Back to Journals » Clinical Ophthalmology » Volume 19
Anatomic and Functional Outcomes of Lamellar Macular Hole and Epiretinal Membrane Foveoschisis Surgery: Predictive Factors and Associated Complications — A Retrospective Interventional Study
Authors Er-reguyeg Y , Doukkali S, Hébert M , You EL, Bourgault S, Caissie M, Tourville É, Dirani A
Received 6 October 2024
Accepted for publication 28 March 2025
Published 24 April 2025 Volume 2025:19 Pages 1365—1376
DOI https://doi.org/10.2147/OPTH.S499493
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Yosra Er-reguyeg,1 Sihame Doukkali,2 Mélanie Hébert,2 Eunice Linh You,2 Serge Bourgault,2 Mathieu Caissie,2 Éric Tourville,2 Ali Dirani2
1Faculty of Medicine, Laval University, Quebec City, Quebec, Canada; 2Department of Ophthalmology, Hospital Saint-Sacrement, Laval University, Quebec City, Quebec, Canada
Correspondence: Ali Dirani, Hôpital du Saint-Sacrement, 1050 Chemin Sainte-Foy, Québec, QC, D2-44C, Canada, Tel +1 418 525 4444, Email [email protected]
Purpose: To analyze the anatomic and functional outcomes of lamellar macular hole (LMH) and epiretinal membrane foveoschisis (ERMF) surgery.
Patients and Methods: This is a retrospective interventional cohort study of ninety patients with unilateral idiopathic LMH or ERMF who underwent pars plana vitrectomy (PPV) with membrane peeling between 2014 and 2021. We evaluated the anatomic and functional success of PPV with membrane peeling for treating LMH and ERMF, compared surgical outcomes between the two entities, and identified predictive factors for anatomical and functional success. Primary outcomes included final postoperative best-corrected visual acuity (BCVA) and LMH or ERMF closure. Variables associated with final BCVA were assessed using a multiple linear regression model.
Results: 51 subjects presented with ERMF, while 39 presented with LMH. LMH or ERMF closure occurred in 80 cases. LMH cases had a lower rate of closure (LMH closure rate: 76.9%, vs ERMF closure rate: 98.0%, p=0.002) and were more at risk of developing a postoperative macular hole (p=0.008). A significant difference was observed between median [Q1, Q3] preoperative BCVA (0.42 [0.26, 0.61] logMAR) and final BCVA (0.31 [0.14, 0.48] logMAR, p=0.024). BCVA varied from 0.52 [0.40, 0.74] logMAR preoperatively to 0.36 [0.30, 0.66] logMAR postoperatively in the LMH subgroup (p=0.060), and from 0.32 [0.20, 0.54] logMAR preoperatively to 0.22 [0.10, 0.40] logMAR postoperatively in the ERMF subgroup (p=0.146). LMH without epiretinal proliferation (β=0.194, p=0.040) was associated with worse final BCVA in multivariate analysis.
Conclusion: Results support the effectiveness of PPV as a treatment for LMH. LMHs had worse anatomic outcomes than ERMFs.
Keywords: epiretinal membrane foveoschisis, pars plana vitrectomy, epiretinal proliferation peeling, internal limiting membrane peeling, vitreoretinal surgery, retina
Introduction
Lamellar macular hole (LMH) and epiretinal membrane foveoschisis (EMRF) are characterized by a partial-thickness defect in the inner layers of the fovea. Since J.D. Gass first documented LMH in 1975, its diagnostic criteria and classification changed considerably.1 Established as the gold standard in LMH assessment, the emergence of spectral domain optical coherence tomography (SD-OCT) led to new diagnostic criteria including an irregular foveal contour, a break in the inner fovea, dehiscence of the inner foveal retina from the outer retina and absence of a full-thickness foveal defect with preservation of foveal photoreceptors.2
Govetto et al later divided LMHs into “tractional” and “degenerative” subtypes.3 Recently, Hubschman et al separated lesions previously called LMH into two entities: ERMF and LMH.4 ERMF, which is comparable to what was previously considered as tractional LMH, occurs in the presence of a contractile epiretinal membrane (ERM) and foveoschisis at the level of the Henle’s fiber layer (HFL), two mandatory diagnosis criteria. Three optional criteria were also suggested: the presence of microcystoid spaces in the inner nuclear layer (INL), retinal thickening, and retinal wrinkling.
In contrast to ERMF, LMH, which is comparable to what was previously considered degenerative LMH, is characterized by the presence of an irregular foveal contour, a foveal cavity with undermined edges, and at least one other sign evoking loss of foveal tissue. Associated pathological changes can include epiretinal proliferation (ERP), foveal bump, and ellipsoid zone (EZ) disruption.
Recent findings suggest that both originate from a tractional event and that the ERP often found in LMH may be a repair process derived from a tractional impairment to the foveolar Müller cells.5–9 While some cases may remain stable, others may evolve into a degenerative configuration over time.5–9 Indeed, Su et al’s findings indicate that in LMHs displaying ERP, epiretinal traction is commonly observed.10
Disagreement remains regarding the surgical treatment of LMH and ERMF involving integral membrane peeling, which refers to the complete removal of both the epiretinal tissue and the internal limiting membrane (ILM). While surgery may prevent visual acuity (VA) loss and further deterioration of the foveal profile, some studies report outcomes that vary based on the morphological features of the inner retinal lesion.11,12 The fovea-sparing and flap embedding techniques are new methods recently developed to address complications commonly associated with standard integral membrane peeling, showing promising postoperative outcomes. However, standard peeling remains widely used in LMH and ERMF surgery.13,14 Thus, we aimed to assess the anatomic and functional outcomes of LMH and ERMF surgery, assess which factors best predict final VA, and document postoperative complications.
Materials and Methods
Study Design and Population
This retrospective interventional study includes medical records of patients with LMH and ERMF who underwent PPV between 2014 and 2021 at the Centre Hospitalier Universitaire (CHU) de Québec – Université Laval. Approval from the Ethics Committee was obtained, and the study adhered to the tenets of the Declaration of Helsinki. Patient consent for the review of medical records was not required considering the retrospective nature of the study involving de-identified data, and all patient information was handled in accordance with institutional data protection standards. We excluded eyes with macular pseudohole (MPH), full thickness macular hole (MH), history of ocular trauma leading to LMH or ERMF formation, advanced glaucoma, high myopia (HM) (>4 diopters), history of retinal detachment (RD), active uveitis, wet age-related macular degeneration (AMD), active proliferative diabetic retinopathy (PDR), and cases associated with other active maculopathies. Patients with less than one month of postoperative follow-up and patients with missing preoperative or postoperative best-corrected VA (BCVA) and OCT data were also excluded.
LMH and ERMF SD-OCT Diagnosis
We selected LMH and ERMF cases based on the OCT diagnostic criteria of Witkin et al, as adopted by the International Vitreomacular Traction Study Group.15 Anatomic characteristics were collected using several high-quality SD-OCT scans (Cirrus HD-OCT; Carl Zeiss Meditec, Inc). Patients were then further classified into ERMFs and LMHs according to Hubschman et al’s consensus.4 We analyzed the following parameters using Cirrus’ OCT 512×128 macular cube scan: preoperative central foveal thickness (CFT), average foveal thickness (AFT), base diameter and apex diameter of the hole, presence of ERM and ERP, EZ disruption, external limiting membrane (ELM) disruption, presence of intraretinal cysts (IRCs), postoperative MH formation (stages 1, 2, 3 and 4), and foveal profile evolution. As previously described, LMH or ERMF closure was defined as a reconstituted foveal contour with no retinal splitting.16 The preoperative CFT and AFT were measured automatically by Zeiss’ OCT software. Base and apex diameters were measured manually using Zeiss’ OCT software caliper. Since lamellar macular defects often exhibit asymmetry and irregularity, measurements of base diameter were made to the largest horizontal extent of the intraretinal split, as performed in similar studies.17,18
Surgical Procedure
All patients underwent 25-gauge PPV by one of five fellowship-trained vitreoretinal surgeons. ERM peeling with or without ILM peeling was performed. Phacoemulsification was combined with primary PPV in cases of clinically significant cataract at the preoperative assessment which either could be expected to compromise visualization intraoperatively or visual outcomes postoperatively, based on the surgeon’s discretion. A conventional integral peeling technique was performed on all patients, consisting of removing the epiretinal membrane or epiretinal proliferation if present from the surface of the retina using staining agents. Indocyanine green or methylene blue were used as staining agents based on surgeons’ preference. ILM peeling and restaining were done following the removal of ERM or ERP. In exceptional cases where the ILM could not be visualized after extensive ERM peeling, the ILM was not removed. Vitrectomy was completed with a 360° inspection of the peripheral retina using scleral depression, followed by complete fluid-air exchange with or without gas tamponade at the surgeon’s discretion. The gases used for tamponade were either air, sulfur hexafluoride (SF6), or perfluoropropane (C3F) for larger LMHs or ERMFs, chosen at the discretion of the operating surgeon. Sclerotomies were sutured if they were not self-sealing and leakage occurred. Patients who received gas tamponade (SF6 and C3F8) were instructed to maintain prone positioning following surgically.
Statistical Analysis
We conducted the statistical analysis using SPSS Statistics Version 26 (IBM, Armonk, NY, USA). The normality of continuous variables was tested with Q-Q plots and Shapiro–Wilk tests. Means and standard deviations were used to present normally distributed continuous variables, medians and quartiles [first quartile (Q1), third quartile (Q3)] for non-normally distributed continuous variables, and percentages for categorical variables. We compared preoperative characteristics and outcomes between patients using independent Student’s t-tests for normally distributed continuous variables, Mann–Whitney U-tests for non-normally distributed continuous variables, Pearson chi-square tests or Fisher’s exact test when the sample size was inferior to 5 for categorical variables, and Wilcoxon signed-rank tests for paired comparisons of preoperative and postoperative continuous variables.
To analyze which variables were most predictive of final postoperative BCVA, we built a multiple linear regression model using backwards elimination with an F-to-remove at 0.2. Variables that we considered for inclusion were age, sex, preoperative BCVA, use of phaco-vitrectomy, type of peel (eg, ERM peel, ILM peel, and/or ERP peel), tamponade agent, staining, LMH or ERMF closure, preoperative central foveal thickness, lesion type (ie, ERMF or LMH), LMH or ERMF base and apex diameters, preoperative OCT characteristics if they were not significantly collinear with lesion type (ie, ERP, EZ disruption, ELM disruption, IRC, vitreomacular traction (VMT), partial/complete posterior vitreous detachment (PVD), cystoid macular edema (CME), and development of postoperative MH). Unstandardized coefficients with 95% confidence intervals (CI) and standardized coefficients were produced for all variables included in the final model. Baseline demographics including preoperative BCVA, age, and sex were retained in the final model to adjust for these variables. The type of lesion (ERMF vs LMH) and LMH or ERMF closure were included to assess the impact of these anatomical characteristics on final BCVA. Additionally, we favored the use of phaco-vitrectomy over other lens status variables and preoperative central foveal thickness over other preoperative thickness parameters (ie, minimal foveal thickness, average foveal thickness) due to their stronger association with final BCVA and to avoid collinearity in the regression model. Statistical significance was set at a p-value of <0.05.
Results
Characteristics of the Studied Population
This study included 90 patients. Out of them, 57 (63.3%) were women. The mean age at surgery was 71 ± 8 years, with the mean age not being significantly different between men (73 ± 10 years) and women (71 ± 8 years, p=0.258). Of these, 51 (56.7%) subjects presented with ERMF, while 39 (43.3%) presented with LMH. Comparisons of baseline characteristics between both types of lesions are presented in Table 1. Out of the 39 patients presenting LMH, 28 (71.8%) were subject to an ERP and ILM peeling, 6 (15.4%) had an ERP peeling, and 5 (12.8%) had an ILM peeling. The subset of LMH patients who underwent solely an ILM peeling did not have ERP at presentation. Out of the subjects presenting ERMF, 46 (90.2%) had an ERM and an ILM peeling, while 5 (9.8%) had only an ERM peeling. In the entire cohort, 58 (64.4%) subjects received SF6, 30 (33.3%) received air, and 2 (2.2%) received C3F8. Concomitant phacoemulsification and intraocular lens (IOL) implantation were performed in 24 (26.7%) subjects. The median [Q1, Q3] follow-up period was 14 [6, 29] months.
 |
Table 1 Comparison of Preoperative Clinical and Optical Coherence Tomography Findings of ERMF and LMH Patients |
Anatomic Outcomes
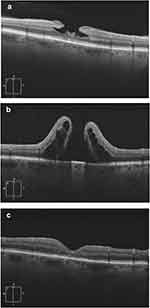
Closure status was achieved in 80 (88.9%) cases. Closure rates differed between LMH (n=30, 76.9% of LMH cases) and ERMF (n=50, 98.0% of ERMF cases), with the ERMF closure rate being significantly higher than LMH (p=0.002). Figure 1 depicts the preoperative and postoperative OCT images of a patient who achieved complete ERMF closure, compared to a patient presenting LMH who did not achieve closure. The characteristics of patients who did or did not achieve closure are summarized in Table 2 for LMH and ERMF patients separately as well as for the overall cohort. In the LMH subgroup, the proportion of patients with ERP that did not achieve closure (n=9, 100% of LMH non-closure cases) was significantly higher than the proportion of patients with ERP that achieved closure (n=18, 60% of LMH closure cases, p=0.023). No variable was predictive of ERMF closure, but statistical analysis was limited given that only one patient in the ERMF subgroup did not achieve closure.
 |
Table 2 Comparison of Patient Characteristics Between Cases Which Did and Did Not Lead to Lamellar Macular Hole (LMH) Closure |
When considering the overall cohort, 7 out of the 10 patients who did not achieve closure were men (70.0%, p=0.020). Subjects who did not achieve closure had significantly worse preoperative BCVA (closure group: 0.40 [0.26, 0.56] vs non-closure group: 0.66 [0.47, 0.80], p=0.009). No significant association was found between closure and the type of tamponade agents. atients who achieved closure had higher CFT values, although not reaching statistical significance (closure group: 354 [299, 413] μm, non-closure group: 295 [271, 360] μm, p=0.065). The preoperative apex diameter was smaller in patients who achieved closure (421 [314, 608] μm) than in the ones who did not (632 [385, 827] μm, p=0.052). Age, preoperative base diameter, preoperative presence of ELM disruption, EZ disruption, vitreomacular traction, intraretinal retinal cysts, and PVD were not significantly associated with LMH or ERMF closure.
In addition to restoring foveal profile, surgery significantly decreased the number of patients presenting IRCs, which went from 60 (66.7%) preoperatively to 32 (35.6%) postoperatively (p<0.001). The number of patients with EZ disruption who achieved restoration of EZ integrity postoperatively was 6 (representing a decrease of 20.7%) (p=0.238), while the number of patients presenting CME decreased from 6 (6.6%) to 5 (5.6%) postoperatively (p=1.000).
Functional Outcomes
Surgery significantly improved BCVA from a preoperative median [Q1, Q3] of (0.42 [0.26, 0.61]) (Snellen: 20/50) to a final postoperative BCVA of (0.31 [0.14, 0.48]) (Snellen: 20/40) (p=0.024). When considering solely the LMH cases, BCVA varied from 0.52 [0.40, 0.74] logMAR preoperatively to 0.36 [0.30, 0.66] logMAR postoperatively (p=0.060). When considering the ERMF subgroup, BCVA changed from 0.32 [0.20, 0.54] logMAR preoperatively to 0.22 [0.10, 0.40] logMAR postoperatively (p=0.146). Table 3 summarizes the factors associated with final BCVA when adjusted for preoperative BCVA in a multiple linear regression model. Variables not included in the model were rejected by the backward elimination method.
 |
Table 3 Multiple Linear Regression Model for Final Best-Corrected Visual Acuity (BCVA) Following Surgery for Lamellar Macular Hole (LMH) and Epiretinal Membrane Foveoschisis (ERMF) in 90 Patients |
Multiple linear regression analysis indicated that, placed in order of magnitude of impact, masculine sex (β=0.358, p<0.001) and LMH with no ERP (β=0.194, p=0.040) were significant predictors negatively affecting final postoperative BCVA. On the other hand, phaco-vitrectomy (β=−0.343, p<0.001) was a significant predictor positively affecting final postoperative BCVA. Considering the opposing effects of male sex and phaco-vitrectomy on final BCVA in the regression model, the proportion of females undergoing phaco-vitrectomy was compared to the proportion who did not to eliminate possible confounding bias. We found that the proportion of female patients undergoing simultaneous cataract surgery (n=12, 50.0%) was not significantly higher than the proportion of females patients that underwent vitrectomy as a standalone procedure (n=45, 68.1%, p=0.113).
Lesion type, whether LMH or ERMF, was not a significant predictor of postoperative BCVA (p=0.665), nor was closure status (p=0.671). All other variables included in the model were not significantly associated with final postoperative BCVA.
Postoperative Complications
Preoperatively, 61 (67.0%) patients were phakic, among whom 24 (26.7%) underwent phaco-vitrectomy. The number of subjects who underwent postoperative cataract surgery was of 22 (24.4%), increasing the number of pseudophakic patients from 53 (58.9%) immediately after vitrectomy to 75 (83.3%) at final follow-up. The number of patients who underwent postoperative cataract surgery did not differ between the LMH (n=10, 25.6% of LMH patients) and the ERMF subgroups (n=12, 23.5% of ERMF patients, p=0.817).
After surgery, one patient (1.1%) developed uveitis. MH occurred in 5 (5.5%) cases postoperatively. Figure 2 depicts preoperative and postoperative OCT images of a patient who experienced an MH postoperatively and subsequently underwent a secondary vitrectomy. Table 4 summarizes the characteristics of the patients who experienced postoperative MH development.
 |
Table 4 Clinical Data and Optical Coherence Tomography (OCT) Characteristics of Postoperative Macular Hole Cases |
Postoperative MH was associated with LMH as all 5 patients who developed the complication were LMH subjects (p=0.008). Sex was also associated with postoperative MH as 4 (80.0%) subjects who developed MH were males (p=0.036). Patients with postoperative MH had a significantly worse preoperative median [Q1, Q3] BCVA (postoperative MH group: 0.76 [0.45, 1.11], no postoperative MH group: 0.42 [0.26, 0.56], p=0.033). Other parameters were not significantly associated with postoperative MH.
Discussion
The purpose of this study was to analyze the functional and anatomic outcomes of LMH and ERMF surgery using the latest classification.4 Results indicate that LMH and ERMF surgery is an effective treatment, resulting in high overall rates of closure (88.9%) and improved visual outcomes (median gain of 1 line on the Snellen chart), which confirms previous meta-analysis findings.19 Comparison of ERMF and LMH surgical outcomes remains a topic of discussion in the literature. Previous studies showed heterogenous results; with some indicating a postoperative discrepancy between the two entities and some not reporting differences.12,19–24 Hubschman et al’s reclassification implied that LMH and ERMF might have different surgical outcomes as they may follow distinct pathological pathways.4 Thus, the present study also aimed at investigating that matter. Our findings indicated that ERMF and LMH exhibit different surgical outcomes, with LMH cases having lower closure rates than ERMF and a higher risk of postoperative macular hole formation. However, surgery had positive functional outcomes for both ERMF and LMH as lesion type was not significantly associated with postoperative final BCVA when adjusted for confounding variables.
Venkatesh et al associated the presence of ERP in LMH with lower visual acuity, larger hole size, thinner residual retinal tissue, larger EZ disruption, and more inner segment/outer segment (IS/OS) defects.23 The results of the present study align with the latter, as cases of LMH presenting with ERP had a significantly higher non-closure rate, and comprised all cases which developed postoperative MH. LMH patients were older and waited longer to undergo surgery than ERMF patients, which may suggest that LMH could be a subsequent manifestation or a more advanced stage of the condition, as proposed by Lee et al.7 Although it remains unclear what causes degeneration and tissue loss in some cases, recent findings by Crincoli et al have identified key predictive factors for LMH progression, including EZ disruption, early volumetric tissue loss, vitreopapillary adhesion, and reduced parafoveal vascular density.25 Omoto et al reported that the presence of ERP was not significantly related to postoperative VA, which contrasts with the findings of the current study.20 When adjusted for preoperative BCVA, LMH patients without ERP had worse final BCVA in the present report. These LMH cases had solely undergone an ILM peeling.
Patients with higher CFT values had better anatomic results, as they presented a milder loss of foveal tissue. While not reaching statistical significance, EZ disruption was related to worse final postoperative BCVA, and subjects who did not achieve LMH closure tended to have larger base diameter and apex diameter of the LMH. These findings emphasize the importance of evaluating anatomic foveal features before proceeding with surgery.
Moreover, a significant worsening predictor of both functional and anatomic outcomes was masculine sex. Age at presentation was not significantly different between sexes, and the ratio of males and females did not significantly differ between LMH and ERMF.
Haave et al suggested that if cataract is present, combining phaco-vitrectomy during surgical intervention could optimize functional outcomes.26 Coassin et al conducted a retrospective study of patients who underwent surgical treatment for symptomatic LMH and ERMF where pseudophakic patients exhibited better outcomes than phakic patients.16 The current study’s results are consistent with those findings as postoperative BCVA was significantly improved with phaco-vitrectomy. Haave et al further reported that gas tamponade should be avoided as patients who were exclusively administered a balanced salt solution (BSS) achieved the most favorable outcomes.26 However, no significant relationship was found between air, SF6 or C3F8 tamponade and postoperative outcomes in the present study. These results suggest that similarly to what was recently reported for idiopathic macular holes in Dervenis et al’s systematic review and meta-analysis, the choice of tamponade does not affect visual outcomes or closure rates in lamellar macular hole surgery.27
All postoperative FTMH cases were LMH cases. Other studies have also reported the occurrence of postoperative FTMH in LMH cases, as the ERP often present in such cases is more challenging to peel.12,28 Thus, the fovea sparing and the flap embedding peeling techniques have emerged as alternatives for treating LMH.13,29 The rationale behind them is to avoid peeling the edges of the LMH which are oftentimes connected to the ERP. Such studies have reported positive outcomes and no postoperative FTMH, but further comparatives studies are warranted to establish the superiority of a peeling technique over the other.13,29
Limitations of this study include its retrospective nature. The surgery was performed by multiple surgeons, which might induce heterogeneity in outcomes. However, we accounted for the possible confounders related to surgical technique in our analysis of anatomic and functional outcomes. Indeed, there were no significant differences in the proportions of tamponade and staining agents used between patients who achieved LMH closure and those who did not. These variables were also adjusted for in our multiple linear regression analysis, along with confounders inherent to the retrospective nature of the study, such as follow-up period duration. Another limitation in the interpretation of our results is related to the associations between male sex and functional and anatomic outcomes, as they are not reported by the literature and should thus be interpreted cautiously. The study’s strengths include its relatively large cohort size (n=90), making it one of the largest using the latest classification of LMH.4 The study also examined how the lesion subtype (ie, LMH or ERMF) and the specific membranes peeled affected visual and anatomical outcomes. Particularly, it highlighted that LMHs without ERP (thus solely undergoing ILM peeling) were predictive of worse final BCVA. Furthermore, the study benefits from a relatively long median follow-up period, enabling comprehensive evaluation of surgical outcomes over an extended duration. Finally, various controversial factors that could influence surgical outcomes were examined, providing valuable insights of the key considerations for evaluating the likelihood of surgical success.
Conclusion
In conclusion, this study sheds light on the characteristics and outcomes of patients undergoing surgery for LMH and ERMF. It further supports the effectiveness of primary vitrectomy as a viable treatment option for LMH and ERMF patients. LMH and ERMF patients had significantly different anatomic characteristics at presentation. Although functional outcomes did not significantly differ between LMH and ERMF, anatomic outcomes were worse in LMH. Our findings suggest that the presence of ERP in LMH should be particularly acknowledged in terms of functional and anatomical improvements. Therefore, specific considerations should be given to OCT biomarkers and patients presenting LMH to optimize surgical outcomes and limit postoperative complications. Future studies should focus on validating such predictive biomarkers reported in the current literature in larger, prospective cohorts to improve the clinical management of LMH and ERMF.
Acknowledgments
The patients included in this study for enabling advancement in the field. This paper has been uploaded to medRxiv as a preprint: https://www.medrxiv.org/content/10.1101/2025.01.09.25320019v1.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Gass JD. Lamellar macular hole A. Complication of cystoid macular edema after cataract extraction. Arch Ophthalmol. 1976;94(5):231–250. doi:10.1001/archopht.1976.03910030391008
2. Witkin AJ, Ko TH, Fujimoto JG, et al. Redefining lamellar holes and the vitreomacular interface: an ultrahigh-resolution optical coherence tomography study. Ophthalmology. 2006;113(3):388–397. doi:10.1016/j.ophtha.2005.10.047
3. Govetto A, Dacquay Y, Farajzadeh M, et al. Lamellar macular hole: two distinct clinical entities? Am J Ophthalmol. 2016;164:99–109. doi:10.1016/j.ajo.2016.02.008
4. Hubschman JP, Govetto A, Spaide RF, et al. Optical coherence tomography-based consensus definition for lamellar macular hole. Br J Ophthalmol. 2020;104(12):1741. doi:10.1136/bjophthalmol-2019-315432
5. Hsia Y, Lee CY, Ho TC, Yang CH, Yang CM. The development and evolution of lamellar macular hole in highly myopic eyes. Eye. 2022. doi:10.1038/s41433-022-02086-3
6. Wu L, Evans T, Arevalo JF. Idiopathic macular telangiectasia type 2 (idiopathic juxtafoveolar retinal telangiectasis type 2A, Mac Tel 2). Surv Ophthalmol. 2013;58(6):536–559. doi:10.1016/j.survophthal.2012.11.007
7. Lee CY, Hsia Y, Yang CM. Formation and evolution of idiopathic lamellar macular hole-a pilot study. BMC Ophthalmol. 2022;22(1):432. doi:10.1186/s12886-022-02669-4
8. Compera D, Entchev E, Haritoglou C, et al. Lamellar hole–associated epiretinal proliferation in comparison to epiretinal membranes of macular pseudoholes. Am J Ophthalmol. 2015;160(2):373–384.e1. doi:10.1016/j.ajo.2015.05.010
9. Bringmann A, Unterlauft JD, Wiedemann R, Rehak M, Wiedemann P. Morphology of partial-thickness macular defects: presumed roles of Müller cells and tissue layer interfaces of low mechanical stability. Int J Retina Vitreous. 2020;6(1):28. doi:10.1186/s40942-020-00232-1
10. Su YT, Yang CM, Lai TT. Multimodel imaging evidence of traction component in lamellar macular hole with epiretinal proliferation. Ophthalmic Res. 2023;66(1):828–838. doi:10.1159/000530529
11. Parolini B, Schumann RG, Cereda MG, Haritoglou C, Pertile G. Lamellar macular hole: a clinicopathologic correlation of surgically excised epiretinal membranes. Invest Ophthalmol Vis Sci. 2011;52(12):9074–9083. doi:10.1167/iovs.11-8227
12. Ko J, Kim GA, Lee SC, et al. Surgical outcomes of lamellar macular holes with and without lamellar hole‐associated epiretinal proliferation. Acta Ophthalmol. 2017;95(3):e221–e226. doi:10.1111/aos.13245
13. Morescalchi F, Russo A, Gambicorti E, et al. Peeling of the internal limiting membrane with foveal sparing for treatment of degenerative lamellar macular hole. Retina. 2020;40(6):1087–1093. doi:10.1097/IAE.0000000000002559
14. Takahashi K, Morizane Y, Kimura S, et al. Results of lamellar macular hole-associated epiretinal proliferation embedding technique for the treatment of degenerative lamellar macular hole. Graefes Arch Clin Exp Ophthalmol. 2019;257(10):2147–2154. doi:10.1007/s00417-019-04425-9
15. Duker JS, Kaiser PK, Binder S, et al. The international vitreomacular traction study group classification of vitreomacular adhesion, traction, and macular hole. Ophthalmology. 2013;120(12):2611–2619. doi:10.1016/j.ophtha.2013.07.042
16. Coassin M, Mastrofilippo V, Stewart JM, et al. Lamellar macular holes: surgical outcome of 106 patients with long-term follow-up. Graefes Arch Clin Exp Ophthalmol. 2018;256:1265–1273. doi:10.1007/s00417-018-3989-6
17. Marques MF, Rodrigues S, Raimundo M, et al. Epiretinal proliferations associated with lamellar macular holes: clinical and surgical implications. Ophthalmologica. 2018;240(1):8–13. doi:10.1159/000486691
18. Parravano M, Oddone F, Boccassini B, et al. Functional and structural assessment of lamellar macular holes. Br J Ophthalmol. 2013;97(3):291. doi:10.1136/bjophthalmol-2011-301219
19. Parisi G, Fallico M, Maugeri A, et al. Primary vitrectomy for degenerative and tractional lamellar macular holes: a systematic review and meta-analysis. PLoS One. 2021;16(3):e0246667. doi:10.1371/journal.pone.0246667
20. Omoto T, Asahina Y, Zhou HP, et al. Visual outcomes and prognostic factors of vitrectomy for lamellar macular holes and epiretinal membrane foveoschisis. PLoS One. 2021;16(2):e0247509. doi:10.1371/journal.pone.0247509
21. Pang CE, Spaide RF, Freund KB. Epiretinal proliferation seen in association with lamellar macular holes: a distinct clinical entity. Retina. 2014;34(8):1513–1523. doi:10.1097/IAE.0000000000000163
22. Lai TT, Chen SN, Yang CM. Epiretinal proliferation in lamellar macular holes and full-thickness macular holes: clinical and surgical findings. Graefes Arch Clin Exp Ophthalmol Albrecht Von Graefes Arch Klin Exp Ophthalmol. 2016;254(4):629–638. doi:10.1007/s00417-015-3133-9
23. Venkatesh R, Pereira A, Jain K, Yadav NK. Structural and functional outcomes of surgery for lamellar macular holes with or without epimacular proliferations. J Ophthalmic Vis Res. 2022;17(1):42–50. doi:10.18502/jovr.v17i1.10169
24. Caretti L, La Gloria Valerio A, Verzola G, Badin G, Monterosso C, Daniele AR. Functional and morphological outcomes after surgery in lamellar macular holes versus epiretinal membrane foveoschisis. Eur J Ophthalmol. 2021;31(6):3294–3299. doi:10.1177/1120672120974287
25. Crincoli E, Parolini B, Catania F, et al. Prediction of functional and anatomic progression in lamellar macular holes. Ophthalmol Sci. 2024;4(6):100529. doi:10.1016/j.xops.2024.100529
26. Haave H, Petrovski BÉ, Zając M, et al. Outcomes from the retrospective multicenter cross-sectional study on lamellar macular hole surgery. Clin Ophthalmol Auckl NZ. 2022;16:1847–1860. doi:10.2147/OPTH.S351932
27. Dervenis N, Dervenis P, Sandinha T, Murphy DC, Steel DH. Intraocular tamponade choice with vitrectomy and internal limiting membrane peeling for idiopathic macular hole: a systematic review and meta-analysis. Ophthalmol Retina. 2022;6(6):457–468. PMID: 35144020. doi:10.1016/j.oret.2022.01.023
28. Figueroa MS, Govetto A, Steel DH, Sebag J, Virgili G, Hubschman JP. Pars plana vitrectomy for the treatment of tractional and degenerative lamellar macular holes: functional and anatomical results. Retina. 2019;39(11):2090–2098. doi:10.1097/IAE.0000000000002326
29. Kanai M, Sakimoto S, Takahashi S, et al. Embedding technique versus conventional internal limiting membrane peeling for lamellar macular holes with epiretinal proliferation. Ophthalmol Retina. 2023;7(1):44–51. doi:10.1016/j.oret.2022.07.009
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Internal Limiting Membrane Peeling and Gas Tamponade For Full-Thickness Macular Holes of Different Etiology – Is It Still Relevant?

Ruban A, Petrovski BÉ, Petrovski G, Lytvynchuk LM
Clinical Ophthalmology 2022, 16:3391-3404
Published Date: 13 October 2022
Economic Impact Analysis of Custom Pak® on Cataract and Vitreoretinal Surgery in the United States
Ayres BD, Gupta OP, Davis JS, Hahn R, Hsiao CW, Kara R, Di Simplicio S
ClinicoEconomics and Outcomes Research 2022, 14:715-730
Published Date: 10 November 2022