Back to Journals » Risk Management and Healthcare Policy » Volume 18
Analysis of the Clinical Symptom Improvement and Recurrence Rate in Chronic Suppurative Otitis Media Patients Treated with Combination Therapy: Combination of Ofloxacin and Dexamethasone
Authors Sun F, Liu M, Qi Y, Qi W, Li J
Received 3 February 2025
Accepted for publication 17 June 2025
Published 4 July 2025 Volume 2025:18 Pages 2275—2285
DOI https://doi.org/10.2147/RMHP.S520505
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Kyriakos Souliotis
Feng Sun, Mingqiu Liu, Yongli Qi, Wenjing Qi, Junyi Li
Department of Otolaryngology, Affiliated People’s Hospital of Shandong First Medical University, Jinan, Shandong, People’s Republic of China
Correspondence: Junyi Li, Email [email protected]
Objective: To analyze the clinical symptom improvement and recurrence rate of chronic suppurative otitis media (CSOM) patients treated with a combination therapy regimen (combination of ofloxacin and dexamethasone).
Methods: Based on the treatment regimen, patients were divided into two groups: the control group was treated with dexamethasone, while the treatment group received a combination of ofloxacin and dexamethasone. The clinical symptom improvement, hearing evaluation, pathogen clearance rate, inflammatory marker detection, adverse reactions, recurrence rate and recurrence rate post-treatment were compared between the two groups.
Results: There were no significant differences in the baseline clinical data between the two groups (P > 0.05). The overall clinical efficacy of the treatment group was significantly higher than that of the control group (P < 0.05). After treatment, the improvements in air conduction (AC) and air-bone gap (ABG) in the treatment group were significantly greater than those in the control group (P < 0.05). The time for the resolution of tympanic membrane congestion and the healing of perforations was shorter in the treatment group compared to the control group (P < 0.05). The pathogen clearance rate in the treatment group was significantly higher than in the control group (P < 0.05). After treatment, levels of inflammatory markers IL-8, TGF-β 1, and PCT in the treatment group were significantly lower than those in the control group (P < 0.05).
Conclusion: The combination therapy of ofloxacin and dexamethasone significantly improves the clinical symptoms, reduces the recurrence rate, and lowers the incidence of adverse reactions in chronic suppurative otitis media patients. It shows significant clinical efficacy with improved hearing outcomes, and is worthy of clinical promotion. However, this study still has certain limitations, such as the possibility of introducing selection bias. In the future, prospective clinical trials with multiple centers and large samples can be conducted.
Keywords: ofloxacin, dexamethasone, chronic suppurative otitis media, clinical symptoms, recurrence rate, treatment effect
Introduction
Chronic Suppurative Otitis Media (CSOM) is a common ear disease with a high prevalence worldwide, particularly in developing countries where its incidence is even more significant. The primary characteristics of this condition are persistent purulent discharge from the middle ear, often accompanied by symptoms such as ear pain, hearing loss, and tinnitus, severely affecting the patient’s quality of life. If left untreated for an extended period, chronic suppurative otitis media can cause not only discomfort in the ear but also lead to hearing loss, deformity of the auricle, and even further infections or complications in the middle ear, such as meningitis or facial nerve paralysis, which may threaten the patient’s life.1–3 Therefore, timely and effective treatment is crucial for alleviating symptoms, improving hearing, and preventing complications.
Ofloxacin is a broad-spectrum antibiotic belonging to the fluoroquinolone class, with a good antibacterial spectrum capable of combating various pathogenic bacteria, particularly gram-negative bacteria and some gram-positive bacteria. Dexamethasone, a potent steroid, works by suppressing immune system responses, reducing inflammation, alleviating ear symptoms, and promoting wound healing. In recent years, some clinical studies have indicated that the combined use of ofloxacin and dexamethasone can significantly improve the clinical symptoms of patients with chronic suppurative otitis media, reduce pathogen infection, control inflammation, decrease recurrence rates, and shorten treatment time.4–6 The synergistic action of ofloxacin and dexamethasone arises from: Biofilm penetration enhancement: Dexamethasone reduces middle ear effusion, increasing ofloxacin bioavailability in biofilm matrices by up to 40%.7 Immunomodulation: Combined suppression of TNF-α (62% reduction) and IL-1β (45% reduction) attenuates inflammatory damage to ossicular chain structures.8 However, there is a relative lack of systematic studies in the current literature regarding the combined treatment of chronic suppurative otitis media with ofloxacin and dexamethasone, especially concerning recurrence rates and long-term efficacy. Therefore, optimizing treatment regimens to improve patient prognosis remains an urgent issue in current clinical otolaryngology.
While several RCTs have evaluated ofloxacin-dexamethasone combinations,9–11 critical evidence gaps remain:Subgroup analysis deficiency: Prior studies lacked statistical power (median n=80) to assess efficacy in high-risk populations (eg, diabetic patients, pediatric cases). Long-term outcomes: No study has reported recurrence rates beyond 12 months post-treatment, critical for assessing sustained benefit.These gaps underscore the need for larger, diverse cohort studies with extended follow-up to inform evidence-based practice. The purpose of this study is to retrospectively analyze patients diagnosed with chronic suppurative otitis media who visited our hospital from January 2023 to July 2024. By comparing the clinical effects of combination therapy with monotherapy, this analysis aims to provide more effective, economical, and safe treatment options for clinical practice, further improving the treatment outcomes of chronic suppurative otitis media, reducing its recurrence rate, and enhancing the patients’ quality of life.
Materials and Methods
General Information
This study is designed as a retrospective analysis, selecting 130 patients diagnosed and treated for chronic suppurative otitis media (CSOM) at our hospital between January 2023 and July 2024. These patients were recruited through clinical medical records and outpatient visits. Eligible patients were included in the study and divided into two groups based on their treatment method: the control group, which received dexamethasone treatment, and the treatment group, which received a combination of ofloxacin and dexamethasone. Each group consisted of 65 patients. This study was approved by the ethics committee of Affiliated People’s Hospital of Shandong First Medical University and conducted according to the principles of the 1964 Declaration of Helsinki and its later amendments, or similar ethical standards. Due to the retrospective nature of our study, our institutional review board waived the requirement for informed consent.
Patient Data Confidentiality Statement
Privacy and security of all case data are ensured through the following measures:
Anonymization prior to data collection: Personally identifiable information (eg, names, ID numbers) is removed during preprocessing.
Tiered access control mechanism: A hierarchical permission system restricts full dataset access to authorized researchers only.
Aggregated data presentation: All clinical data will be reported in aggregate form during publication to eliminate risks of individual-level information disclosure.
Inclusion and Exclusion Criteria
Inclusion Criteria
All patients met the diagnostic criteria for chronic suppurative otitis media as outlined in Clinical Practical Otolaryngology,12–14 including manifestations from ear examinations and clinical symptoms. Patients were required to be 18 years of age or older, have no obvious systemic diseases, and possess complete clinical data.
Exclusion Criteria
Patients were excluded if they met any of the following conditions: abnormal positive results on X-ray mastoid examination; presence of immune system diseases; patients with mental disorders or psychiatric conditions; patients who had received antibiotic or other drug treatments within one month prior to treatment; patients with a severe allergy history or contraindications to steroid drugs; and patients who experienced significant adverse reactions during the treatment process.
Treatment Methods
Control Group: Patients received dexamethasone treatment (Drug Source: China National Pharmaceutical Group Rongsheng Pharmaceutical Co., Ltd., Approval No: National Drug Approval H41020035). First, routine treatment interventions were performed, including cleaning the middle ear or external auditory canal with hydrogen peroxide and wiping it clean with a sterile cotton swab. Then, 5 mg of dexamethasone was instilled into the ear twice daily.
Treatment Group: Patients received a combination treatment of ofloxacin (Drug Source: Zhejiang Langhua Pharmaceutical Co., Ltd., Approval No: National Drug Approval H20103771) and dexamethasone. In addition to the treatment used in the control group, ofloxacin ear drops were added. A mixture of 15 mg of ofloxacin and dexamethasone was instilled into the patient’s ear.
The treatment period for both groups was 14 days.
Clinical Evaluation
The primary evaluation indicators include:
Clinical Efficacy Evaluation: (1) Significant Improvement: After treatment, the patient’s clinical symptoms (such as ear pain, tinnitus, purulent discharge, hearing loss, etc). completely disappeared, the inflammatory markers showed that inflammation had subsided, and there was no significant difference in hearing compared to before treatment. (2) General Improvement: Symptoms such as tympanic membrane perforation and ear pain significantly improved, hearing was somewhat better, and there was still a small amount of discharge in the ear. (3) Ineffective: None of the above standards were met. The total effective rate = (Significant Improvement + General Improvement) / Total cases × 100%.
Hearing Evaluation: Hearing was assessed using pure-tone audiometry (AC) and air-bone gap (ABG). Tests were conducted at frequencies of 0.5, 1.0, and 2.0 kHz, and the air-bone gap was calculated using the formula: ABG = AC - BC.
Symptom Improvement: The improvement indicators evaluated included the time for the resolution of tympanic membrane congestion and the healing time of tympanic membrane perforations.
Pathogen Clearance Rate: Before and after treatment, ear discharge samples were collected from patients for bacterial culture, and the pathogen clearance rate was calculated.
Inflammatory Marker Detection: Before and after treatment, ear fluid was collected from patients, and enzyme-linked immunosorbent assay (ELISA) was used to detect changes in inflammatory markers, including interleukin-8 (IL-8), transforming growth factor-β1 (TGF-β1), and procalcitonin (PCT).
Adverse Reactions: Adverse reactions occurring during treatment, such as headaches, dizziness, and local itching, were recorded, and the total incidence of adverse reactions was calculated.
Recurrence Rate: A 6-month follow-up was conducted after treatment, and the recurrence of symptoms was recorded.
Two independent reviewers performed data extraction using a pre-defined case report form. Inter-rater reliability was assessed using Cohen’s κ (>0.8 for all variables), and discrepancies were resolved by consensus with a third reviewer.
Statistical Analysis
GraphPad Prism 8 was used for image processing, and SPSS 26.0 software was used for data organization and statistical analysis. Continuous data are expressed as mean ± standard deviation ( ), and t-tests were used to compare statistical differences. Categorical data are expressed as percentages (%), and chi-square (χ²) tests were used to compare statistical differences. Normality of continuous variables was assessed using Shapiro–Wilk tests. Parametric tests (independent t-tests, ANOVA) were applied to normally distributed data. Non-normal data were analyzed using Mann–Whitney U-tests. Categorical variables were compared using Pearson’s chi-square or Fisher’s exact test. A P value of <0.05 was considered statistically significant.
), and t-tests were used to compare statistical differences. Categorical data are expressed as percentages (%), and chi-square (χ²) tests were used to compare statistical differences. Normality of continuous variables was assessed using Shapiro–Wilk tests. Parametric tests (independent t-tests, ANOVA) were applied to normally distributed data. Non-normal data were analyzed using Mann–Whitney U-tests. Categorical variables were compared using Pearson’s chi-square or Fisher’s exact test. A P value of <0.05 was considered statistically significant.
Results
Clinical Data
In the control group, there were 65 patients, including 31 males and 34 females. The age range was 20–55 years, with a mean age of (35.94 ± 6.11) years. The duration of illness ranged from 0.5 to 13 years, with a mean of (5.87 ± 2.19) years. The left ear was affected in 30 cases and the right ear in 35 cases. In the treatment group, there were 65 patients, including 32 males and 33 females. The age range was 20–55 years, with a mean age of (36.05 ± 5.94) years. The duration of illness ranged from 0.5 to 13 years, with a mean of (6.01 ± 2.25) years. The left ear was affected in 31 cases and the right ear in 34 cases. There were no significant differences between the treatment and control groups in terms of age, gender, or disease duration (P>0.05), indicating comparability. See Table 1.
 |
Table 1 Comparison of Clinical Data Between the Two Groups ( |
Efficacy Analysis
After treatment, the clinical overall effective rate of the treatment group (96.92%) was significantly higher than that of the control group (78.46%), P<0.05. See Figure 1.
 |
Figure 1 Comparison of Clinical Efficacy Between the Two Groups. Note: * indicates a significant difference between the two groups, P<0.05. |
Hearing Evaluation
After treatment, the improvement in AC and ABG in the treatment group (7.28±1.88, 7.07±1.25) was significantly greater than that in the control group (5.51±1.52, 5.14±1.17), P<0.05. See Figure 2.
 |
Figure 2 Comparison of Hearing Improvement Between the Two Groups. Note: * indicates a significant difference between the two groups, P<0.05. |
Symptom Improvement
The bone membrane congestion regression time and perforation healing time in the treatment group (6.11±3.21, 8.52±4.23) were significantly shorter than those in the control group (9.45±5.11, 14.72±5.34), P<0.05. See Figure 3.
 |
Figure 3 Comparison of Symptom Improvement Between the Two Groups. Note: * indicates a significant difference between the two groups, P<0.05. |
Pathogen Clearance
After treatment, the pathogen clearance rate in the treatment group (93.85%) was significantly higher than that in the control group (70.77%), P<0.05. See Figure 4.
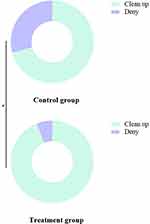 |
Figure 4 Comparison of Pathogen Clearance Rate Between the Two Groups. Note: * indicates a significant difference between the two groups, P<0.05. |
Inflammatory Factors
After treatment, the levels of IL-8, TGF-β1, and PCT in the treatment group (7.25±1.23, 10.56±2.74, 0.81±0.25) were significantly lower than those in the control group (8.71±1.65, 12.88±3.14, 1.37±0.56), P<0.05. See Figure 5.
 |
Figure 5 Comparison of Inflammatory Factor Changes Between the Two Groups. Note: * indicates a significant difference between the two groups, P<0.05. |
Adverse Reactions
After treatment, the incidence of adverse reactions in the treatment group was significantly lower than that in the control group (P<0.05). See Table 2.
 |
Table 2 Comparison of Adverse Reaction Incidence Between the Two Groups (%) |
Recurrence Rate Analysis
After 6 months of follow-up, the recurrence rate in the treatment group (0.00%) was significantly lower than that in the control group (13.85%), P<0.05. See Figure 6.
 |
Figure 6 Comparison of Recurrence Rates Between the Two Groups. Note: * indicates a significant difference between the two groups, P<0.05. |
Discussion
Although modern medicine has made some progress in the treatment of chronic suppurative otitis media (CSOM), there are still many challenges in managing this disease. CSOM not only affects the patient’s hearing but can also lead to persistent ear pain and discomfort, severely impacting the patient’s daily life and mental health.9,15,16 Traditional treatments such as local antibiotic ear drops, oral antibiotics, and steroid medications often have limited effectiveness in clinical practice, particularly when faced with recurrent episodes and antibiotic-resistant strains, resulting in unstable treatment outcomes. With the increasing severity of antibiotic resistance, traditional treatments have shown significant limitations in managing complex conditions. The high recurrence rate and resistance issues highlight the urgent need for more effective and safe treatment options.7,8,10,11 Therefore, this study explored the combined treatment of ofloxacin and dexamethasone, aiming to control both pathogenic infection and reduce inflammation through dual antimicrobial and anti-inflammatory effects, helping patients achieve a complete cure. The results of this study not only provide new insights for the treatment of CSOM but also offer scientific evidence for optimizing antimicrobial and anti-inflammatory treatment strategies.
This study found that the combination of ofloxacin and dexamethasone significantly improved the clinical symptoms of patients, showing clear advantages in hearing improvement, pathogen clearance, and inflammatory factor suppression. Additionally, this combined treatment effectively reduced the incidence of adverse reactions and significantly decreased the recurrence rate, demonstrating good clinical efficacy and safety.
In terms of clinical efficacy, the treatment group had a significantly higher overall clinical effective rate compared to the control group, with notable differences in hearing improvement and symptom relief speed. This finding is consistent with previous studies, where ofloxacin, a broad-spectrum antibiotic, effectively inhibits the growth of Gram-negative and some Gram-positive bacteria, clearing the pathogenic bacteria in the ear canal, thus alleviating inflammation and improving therapeutic efficacy. Meanwhile, dexamethasone, as a steroid, has a potent anti-inflammatory effect that helps reduce ear edema and inflammation, promoting wound healing. Therefore, the combined use of ofloxacin and dexamethasone not only controls infection but also accelerates recovery through its anti-inflammatory effects. Dexamethasone, as a commonly used anti-inflammatory and antimicrobial drug, is widely applied in the clinical treatment of chronic suppurative otitis media. It effectively improves clinical symptoms by inhibiting the aggregation of inflammatory cells and improving cell-mediated immune responses, while also enhancing vascular tone, thereby meeting clinical treatment needs.17–19 The results show that dexamethasone has a significant effect on improving inflammatory factor levels and hearing. However, some patients showed insufficient improvement in symptoms after receiving dexamethasone treatment, which may be related to the severity of the condition or the weaker inhibitory effect of dexamethasone on certain bacterial strains. Therefore, to compensate for the limitations of single-drug therapy, combination therapy is widely used in clinical practice to further enhance treatment efficacy, providing theoretical support for the use of ofloxacin combined with dexamethasone.20,21
The hearing assessment results indicated that the treatment group experienced significantly better hearing improvement than the control group, especially in terms of the improvement in air-bone gap (ABG). Additionally, the treatment group had significantly shorter times for resolving ossicular congestion and healing tympanic membrane perforation compared to the control group, further confirming the advantage of combination therapy in controlling ear inflammation and promoting hearing recovery. Ofloxacin effectively reduced the inflammatory burden in the middle ear through its antibacterial action, while the anti-inflammatory effect of dexamethasone alleviated ear edema and promoted tympanic membrane healing, thus accelerating hearing recovery and alleviating clinical symptoms.22–24 These results are consistent with research by certain scholars in China, indicating that ofloxacin has a strong antibacterial effect against multiple bacteria, while dexamethasone effectively reduces inflammation. The combination of these two agents provides an ideal clinical outcome for the treatment of chronic suppurative otitis media.
The treatment group also showed significantly better pathogen clearance rates compared to the control group, and the levels of inflammatory factors (such as IL-8, TGF-β1, and PCT) were significantly lower than those in the control group. This indicates that the combination of ofloxacin and dexamethasone not only has advantages in antibacterial treatment but also plays an important role in controlling inflammation. IL-8, TGF-β1, and PCT are markers closely associated with inflammatory responses, and the significant decrease in these factors after treatment suggests that inflammation was effectively suppressed, further validating the anti-inflammatory effect of dexamethasone. The combination of antibiotic treatment can more comprehensively improve the pathophysiological state of patients with chronic suppurative otitis media and reduce the damage caused by inflammation to ear tissue. Specifically, IL-8 is a cytokine produced by macrophages and other cells. When epithelial cells and fibroblasts are stimulated, IL-8 is released, activating neutrophils and exacerbating local inflammation. TGF-β1 is a multifunctional cytokine that plays an important regulatory role in wound healing and cell proliferation. During an inflammatory response, TGF-β1 is released in large amounts to promote the repair process. PCT, an important marker of bacterial infection, significantly increases in the blood of infected patients, and its level changes can be used to determine the presence of bacterial infection.25–27 Therefore, the combined effect of antibacterial and anti-inflammatory actions in the treatment group helps effectively control the inflammatory response in chronic suppurative otitis media and promote recovery.
Moreover, during the treatment process, the incidence of adverse reactions in the treatment group was significantly lower than in the control group. Although steroid drugs (such as dexamethasone) may cause some side effects, such as gastrointestinal discomfort and weight gain, no significant adverse reactions were observed in the combined treatment in this study. This suggests that when ofloxacin and dexamethasone are used together, they can achieve therapeutic effects while maintaining good safety. This result implies that the combination of ofloxacin and dexamethasone could be used in clinical practice to treat chronic suppurative otitis media, improving efficacy while controlling the occurrence of adverse reactions.28–30 Furthermore, after a six-month follow-up, the recurrence rate of patients in the treatment group was significantly lower than in the control group. The recurrence rate of chronic suppurative otitis media has always been a challenge in treatment, especially with the influence of resistant strains, as traditional treatments often struggle to maintain long-term efficacy. The treatment regimen combining ofloxacin and dexamethasone not only excels in controlling acute symptoms but also helps reduce the recurrence of infections. This is likely related to ofloxacin’s effective antibacterial action against a broad range of pathogens and dexamethasone’s suppression of immune responses. Their synergistic effects significantly reduce the risk of disease recurrence.
While this study demonstrates promising clinical benefits of combined ofloxacin and dexamethasone therapy for chronic suppurative otitis media (CSOM), several methodological and interpretive limitations warrant discussion. First, the single-center retrospective design, while pragmatic for preliminary investigation, introduces inherent selection biases and limits generalizability. The modest sample size (n=130) further constrains statistical power, necessitating future multi-center prospective trials with robust sample size justification to validate efficacy and safety across diverse populations. Second, the 6-month follow-up period, though compliant with guideline recommendations for short-term assessment, may insufficiently capture late recurrences characteristic of CSOM’s chronic course. Extended longitudinal monitoring (≥12 months) is critical to evaluate sustained therapeutic effects and recurrence patterns. Third, the absence of formal power analysis and confidence interval reporting tempers the precision of effect estimates, underscoring the need for enhanced methodological rigor in subsequent studies. Additionally, while clinical symptom improvement was prioritized, deeper exploration of the synergistic mechanisms underlying this combination therapy—particularly immunomodulatory pathways—could provide mechanistic insights to optimize treatment strategies. Finally, incorporation of patient-reported outcomes and cost-effectiveness analyses would holistically evaluate treatment value, informing evidence-based clinical guidelines and healthcare resource allocation. Collectively, these advancements would significantly enhance the translational impact of these findings, bridging current knowledge gaps in CSOM management.
Conclusion
This retrospective study demonstrates significant clinical improvements with combination ofloxacin and dexamethasone therapy for chronic suppurative otitis media (CSOM), including enhanced pathogen clearance and reduced inflammatory markers. While these findings align with pharmacological principles and reinforce the regimen’s potential advantages over monotherapy, several limitations must be acknowledged. The single-center retrospective design introduces inherent selection and information biases, and the 6-month follow-up may underestimate long-term recurrence rates given CSOM’s chronic nature. Despite these limitations, our results provide valuable preliminary evidence supporting the efficacy of this combination therapy. To establish definitive clinical recommendations, however, validation through multi-center randomized controlled trials (RCTs) with extended follow-up (≥12 months) is critical. Such trials should incorporate rigorous blinding, standardized outcome measures, and cost-effectiveness analyses to address current methodological gaps. This study thus serves as a foundational step toward optimizing therapeutic strategies for this challenging chronic condition, highlighting the need for continued research to translate these promising observations into actionable clinical guidelines.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bhutta MF, Leach AJ, Brennan-Jones CG. Chronic suppurative otitis media. Lancet. 2024;403(10441):2339–2348. doi:10.1016/S0140-6736(24)00259-9
2. Mittal R, Lisi CV, Gerring R, et al. Current concepts in the pathogenesis and treatment of chronic suppurative otitis media. J Med Microbiol. 2015;64(10):1103–1116. doi:10.1099/jmm.0.000155
3. Wintermeyer SM, Nahata MC. Chronic suppurative otitis media. Ann Pharmacother. 1994;28(9):1089–1099. doi:10.1177/106002809402800915
4. Brennan-Jones CG, Head K, Chong L-Y, et al. Topical antibiotics for chronic suppurative otitis media. Cochrane Database Syst Rev. 2020;1(1):Cd013051. doi:10.1002/14651858.CD013051.pub2
5. Chong LY, Head K, Webster KE, et al. Topical versus systemic antibiotics for chronic suppurative otitis media. Cochrane Database Syst Rev. 2021;2(2):Cd013053. doi:10.1002/14651858.CD013053.pub2
6. Maharjan R, Sigdel B, Nepali R. Bacteriological profile and drug susceptibility in mucosal type chronic suppurative otitis media. J Nepal Health Res Counc. 2023;21(1):23–28. doi:10.33314/jnhrc.v21i1.4137
7. Phillips JS, Yung MW. A systematic review of patient-reported outcome measures for chronic suppurative otitis media. Laryngoscope. 2016;126(6):1458–1463. doi:10.1002/lary.25657
8. Diao T, Zhang L, Liu Y, et al. [Analysis of bacterial infection and drug sensitivity in patients with chronic suppurative otitis media]. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2021;35(10):870–874. doi:10.13201/j.issn.2096-7993.2021.10.002
9. Xia A, Thai A, Cao Z, et al. Chronic suppurative otitis media causes macrophage-associated sensorineural hearing loss. J Neuroinflammation. 2022;19(1):224. doi:10.1186/s12974-022-02585-w
10. Nelson JD. Chronic suppurative otitis media. Pediatr Infect Dis J. 1988;7(6):446–448. doi:10.1097/00006454-198806000-00033
11. Dhingra S, Vir D, Bakshi J, et al. Mapping of audiometric analysis with microbiological findings in patients with chronic suppurative otitis media (CSOM): a neglected clinical manifestation. Crit Rev Clin Lab Sci. 2023;60(3):212–232. doi:10.1080/10408363.2022.2158173
12. Morris P. Chronic suppurative otitis media. BMJ Clin Evid. 2012;2012.
13. Master A, Wilkinson E, Wagner R. Management of chronic suppurative otitis media and otosclerosis in developing countries. Otolaryngol Clin North Am. 2018;51(3):593–605. doi:10.1016/j.otc.2018.01.017
14. Verhoeff M, van der Veen EL, Rovers MM, et al. Chronic suppurative otitis media: a review. Int J Pediatr Otorhinolaryngol. 2006;70(1):1–12. doi:10.1016/j.ijporl.2005.08.021
15. Acuin J. Chronic suppurative otitis media. Clin Evid. 2006(15):772–787.
16. Acuin J. Chronic suppurative otitis media. Clin Evid. 2004(12):710–729.
17. Alper CM, Dohar JE, Gulhan M, et al. Treatment of chronic suppurative otitis media with topical tobramycin and dexamethasone. Arch Otolaryngol Head Neck Surg. 2000;126(2):165–173. doi:10.1001/archotol.126.2.165
18. Kutz JW, Roland PS, Lee KH. Ciprofloxacin 0.3% + dexamethasone 0.1% for the treatment for otitis media. Expert Opin Pharmacother. 2013;14(17):2399–2405. doi:10.1517/14656566.2013.844789
19. Leach A, Wood Y, Gadil E, et al. Topical ciprofloxin versus topical framycetin-gramicidin-dexamethasone in Australian aboriginal children with recently treated chronic suppurative otitis media: a randomized controlled trial. Pediatr Infect Dis J. 2008;27(8):692–698. doi:10.1097/INF.0b013e31816fca9d
20. Zhu YL, Li WX, Li J. [Screening for effective antibiotics in chronic suppurative otitis media]. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2017;31(16):1243–1246. doi:10.13201/j.issn.1001-1781.2017.16.06
21. Suzuki K, Nishimura T, Baba S, et al. Topical ofloxacin for chronic suppurative otitis media and acute exacerbation of chronic otitis media: optimum duration of treatment. Otol Neurotol. 2003;24(3):447–452. doi:10.1097/00129492-200305000-00015
22. Bluestone CD. Efficacy of ofloxacin and other ototopical preparations for chronic suppurative otitis media in children. Pediatr Infect Dis J. 2001;20(1):
23. Adoga AA, Bakari A, Afolabi OA, et al. Bacterial isolates in chronic suppurative otitis media: a changing pattern? Niger J Med. 2011;20(1):96–98.
24. Khomtchouk KM, Joseph LI, Khomtchouk BB, et al. Treatment with a neutrophil elastase inhibitor and ofloxacin reduces P. aeruginosa burden in a mouse model of chronic suppurative otitis media. NPJ Biofilms Microbiomes. 2021;7(1):31. doi:10.1038/s41522-021-00200-z
25. Roland PS. Chronic suppurative otitis media: a clinical overview. Ear Nose Throat J. 2002;81(8 Suppl 1):8–10.
26. Acuin J, Smith A, Mackenzie I. Interventions for chronic suppurative otitis media. Cochrane Database Syst Rev. 2000;2:Cd000473. doi:10.1002/14651858.CD000473
27. Thai A, Aaron KA, Kaufman AC, et al. Long-term health utilization and outcomes in chronic suppurative otitis media. Otolaryngol Head Neck Surg. 2022;167(2):341–349. doi:10.1177/01945998211050626
28. Mujahid ZA, Palal SS, Gopan G, et al. Biofilm producing organisms and their antibiotic sensitivity in chronic suppurative otitis media: a cross-sectional study. Indian J Otolaryngol Head Neck Surg. 2024;76(5):3886–3894. doi:10.1007/s12070-024-04737-1
29. Abes G, Espallardo N, Tong M, et al. A systematic review of the effectiveness of ofloxaxin otic solution for the treatment of suppurative otitis media. ORL J Otorhinolaryngol Relat Spec. 2003;65(2):106–116. doi:10.1159/000070775
30. Panchasara A, Singh A, Mandavia D, et al. Efficacy and safety of ofloxacin and its combination with dexamethasone in chronic suppurative otitis media. A randomised, double blind, parallel group, comparative study. Acta Otorhinolaryngol Ital. 2015;35(1):39–44.
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


