed written consent was obtained from each eligible participant (adults attending selected community clinics and their general practitioner), and general practitioners consented to the use of their anonymized responses/direct quotes for research purposes. The protocol was approved by the Metro South Health Human Research Ethics Committee (HREC/2021/QMS/72731 and HREC/2022/QMS/82418).
Acknowledgments
We thank the staff and patients of the participating clinics for their assistance and cooperation in performing the current study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was funded by the Metro South Health Research Support Scheme (2022 Program Grant). Access to a mobile community FibroScan was provided by a Medical Research Future Fund (MRFF) Keeping Australians Out of Hospital grant (GNT 1175567). The Queensland Government Gambling Community Benefit Fund provided support to purchase an M+ FibroScan probe.
Disclosure
There are no financial disclosures. The funders had no role in study design, data collection, data analysis, interpretation, or writing of the manuscript.
References
1. Farrell AM, Magliano DJ, Shaw JE, et al. A problem of proportions: estimates of metabolic associated fatty liver disease and liver fibrosis in Australian adults in the nationwide 2012 ausdiab study. Sci Rep. 2022;12(1):1956. doi:10.1038/s41598-022-05168-0
2. Vaz K, Kemp W, Majeed A, et al. Non-alcoholic fatty liver disease prevalence in Australia has risen over 15 years in conjunction with increased prevalence of obesity and reduction in healthy lifestyle. J Gastroenterol Hepatol. 2023;38(10):1823–1831. doi:10.1111/jgh.16314
3. O’Beirne J, Skoien R, Leggett BA, et al. Diabetes mellitus and the progression of non-alcoholic fatty liver disease to decompensated cirrhosis: a retrospective cohort study. Med J Aust. 2023;219(8):358–365. doi:10.5694/mja2.52104
4. Elangovan H, Rajagopaul S, Williams SM, et al. Nonalcoholic fatty liver disease: interface between primary care and hepatology clinics. Hepatol Commun. 2020;4(4):518–526. doi:10.1002/hep4.1486
5. Bertot LC, Jeffrey GP, Wallace M, et al. Nonalcoholic fatty liver disease-related cirrhosis is commonly unrecognized and associated with hepatocellular carcinoma. Hepatol Commun. 2017;1(1):53–60. doi:10.1002/hep4.1018
6. Taylor RS, Taylor RJ, Bayliss S, et al. Association between fibrosis stage and outcomes of patients with nonalcoholic fatty liver disease: a systematic review and meta-analysis. Gastroenterology. 2020;158(6):1611–1625e1612. doi:10.1053/j.gastro.2020.01.043
7. Abeysekera KWM, Macpherson I, Glyn-Owen K, et al. Community pathways for the early detection and risk stratification of chronic liver disease: a narrative systematic review. Lancet Gastroenterol Hepatol. 2022;7(8):770–780. doi:10.1016/S2468-1253(22)00020-6
8. Ilagan-Ying YC, Banini BA, Do A, Lam R, Screening LJK. Diagnosis, and staging of Non-Alcoholic Fatty Liver Disease (NAFLD): application of society guidelines to clinical practice. Curr Gastroenterol Rep. 2023;25(10):213–224. doi:10.1007/s11894-023-00883-8
9. Gracen L, Hayward KL, Irvine KM, Valery PC, Powell EE. Low accuracy of FIB-4 test to identify people with diabetes at low risk of advanced fibrosis. J Hepatol. 2022;77(4):1219–1221. doi:10.1016/j.jhep.2022.06.016
10. Boursier J, Hagström H, Ekstedt M, et al. Non-invasive tests accurately stratify patients with NAFLD based on their risk of liver-related events. J Hepatol. 2022;76(5):1013–1020. doi:10.1016/j.jhep.2021.12.031
11. Lomonaco R, Godinez Leiva E, Bril F, et al. Advanced liver fibrosis is common in patients with type 2 diabetes followed in the outpatient setting: the need for systematic screening. Diabetes Care. 2021;44(2):399–406. doi:10.2337/dc20-1997
12. Castera L, Laouenan C, Vallet-Pichard A, et al. High Prevalence of NASH and advanced fibrosis in type 2 diabetes: a prospective study of 330 outpatients undergoing liver biopsies for elevated ALT, using a low threshold. Diabetes Care. 2023;46(7):1354–1362. doi:10.2337/dc22-2048
13. Gracen L, Hayward KL, Aikebuse M, et al. An exploration of barriers and facilitators to implementing a nonalcoholic fatty liver disease pathway for people with type 2 diabetes in primary care. Diabet Med. 2022;39(6):e14799. doi:10.1111/dme.14799
14. Gracen L, Hayward KL, Aikebuse M, et al. Implementing the right care in the right place at the right time for non-alcoholic fatty liver disease (NAFLD-RRR study): a study protocol for a community care pathway for people with type 2 diabetes. BMC Health Serv Res. 2022;22(1):487. doi:10.1186/s12913-022-07808-7
15. Bradley KA, DeBenedetti AF, Volk RJ, Williams EC, Frank D, Kivlahan DR. AUDIT-C as a brief screen for alcohol misuse in primary care. Alcohol Clin Exp Res. 2007;31(7):1208–1217. doi:10.1111/j.1530-0277.2007.00403.x
16. Karlas T, Petroff D, Sasso M, et al. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol. 2017;66(5):1022–1030. doi:10.1016/j.jhep.2016.12.022
17. Braun V, Clarke V. Using thematic analysis in psychology. Qual Research Psychol. 2008;3(2):77–101
18. Ajmera V, Cepin S, Tesfai K, et al. A prospective study on the prevalence of NAFLD, advanced fibrosis, cirrhosis and hepatocellular carcinoma in people with type 2 diabetes. J Hepatol. 2023;78(3):471–478. doi:10.1016/j.jhep.2022.11.010
19. Krag A, Buti M, V. LJ, et al. Uniting to defeat steatotic liver disease: a global mission to promote healthy livers and healthy lives. J Hepatol. 2023;79(5):1076–1078. doi:10.1016/j.jhep.2023.07.029
20. Wieland AC, Mettler P, McDermott MT, Crane LA, Cicutto LC, Bambha KM. Low awareness of nonalcoholic fatty liver disease among patients at high metabolic risk. J Clin Gastroenterol. 2015;49(1):e6–e10. doi:10.1097/MCG.0000000000000075
21. Alemany-Pagès M, Moura-Ramos M, Araújo S, et al. Insights from qualitative research on NAFLD awareness with a cohort of T2DM patients: time to go public with insulin resistance? BMC Public Health. 2020;20(1):1142. doi:10.1186/s12889-020-09249-5
22. Kwok R, Choi KC, Wong GLH, et al. Screening diabetic patients for non-alcoholic fatty liver disease with controlled attenuation parameter and liver stiffness measurements: a prospective cohort study. Gut. 2016;65(8):1359–1368. doi:10.1136/gutjnl-2015-309265
23. Arai T, Takahashi H, Seko Y, et al. Accuracy of the enhanced liver fibrosis test in patients with type 2 diabetes mellitus and its clinical implications. Clin Gastroenterol Hepatol. 2024;22(4):789–797.e8. doi:10.1016/j.cgh.2023.11.022
24. Ishiba H, Sumida Y, Seko Y, et al. Type IV collagen 7s is the most accurate test for identifying advanced fibrosis in NAFLD with type 2 diabetes. Hepatol Commun. 2020;5(4):559–572. doi:10.1002/hep4.1637
25. Ito T, Nguyen VH, Tanaka T, et al. Poor diagnostic efficacy of noninvasive tests for advanced fibrosis in obese or younger than 60 diabetic NAFLD patients. Clin Gastroenterol Hepatol. 2023;21(4):1013–1022.e6. doi:10.1016/j.cgh.2022.05.015
26. Dunn W, Song X, Koestler D, et al. Patients with type 2 diabetes and elevated fibrosis-4 are under-referred to hepatology and have unrecognized hepatic decompensation. J Gastroenterol Hepatol. 2022;37(9):1815–1821. doi:10.1111/jgh.15900
27. Fox RK, Chu JN, Goldman ML, Islam KB, Brandman D. Prospective study of a case-finding algorithm to detect NAFLD with advanced fibrosis in primary care patients. Hepatol Commun. 2023;7(2):e0024. doi:10.1097/HC9.0000000000000024
28. Pryke R, Guha IN. Time to focus on chronic liver diseases in the community: a review of primary care hepatology tools, pathways of care and reimbursement mechanisms. J Hepatol. 2023;78(3):663–671. doi:10.1016/j.jhep.2022.10.010
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.