Back to Journals » Journal of Inflammation Research » Volume 17
Anti-Inflammatory Effects of Helminth-Derived Products: Potential Applications and Challenges in Diabetes Mellitus Management
Authors Zhu Y, Chen X, Zheng H, Ma Q, Chen K , Li H
Received 5 September 2024
Accepted for publication 15 December 2024
Published 28 December 2024 Volume 2024:17 Pages 11789—11812
DOI https://doi.org/10.2147/JIR.S493374
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Adam Bachstetter
Yunhuan Zhu,1 Xintong Chen,1 Hezheng Zheng,1 Qiman Ma,1 Keda Chen,1 Hongyu Li1,2
1Key Laboratory of Artificial Organs and Computational Medicine in Zhejiang Province, Shulan International Medical College, Zhejiang Shuren University, Hangzhou, Zhejiang, People’s Republic of China; 2Ocean College, Beibu Gulf University, Qinzhou, Guangxi, People’s Republic of China
Correspondence: Hongyu Li, Key Laboratory of Artificial Organs and Computational Medicine in Zhejiang Province, Shulan International Medical College, Zhejiang Shuren University, Hangzhou, Zhejiang, People’s Republic of China, Email [email protected]
Abstract: The global rise in diabetes mellitus (DM), particularly type 2 diabetes (T2D), has become a major public health challenge. According to the “hygiene hypothesis”, helminth infections may offer therapeutic benefits for DM. These infections are known to modulate immune responses, reduce inflammation, and improve insulin sensitivity. However, they also carry risks, such as malnutrition, anemia, and intestinal obstruction. Importantly, helminth excretory/secretory products, which include small molecules and proteins, have shown therapeutic potential in treating various inflammatory diseases with minimal side effects. This review explores the anti-inflammatory properties of helminth derivatives and their potential to alleviate chronic inflammation in both type 1 diabetes and T2D, highlighting their promise as future drug candidates. Additionally, it discusses the possible applications of these derivatives in DM management and the challenges involved in translating these findings into clinical practice.
Keywords: diabetes mellitus, helminth-derived products, anti-inflammatory, immune modulation
Introduction
Over the past three decades, the prevalence of diabetes mellitus (DM) has risen sharply across the globe (Figure 1). Calculation using data from multiple sources indicates that the average incidence of DM increased by 3.73 cases per 100,000 people per year between 1990 and 2019, highlighting its rise as one of the essential global major public health problems.1 In 2019 alone, new reports from around the world indicated some 22 million new cases of DM, with the burden projected to increase notably in the coming years.2
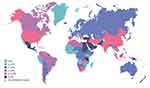 |
Figure 1 Estimated age-adjusted comparative prevalence of diabetes. Notes: In adults aged 20–79 years in 2021. Reproduced from International Diabetes Federation. IDF Diabetes Atlas, 10th edn. Brussels, Belgium: 2021. Available at: https://www.diabetesatlas.org.3 |
The interplay between type 1 diabetes (T1D) and type 2 diabetes (T2D) is significantly modulated by chronic inflammation, a pivotal factor in the pathogenesis of both conditions. T1D is predominantly marked by the autoimmune destruction of insulin-producing pancreatic β-cells, driven by immune cell infiltration that induces chronic inflammation in the islets of Langerhans.4,5 This process is primarily mediated by autoreactive T cells and the release of pro-inflammatory cytokines, which not only lead to β-cell destruction but also sustain the autoimmune cascade.6 Conversely, T2D is characterized by insulin resistance (IR) and metabolic dysfunction, with chronic low-grade inflammation playing a crucial role in its progression.7 In T2D, obesity-associated chronic inflammation is a key contributor, as adipose tissue functions as an endocrine organ, releasing pro-inflammatory cytokines that exacerbate IR.8 The inflammatory response involves mechanisms such as inflammasome activation and the release of damage-associated molecular patterns, which act as endogenous triggers of inflammation.9 Inflammatory mediators, including IL-1 and TNF-α, have been demonstrated to inhibit insulin signaling, further complicating the metabolic dysregulation observed in T2D.10 Elevated levels of pro-inflammatory cytokines, such as TNF-α and IL-6, are closely associated with impaired insulin signaling pathways in T2D patients.11,12 Furthermore, increased concentrations of inflammatory biomarkers—including C-reactive protein, TNF-α, and IL-6—correlate with the transition from prediabetes to DM.13,14
The “hygiene hypothesis” suggests that live helminths may have potential therapeutic benefits, especially for T2D. Indeed, recent research provides growing evidence for the ability of helminth infections to modify immune responses and suppress inflammation, hence improving insulin sensitivity. Recent studies indicate that some helminth infections reduce the risk of developing DM but increase the risk of adverse effects such as malnutrition,15 anemia,16 or intestinal obstruction.17 Importantly, helminths secrete excretory/secretory products (ESPs), which transform the host immune response into an anti-inflammatory state, hence playing a role in controlling autoimmune diseases.18,19 These ESPs consist of low molecular weight molecules and proteins that have shown therapeutic effects in several inflammatory diseases, such as rheumatoid arthritis and multiple sclerosis, with hardly any side effects reported.20,21 Several studies have documented the anti-inflammatory features of helminth-derived products in the treatment of DM through a skew to anti-inflammatory pathways that oppose and can potentially correct the chronic inflammation that comes with both T1D and T2D. This immune modulation can enhance insulin sensitivity and preserve β-cell function with relatively few adverse effects.22–24 Currently, anti-inflammatory activities by helminths have been proposed from the observation that they release helminth-derived molecules on host-parasite interactions called helminth defense molecules (HDM) with broad anti-inflammatory properties in the absence of cytotoxicity thus, maybe a potential.
Initially, scientists observed that parasitic infections in many rural areas seemed to confer protection against metabolic diseases such as DM and atherosclerosis.25,26 For instance, the prevalence of metabolic syndrome was significantly lower at 18.28% among individuals who had been infected with schistosomes, compared to 34.01% in those who had never been infected.27 This observation aligns with the “hygiene hypothesis”, which suggests that the incidence of DM is markedly higher in middle- and high-income countries than in low-income nations. This hypothesis is reinforced by epidemiological studies showing a higher incidence of DM in wealthier countries where parasitic infections are less common.23,28 Such contrasting findings indicate a potential inverse relationship between the prevalence of parasitic infections and the incidence of DM.
Subsequent studies suggest that helminth infections may protect against DM by modulating metabolic processes. A randomized trial in Indonesia found that individuals infected with helminths had a 16% lower IR index (HOMA-IR) compared to uninfected participants, with the improvement reducing by 10% after deworming treatment.29 A cross-sectional study in rural China reported a lower prevalence of DM (14.9%) and metabolic syndrome (14.0%) among individuals previously infected with schistosomes, compared to those without prior infection (25.4% and 35.0%, respectively).26 Similarly, an Australian study found a 13% lower incidence of T2D in individuals infected with Strongyloides stercoralis (S. stercoralis), with risk increasing by 10% post-deworming.30 A trial in Uganda showed that Schistosoma mansoni (S. mansoni) infection lowered LDL cholesterol levels by 0.26 mmol/L, potentially reducing cardiovascular risk.31 These findings indicate that helminth infections may protect against T2D and metabolic syndrome through metabolic and immune regulation.32–35 Research has also shown that hookworm infections can modulate inflammatory diseases and metabolic syndrome. While studies on hookworm’s direct effect on T2D are limited, they suggest that these infections alter the gut microbiome36 and elicit immune responses,37 which may benefit T2D. An Australian trial involving hookworm larvae reported improved IR and reduced fasting blood glucose after one year, with a reduction in body weight after two years.38 Helminths have also been shown to affect fat metabolism by lowering leptin and increasing adiponectin levels, which enhances insulin sensitivity.39,40 Furthermore, helminths may influence T2D progression by modulating angiogenesis and immune responses. For example, S. stercoralis infection reduced pro-inflammatory cytokines (IL-1β, IL-6, TNF-α) in T2D patients, with levels returning to normal after antiparasitic treatment.41,42 Additionally, hookworm infections increased anaerobic bacteria such as Lactobacillus and Bifidobacterium, which are linked to reduced inflammation and improved insulin sensitivity.42 Recent studies in 2024 indicate that S. stercoralis may also modulate the complement system, reducing inflammation through regulation of complement proteins.43 Collectively, these findings suggest that helminth infections offer protective effects against metabolic diseases like T2D through immune modulation, lipid metabolism regulation, and gut microbiota changes.
Contrarily, not all parasitic infections confer protection against DM; some may trigger its onset. The prevalence of intestinal parasitic infections in such case studies was higher in the affected population than in controls, with a noted correlation (OR, 1.80).44 Additional meta-analytical research is required to explore the interplay between T1D-induced immunological changes and the risk of T. gondii infection, and vice versa.22,45,46 Moreover, a case report highlighted the death of a 40-year-old diabetic patient with a history of alcoholism due to severe S. stercoralis infection.47 Most experts agree that patients with T2D should be shielded from parasitic infections due to their compromised glucose regulation,48,49 which could exacerbate susceptibility to infections like those caused by acarids, potentially accelerating parasite growth and enhancing virulence.50,51
Parasitic infections typically result in pathological conditions; however, the therapeutic use of helminth-derived molecules in the treatment of DM has garnered increasing interest in recent years. These molecules regulate the host immune response through various mechanisms and have demonstrated potential therapeutic effects in both T1D and T2D.52–54 Recent studies have shown that secretions from Fasciola hepatica possess significant immunomodulatory properties, which can prevent T1D in Non-Obese Diabetic (NOD) mice.55 These secretions achieve this by inducing M2 and Tregs, which suppress inflammatory responses in the pancreas, thereby reducing autoimmune damage and effectively halting the progression of DM.55 An increasing number of helminth-derived molecules have been experimentally demonstrated to modulate adipose tissue secretion or reduce inflammation.52,56–60 This immunoregulatory mechanism underscores the potential of helminth-derived molecules in DM treatment, similar to the effects of live helminths, while also enhancing their acceptance by the general population.
Mechanism of Action
Impact on Adipose Tissue Secretions
Worms and their proteins can profoundly influence adipose tissue secretions, particularly adipokines, by modulating immune responses and metabolic pathways.
Studies by Queiroz-Glauss and Su. have shown that Heligmosomoides polygyrus (H. polygyrus) infection in high-fat diet-induced obese mice reduces glucose and triglyceride levels. This infection also decreases pro-inflammatory adipokines, such as leptin and resistin, while enhancing anti-inflammatory factors, including uncoupling protein 1 and adiponectin. These changes mitigate obesity-associated inflammation and improve insulin sensitivity.61,62 Research by Kang demonstrated that Trichinella spiralis lysates significantly reduce lipid droplet accumulation during adipocyte precursor cell (3T3-L1) differentiation. The lysates inhibit the expression of key adipogenic regulators, including peroxisome proliferator-activated receptor gamma, CCAAT-enhancer-binding protein alpha and adipocyte protein 2, thereby reducing intracellular lipid storage.63 Findings from Hussaart revealed that chronic worm infections lead to significant reductions in weight gain (−62%), fat mass (−89%), and adipocyte size. These infections also decrease systemic IR (−23%) and impaired glucose tolerance (−16%), while enhancing peripheral glucose uptake (+25%) and WAT insulin sensitivity.64 Further evidence suggests that Trichinella spiralis infection promotes elevated adiponectin expression in the small intestine. This increase correlates strongly with epithelial-derived cytokines such as IL-25, IL-33, and thymic stromal lymphopoietin.65
Helminth-derived proteins also demonstrate similar regulatory effects. Molecules in pseudocoelomic fluid from Ascaris suum (A. suum) upregulate gene expression in small intestinal tuft cells, including genes involved in runt-related transcription factor 1 and organic cation transporter regulation. These processes link tuft cell activity to the fat-to-lean mass ratio and eosinophil function in epididymal white adipose tissue, ultimately regulating body fat composition.66 The fatty acid and retinol-binding proteins from Steinernema carpocapsae consume lipid signaling precursors in vivo and bind to these fatty acids in vitro. This activity alters the availability of signaling molecules required for effective immune responses, thus mediating immunomodulation.67 Additionally, the glycoprotein omega-1 (ω1) from S. mansoni enhances metabolic homeostasis by inducing type 2 immune responses and suppressing food intake, which reduces body fat and improves insulin sensitivity.57,58 Collectively, these findings highlight the potential of worm proteins to modulate adipose tissue secretions and adipokine profiles, alleviating obesity and metabolic syndrome through intricate immune-metabolic interactions. The induction of regulatory immune cells plays a central role in maintaining metabolic stability, reducing adipokine production, and facilitating immune regulation.68
Nevertheless, the potential adverse effects of worm infections should not be overlooked. Hookworm infection in obese mice has been associated with elevated cholesterol and triglyceride levels, as well as increased inflammation, exacerbating metabolic disorders.69 Furthermore, studies indicate that infections with Ascaris or hookworm can lead to increased fat deposition, particularly in the abdominal region, among children.70 The dualistic nature of worm infections emphasizes the need for further investigation into the therapeutic applications of worm-derived molecules for treating obesity and DM.71
Direct Anti-Inflammatory Effects
The critical role of parasite ESPs in parasite-host interactions has increasingly come to light, with extracellular vesicles (EVs) receiving particular attention.59 Through optimized extraction methods, high-purity EVs have been successfully isolated from parasites such as S. mansoni, revealing significant anti-inflammatory and immunomodulatory potential.72 For example, EVs derived from Necator brasiliensis (N. brasiliensis) suppress pro-inflammatory cytokines associated with colitis, including IL-6, IL-1β, IFNγ, and IL-17a, while promoting the anti-inflammatory cytokine IL-10.73 Similarly, EVs from A. suum specifically target epithelial cells, and the ES proteins (Ts-MES) of Trichinella spiralis (T. spiralis) have been shown to protect murine cardiac tissue from septic injury.74 Advanced research further delves into the intricate interactions between helminth-derived proteins and various immune cell populations.
Effects on Lymphocytes
The balance of Th1 and Th2 immune responses, regulated by CD4+ T-cell differentiation, is crucial for maintaining immune homeostasis.75 Numerous studies have confirmed that various worm proteins can shift adipose tissue inflammation, which is dominated by Th1 and Th17 cells, to an anti-inflammatory state driven by Th2 and Treg cells76–78 (Figure 2).
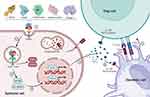 |
Figure 2 Helminth proteins regulate immune responses by modulating DCs and Tregs. Initially, helminth proteins are identified by pattern recognition receptors on the surface of host epithelial cells, which trigger specific signaling pathways, notably TLR2 and TLR4. This interaction activates NF-κB and interferon regulatory factors (IRF1 and IRF4), crucial for driving the expression of anti-inflammatory cytokines like IL-10 and TGF-β. Once released into the surrounding environment, these cytokines bind to their respective receptors, IL-10R and TGF-β receptors, on DCs. This binding modulates DC functions, facilitating the activation and proliferation of regulatory Treg cells. These Treg cells are essential for maintaining immune tolerance and preventing overactive immune responses, thereby playing a pivotal role in the immune system’s ability to manage parasitic infections effectively. Created in BioRender. Yunhuan Zhu (2025) https://BioRender.com/x98w137. |
Experimental treatments with cystatin-like protein have been observed to increase the secretion of the regulatory cytokine IL-10, highlighting its anti-inflammatory potential.79 Recent research has further highlighted the significance of parasite-derived protease inhibitors, such as cystatins, in regulating key immunological processes, including antigen processing, activation of TLRs, and secretion of cytokines.80–83 Additionally, compounds such as prostaglandin E2, produced by parasites like Trichuris suis (T. suis) and S. mansoni, play critical roles by promoting Th2 immune responses and suppressing responses from ILC2, which are vital for the therapeutic management of conditions such as atopic dermatitis.84,85
Studies on mice treated with soluble egg antigens (SEA) from Schistosoma japonicum (S. japonicum) demonstrate a significant decrease in IR and increased frequency of Tregs in the spleen. These changes coincide with enhanced secretion of IL-4 and IL-5 from spleen cells.86
The ES products from Echinococcus multilocularis (E. multilocularis) larvae are capable of inducing the formation of Foxp3+ Treg cells from CD4+ T cells in a TGF-β-dependent manner in vitro. During interactions with these larval products, T cells also secrete increased levels of the immunosuppressive cytokine IL-10.87 As noted by Pineda, the phosphocholine glycoprotein ES-62 from filarial and nematode parasites is a potent inducer of the Th2 immune response.88 ES-62 helps restore the function of IL-10-producing regulatory B cells and reduces chronic inflammation driven by Th1 and Th17 cells.89 Additionally, parasitic molecules manage excessive B cell and T cell activities, reducing cytokine output, receptor expression on the cell surface, and cell proliferation, thus fostering an environment conducive to anti-inflammatory and regulatory immune responses.90,91
Effects on Dendritic Cells and Macrophages
It is well established that dendritic cells (DCs) and macrophages (MΦ) play pivotal roles in antigen presentation, a key process in the initiation of immune inflammatory responses.92,93 The Glutathione S-transferase ω2 antigen from the liver fluke has been shown to modulate MΦ function, contributing to immunoregulation and anti-inflammatory effects.94 Additionally, lipid mediators derived from Fasciola hepatica (F. hepatica) are known to activate an alternative pathway in MΦ, which suppresses the activation typically induced by Lipopolysaccharide (LPS).60
ES-62, a molecule derived from the genus Acanthocheilonema, has been shown to inhibit DCs and MΦ functions by sequestering MyD88, suppressing most pro-inflammatory TLRs except TLR3, and modulating IL-33 signaling. Additionally, it facilitates antigen processing and presentation, highlighting its multifaceted role in immune regulation.95–97
F. hepatica helminth defense molecule 1 (FhHDM-1), a protein from liver flukes, has been found to promote pancreatic β-cell survival under pro-inflammatory stress by activating the PI3K/Akt signaling pathway. It also protects human islets from cytokine-induced apoptosis.98 Interestingly, MΦ treated with FhHDM-1 do not express typical M2 phenotype markers, indicating that its effects extend beyond merely promoting M2 polarization. Instead, FhHDM-1 appears to directly modulate inflammatory responses. Moreover, it regulates lysosomal pH, revealing a novel functional link between lysosomal and mitochondrial metabolism, underscoring its potential as a therapeutic anti-inflammatory agent.99
Helminth infections caused by Schistosoma species induce M2 polarization through mechanisms such as the secretion of excretory products and EVs that modulate MΦ activity. These helminth-derived molecules shift MΦ metabolism toward oxidative phosphorylation, lipid oxidation, and amino acid metabolism.100 Furthermore, antigens from helminths, such as Ascaris and S. mansoni proteins, enhance the early control and clearance of pathogens like Mycobacterium tuberculosis by modulating the monocyte-macrophage axis.101 Helminth proteins also influence MΦ polarization via signaling pathways such as mTORC2, promoting M2 differentiation and regulating systemic metabolism and thermogenesis.102
Specific helminth proteins, including Ts-Cys,103 have been shown to mitigate sepsis-induced inflammation by inhibiting inflammatory cytokine production (MyD88 and mTOR) in MΦ. This effect is mediated by suppressing the PI3K/Akt pathway, which also induces autophagy in DCs and dampens TLR-mediated inflammation (Figure 2).104–108 These processes are further associated with increased mannose receptor (MR) expression on MΦ,109 similar to mechanisms seen when protoscoleces from multilocular hydatid cysts drive MΦ toward an M2 phenotype characterized by elevated metabolic proteins such as HK1, PFKL, PKM2, phosphorylated Akt and mTOR.110
Next, ES-62 also activates anti-inflammatory signaling pathways through the TLR4-TRIF-TRAF3-IRF3 axis, while simultaneously suppressing NF-κB activity.111–113 This results in reduced levels of inflammatory cytokines, including IFN-γ, and TNF-α, making ES-62 a promising therapeutic candidate for inflammatory and autoimmune diseases.114,115 Upon binding to human TLR4, ES-62 suppresses NF-κB via pathways involving transcription factors such as p38, MAPK, JAK, and Erk.116,117 Similarly, the filarial protein BM-CPI-2 disrupts the processing of human MHC-II antigens by inhibiting asparaginyl endopeptidase and modulating the TLR-NF-κB pathway.118,119
The Jmjd3-Irf4 axis represents another critical pathway in M2 polarization, underscoring the importance of histone demethylation in MΦ differentiation and the anti-helminth immune response.120 In addition, peptides derived from helminths, such as HDM, can traverse the MΦ membrane and enter the lysosomal system. Once inside, these peptides inhibit vacuolar (v)-ATPase activity, which reduces lysosomal acidification and subsequently blocks MΦ activation via TLR 4 signaling.121
Despite the promising potential of helminth-derived products in treating autoimmune and inflammatory diseases, their clinical application remains controversial. While their safety has been demonstrated in clinical trials, their therapeutic efficacy has yet to be conclusively established.122,123 The complexity of their use stems from the diverse mechanisms by which parasites modulate host biology, including neutralizing danger signals, regulating DCs and MΦ activities, and modifying the adaptive immune response.124
Effects on Granulocytes
Numerous immunologically active molecules derived from parasites effectively inhibit inflammation. For example, the serine protease inhibitor SmKI-1 from S. mansoni interacts with elastase, impairing neutrophil function and reducing inflammation.125 Neutrophil extracellular traps (NETs), composed of DNA fibers and antimicrobial proteins, are formed through a process known as NETosis, playing a crucial role in the innate immune response against pathogens, including helminths. During helminth infections, NETs primarily immobilize parasites rather than killing them.126–130 For instance, canine neutrophils exposed to Toxocara canis (T. canis) larvae produce NETs that trap the larvae but do not affect their viability, suggesting a containment function rather than direct eradication.131 Similarly, NETs generated in response to S. stercoralis larvae capture the parasites, facilitating their clearance by other immune cells, as NETs alone lack the ability to eliminate them.132 Additionally, helminth-derived factors, such as parasitic ligands, can inhibit NET formation. These factors block the activation of the transient receptor potential melastatin 2 channel, a key mediator of ROS-induced NETosis.132,133 Furthermore, ESPs from adult Trichinella spiralis suppress NETosis and modulate cytokine production in neutrophils.134 Such findings may provide insight into the previously reported reduction of vincristine-induced neuroinflammation in mice following T. spiralis infection.135
Eosinophils play multifaceted roles in the response to helminth proteins, acting as both defenders and regulators in immune responses. During helminth infections, eosinophils are primarily associated with Th2-type immune responses, characterized by the production of interleukin (IL)-5, IL-4, and IL-13, which promote their activation and recruitment.129,136 These cells directly kill the larval stages of helminths by releasing granule proteins such as eosinophil peroxidase and major basic protein-1, although they do not completely eliminate the parasites.102,103,137,138 Eosinophils also facilitate immune regulation, including modulating IgE responses and influencing goblet cell mucus production.137,139 Research by Driss demonstrated that eosinophils, driven by the schistosome P28GST protein, can alleviate inflammatory diseases such as colitis by promoting Th2 responses and reducing the expression of pro-inflammatory cytokines.140 Furthermore, helminth ESPs, such as those from Nippostrongylus brasiliensis (N. brasiliensis), have been shown to improve glucose tolerance and reduce weight gain by inducing type 2 immune responses, which include increased eosinophils and IL-5 as well as decreased IL-6 in adipose tissue.56
Following sensitization by helminth proteins, basophils are activated to release key cytokines, including IL-4 and IL-13, which promote Th2 immune responses and IgE production.141,142 This activation is accompanied by the mobilization and recruitment of basophils to lymphatic and peripheral tissues, where they execute their effector functions. In addition to their role in cytokine release, basophils serve as APCs by expressing MHC-II and co-stimulatory molecules such as CD80/86.143 This enables basophils to present antigens to CD4+ T cells and drive Th2 cell differentiation, particularly in response to protein antigens and antigen-IgE complexes, further amplifying Th2 responses.143,144 The Notch signaling pathway is pivotal in regulating basophil responses during helminth infections. By controlling gene expression and cytokine production, this pathway is essential for mounting an effective immune response and achieving helminth clearance.145,146 However, genetic background influences these mechanisms, as demonstrated in AKR/J mice infected with Trichuris muris (T. muris), where basophils expand but show impaired Notch2 receptor upregulation, compromising their functionality.147 Additionally, IPSE, a protein isolated from S. mansoni egg extracts, has been shown to directly stimulate basophils to release IL-4 and histamine, further activating Th2 immune responses.142
Effects on Epigenetics
Derivatives of worms, including those from the ESPs, can also influence the host epigenetically. From the standpoint of pharmaceutical development, one of the most compelling aspects is the high concentration of miRNAs within the EVs of parasites. These miRNAs are anticipated to target host genes, particularly those involved in immune processes. Host cells can actively uptake these parasite EVs,73,148 facilitating a mechanism by which parasites transmit genetic materials to the host to manipulate gene expression 265 actively. Literature increasingly substantiates parasite-specific miRNAs secreted by worms, although detailed interactions with host genes remain largely unexplored. It has been shown that miRNAs from H. polygyrus can downregulate the mouse phosphatase gene dusp1 expression, the human equivalent of MKP-1, a critical regulator of MAPK signaling and Th1 responses to TLR ligands.149 Research has demonstrated that the liver fluke, F. hepatica, and ESPs significantly influence mouse MΦ function. Exposure of MΦ to whole worm extracts and adult worm ESPs stimulates these cells to produce higher levels of anti-inflammatory cytokines IL-1RA and IL-10 while decreasing pro-inflammatory cytokine production.150 This modulation reflects the parasites’ capability to induce an anti-inflammatory shift in the host immune system and suggests potential epigenetic regulation mechanisms involved, such as histone methylation.151 These findings deepen our understanding of parasite-host interaction mechanisms and could lay the groundwork for new immunomodulatory therapeutic strategies. Studies have also indicated that the ESPs named ES-62 from the rodent filarial worm Acanthocheilonema vitae exerts substantial immunomodulatory effects on host immune cells, mainly by inhibiting the secretion of the pro-inflammatory cytokine IL-12 by antigen-presenting cells upon stimulation.152 This effect persists both in vitro and in vivo, with continuous exposure to ES-62 eliciting similar anti-inflammatory responses in mice.153,154 Further research has revealed that ES-62 induces epigenetic alterations in non-immune cells, including synovial fibroblasts, aiding in mitigating inflammatory responses in rheumatoid arthritis models.155–157 Moreover, ES-62 and its small molecule analogs reprogram immune cells through epigenetic mechanisms, exhibiting therapeutic potential in various inflammatory, autoimmune, and allergic conditions.88,89 Additionally, recent research has highlighted that cystatin upregulates pathways involved in mevalonate and cholesterol biosynthesis and immune regulation genes in human monocyte-derived DCs, manifesting an epigenetic impact.158
Infections with nematodes like Strongyloides venezuelensis (S. venezuelensis) lead to changes in the intestinal microbiome, notably an increase in the population of Lactobacillus species. These microbiome alterations modify host metabolism by elevating anti-inflammatory cytokine levels, transitioning MΦ in adipose tissues from an M1 to an M2 phenotype, enhancing the expression of tight junction proteins in intestinal cells (thereby reducing permeability), and decreasing serum levels of LPS.159 Research by Ying et al demonstrated that exposure to inhibits MΦ secretion of pro-inflammatory cytokines such as IL-1β, TNF, IFNγ, and IL-6 while enhancing the secretion of IL10 and TGF-β, promoting an anti-inflammatory M2 phenotype.160 Studies have identified a liver fluke molecule, FhHDM-1, which prevents the activation of the NLRP3 inflammasome by inhibiting lysosomal acidification within MΦ.161 Similar effects are achievable with exogenous antigens. Worms and worm-derived molecules can regulate the phenotype and functions of DCs, including their surface markers (MHC-II molecules, CD80, CD86)162 and cytokines (IL-12, TGF-β, IL-10).163 For instance, Ascaris cystatin induces human DCs to secrete IL-10,164 facilitating their migration to lymph nodes to drive the differentiation of naive CD4+ T cells. Dioscin can reverse the elevated secretion of IL-6 and IL-12 and the decreased secretion of IL-10 in DCs under high glucose conditions.165 Furthermore, natural liver fluke extracts and recombinant FhFABPs (F. hepatica Fatty Acid Binding Proteins) significantly modulate the immunoregulatory functions of human monocyte-derived DCs. The underlying mechanism involves FhFABP1 inducing a tolerant-like phenotype in moDCs upon LPS stimulation, characterized by a dose-dependent increase in the tolerance marker CD103 and IL-10 secretion without affecting DC co-stimulatory markers. Additionally, there is a significant decrease in the secretion of pro-inflammatory cytokines IL-12p70 and IL-6. These successful immunoregulations by recombinant proteins from natural liver flukes suggest potential therapeutic applications, though further research is needed to evaluate their effects on improving insulin sensitivity or glucose homeostasis.166 These findings emphasize parasites’ capacity and excretions to induce epigenetic modifications in host immune cells, offering crucial insights for developing new therapeutic strategies.167
Helminth ESPs can induce epigenetic changes in host cells, potentially leading to stable phenotypic modifications, although these phenotypes are not always beneficial.167 The regulation of synovial fibroblasts by ES-62 alters their inflammatory phenotype, which may impact joint health and inflammation in diseases such as rheumatoid arthritis.168 Moreover, while helminths can prevent excessive tissue pathology, their presence and the immune regulation they induce may sometimes result in tissue damage, particularly when immune responses are inadequately controlled.167,169
Widely Used Helminth Products
The development of products derived from parasitic worms is advancing rapidly, with increasing interest in their potential therapeutic applications, particularly in the treatment of metabolic disorders like DM. As detailed in Table 1, various worm-derived products have shown promising results in experiments conducted on diabetic mouse models. N. brasiliensis ESPs, such as adult stage and third-stage larvae ESPs, significantly decreased fasting blood glucose and improved glucose metabolism in HFD-fed C57BL/6 mice, while also modulating immune responses, including an increase in eosinophils and IL-5, and a decrease in IL-6 in adipose tissue.170 Similarly, Fasciola hepatica protein FhHDM-1 has demonstrated the ability to activate the PI3K/Akt pathway and preserve β-cell mass in NOD mice, offering protection against cytokine-induced apoptosis and enhancing insulin secretion.98 Other products like S. mansoni SEA and ω1 have shown improvements in metabolic homeostasis and insulin sensitivity through immune modulation, including the promotion of Th2 cells, eosinophils, and M2 in adipose tissue.57,171
 |
Table 1 Basic Experiments of Helminth-Related Molecules in Diabetic Mouse Models |
These results highlight the therapeutic potential of worm-derived molecules, which modulate both metabolic and immune pathways, offering new strategies for managing DM and related metabolic conditions. The diversity of impacts across different parasitic molecules and models, as illustrated in Table 1, reflects their complex mechanisms of action.
ES-62
ES-62 is renowned for its anti-inflammatory properties and potential to modulate immune responses, which have been investigated across various disease models. In studies using mouse models of obesity-induced accelerated aging, ES-62 has shown promise in extending both health span and lifespan. Obesity-induced accelerated aging is often linked to T2D and other metabolic disorders. In these models, ES-62 has been observed to improve parameters related to inflammation, metabolism, and the microbiome, suggesting that targeting chronic inflammation and metabolic dysfunction may yield indirect benefits for conditions such as T2D.89,175,176 This is particularly important given the strong association between T2D and chronic inflammation, with ES-62 appearing to mitigate these inflammatory processes. However, the direct effects of ES-62 on DM, especially T1D, are less encouraging. Research indicates that ES-62 does not confer protection in mouse models of T1D, a condition characterized by the autoimmune destruction of insulin-producing β cells in the pancreas.177 This indicates that although ES-62 may positively influence inflammation and metabolic health, it does not directly address the autoimmune mechanisms underlying T1D.
HSP70
Heat shock protein 70 (HSP70) has been identified as a promising therapeutic target for mitigating the effects of DM, particularly T2D. Research indicates that HSP70 plays a vital role in the pathogenesis of IR, a critical factor in T2D development. As a cytoprotective molecular chaperone, HSP70 is involved in protein folding and degradation, and its regulation has shown potential in enhancing insulin sensitivity and glycemic control.178 The intracellular form of HSP70 (iHSP70) is crucial for maintaining normal insulin signaling and inhibiting protein aggregation, contributing to its anti-inflammatory properties. On the other hand, the extracellular form (eHSP72) is associated with pro-inflammatory conditions and IR.179 This dual role suggests that the therapeutic potential of HSP70 may rely on maintaining the balance between its intracellular and extracellular forms. An imbalance in the ratio of eHSP72 to iHSP70 has been linked to vascular dysfunction in DM, emphasizing the importance of preserving this balance for vascular health and effective DM management.180 Moreover, strategies aimed at increasing HSP70 expression—such as long-term physical exercise, hot water immersion therapy, and the administration of HSP70 derived from alfalfa—have demonstrated potential for both the treatment and prevention of T2D.178 Additionally, heat stimulation has been proposed as a method to induce HSP70 expression, which may enhance insulin sensitivity and reduce chronic inflammation, addressing the underlying pathology of T2D and its related metabolic disorders.181
FhHDM-1
Helminths, such as F. hepatica, secrete various molecules that modulate immune responses, primarily by enhancing anti-inflammatory pathways and suppressing pro-inflammatory reactions.182,183 These secreted molecules, which include EVs and ESPs, are essential for the helminths’ survival within the host as they help evade immune detection and reduce inflammation.182,183 One specific protein derived from F. hepatica, FhHDM-1, has exhibited notable anti-inflammatory properties. It has been shown to prevent the onset of T1D in mouse models by modulating the activity of MΦ and neutrophils, thus inhibiting the release of pro-inflammatory cytokines and chemokines.184 This modulation occurs through the activation of the PPAR-γ pathway, which is known for its role in mitigating inflammation and enhancing insulin sensitivity,185 offering a potential therapeutic approach for DM management.
SmEA
Research has shown that S. mansoni egg antigen (SmEA) exhibits potential in mitigating DM, particularly T1D, through its immunomodulatory effects. Several studies have investigated the influence of SmEA on autoimmune DM models, yielding promising results. For instance, a study by Yahia examined the impact of SmEA on streptozotocin-induced T1D in mice. The findings revealed that SEA treatment significantly reduced blood glucose levels and increased insulin levels, indicating a protective effect against T1D. This protective effect was associated with elevated levels of the anti-inflammatory cytokine IL-10, suggesting a shift towards a Th2 immune response, which is less damaging to pancreatic β cells compared to the Th1 response typically linked to T1D.186 El-Gebaly found that the administration of SEA in diabetic mice resulted in significantly lower blood glucose levels and increased IL-10 production. Histopathological analysis further confirmed reduced inflammation of the pancreas, supporting the protective role of SEA in T1D.187 These results are consistent with the research by Zaccone, who demonstrated that SmEA induces Th2 and Treg responses, which are crucial for establishing immune tolerance and preventing DM in NOD mice. The study also highlighted the crucial role of TGF-β in the expansion of Tregs, a key mechanism driving the immunomodulatory effects of SEA.188
Other Molecules Derived from Helminths
H. polygyrus, a gastrointestinal helminth, is recognized for its ability to modulate host immune responses, primarily by inducing a Th2-skewed immune response and expanding regulatory Tregs.2 This immunomodulation occurs through the secretion of ESPs, which include immunoregulatory proteins such as TGF-β mimics and galectins.4 These findings suggest that while H. polygyrus ESPs can modulate immune responses, but its effectiveness may vary depending on the specific disease and immune pathways involved. Although this abstract does not directly address DM, the immunoregulatory properties of H. polygyrus ESPs—particularly its ability to induce Th2 responses and expand Tregs—may offer potential therapeutic benefits for autoimmune DM, where immune regulation is impaired. Filarial cystatin has been shown to modulate immune responses by upregulating NO production in interferon-γ-activated MΦ, as evidenced by studies on Onchocerca volvulus (O. volvulus) and Acanthocheilonema viteae (A. viteae). This upregulation partially depends on interleukin-10 and tumor necrosis factor-α, reflecting complex interactions within the immune system.189 Nitric oxide, a critical signaling molecule in processes such as vasodilation and immune regulation, could theoretically influence metabolic pathways related to DM. Furthermore, filarial cysteine protease inhibitors have been found to suppress T cell proliferation and promote an anti-inflammatory cytokine profile, such as increased interleukin-10 production.189 This anti-inflammatory action has the potential to mitigate chronic inflammation, a contributing factor in IR and T2D. HpARI, a protein secreted by the parasitic nematode H. polygyrus, binds to the cytokine IL-33 and DNA, thus modulating immune responses. The full-length HpARI protein inhibits IL-33 activity by sequestering it within the nuclei of necrotic cells, preventing its release and subsequent immune activation.190 This inhibition of IL-33 could have significant implications for treating inflammatory conditions, including DM, which is characterized by chronic inflammation. Interestingly, a truncated form of HpARI (HpARI_CCP1/2) has been shown to stabilize IL-33, enhancing rather than suppressing immune responses.191 This truncated form cannot prevent IL-33 from interacting with its receptor, thereby amplifying IL-33-dependent immune reactions. This suggests that the regulatory effects of HpARI on IL-33 vary depending on the specific form of the protein and its interactions.
Advancements in Helminth-Derived Products
Optimization of Drug Delivery
Distinct families of biomacromolecules derived from worms demonstrate unique capabilities to address challenges associated with the therapeutic properties of worm-derived products and the mechanisms for effectively delivering these proteins to target tissues or organs. In recent years, the development of novel delivery technologies has revitalized the therapeutic potential of various drugs (Figure 3).
 |
Figure 3 Common drug delivery systems. Oral administration involves lipid nanoparticles, which deliver drugs such as curcumin. These particles are ingested and enter the stomach. In the case of transdermal administration, drug-laden gels, such as those containing meloxicam, employ microneedles to administer drug particles through the skin. For intravenous administration, drugs like methotrexate are loaded into polyamidoamine (PAMAM) dendrimers, which are then injected directly into the bloodstream. Recently, innovative methods using nanomaterials for drug delivery have been developed, including releasing drugs controlled by various external stimuli such as light, heat, pH, oxygen, and magnetic fields. These new approaches offer precise control over when and where drugs are released, enhancing treatment effectiveness and reducing potential side effects. Created in BioRender. Yunhuan Zhu (2025) https://BioRender.com/p89w615. |
Microneedle arrays have been specifically engineered to penetrate the stratum corneum, creating microchannels that enable efficient transport into the dermis, thereby facilitating the delivery of macromolecular drugs such as proteins.192–194 This delivery method is particularly advantageous for protein-based therapeutics, which are typically unstable and susceptible to degradation when administered orally. By circumventing the gastrointestinal tract and first-pass metabolism, microneedles provide a non-invasive and pain-free alternative to conventional injections.195,196 In Japan, a novel adjuvant-free immunotherapy for T1D employs S. japonicum egg tip-loaded asymmetric microneedle patch loaded at the tips of S. japonicum eggs.197
Nanomedicine, propelled by advances in nanodelivery technologies for targeted drug delivery,198 cancer therapy, and crossing the blood-brain barrier,199 is increasingly being explored in helminthic research. This includes the delivery of immunomodulatory compounds derived from helminths to treat autoimmune and allergic diseases. Emerging technologies, such as intelligent targeted nanodelivery systems, demonstrate the ability to activate the Wnt signaling pathway (via Gsk3β inhibition), suppress inflammasome activation (via NLRP3 inhibition), and stimulate autophagic pathways (by protecting Atg3/Atg7). These mechanisms collectively support β-cell development and mitigate the severity of T2D.200 Nanoparticles have shown potential in delivering bioactive peptides, addressing challenges such as rapid degradation in vivo and the traversal of biological barriers.201 Additionally, food-grade colloidal systems—including microemulsions, emulsions, and nanoemulsions—enhance the stability and absorption efficiency of proteins and peptides.202 Self-assembled peptide nanostructures, characterized by their chemical versatility and precise targeting capabilities, exhibit promising potential in drug delivery applications.203 Furthermore, nanoparticles and nanocapsules improve the solubility and cellular uptake efficiency of biomacromolecules.204
The miRNAs and exosomes derived from parasitic worms not only modulate immune responses and control inflammation,205,206 but also represent a promising new approach to enhance drug delivery efficiency.207 EVs, naturally occurring vesicles with high biocompatibility and stability, can encapsulate both hydrophilic and lipophilic drugs, protecting them from degradation and improving their bioavailability.208,209 Furthermore, EVs are capable of crossing the blood-brain barrier,208,210 and their surface proteins can be engineered to facilitate targeted delivery to specific cell types or tissues,211,212 offering advantages over synthetic nanoparticles.213 Hawdon’s research team has explored transgenic nematodes as novel biological vectors for therapeutic delivery, showcasing the potential of worms in this context.214 The delivery of parasitic miRNAs and EVs can be further enhanced by various innovative strategies. Exosomes, particularly small exosomes (sEVs), have gained attention as promising miRNA delivery vehicles due to their natural ability for intercellular communication.215,216 For instance, the combination of graphene quantum dots with sEVs enables real-time visualization and enhances the delivery of miRNA (such as miR-193a-3p).217 Electroporation, an optimized technique, has been used to improve the loading and delivery efficiency of synthetic miRNA mimics into plasma-derived exosomes.215 Another alternative approach, temperature-controlled co-incubation, has been shown to effectively load miRNA into serum-derived exosomes.218 In addition, regulating miRNA biogenesis pathways can enhance miRNA localization within exosomes, as demonstrated in the case of miR-146a-5p, which improved the therapeutic efficacy of exosomes derived from induced pluripotent stem cells.219 The chemical transfection method Exo-Fect Transfection Reagent has also been reported to significantly increase miRNA loading efficiency in small exosomes, boosting the target miRNA expression by more than 1000-fold, thereby enhancing its delivery and functional effects in target cells.220 These studies highlight the high efficiency and multifunctionality of EVs and miRNAs in helminth-derived therapeutics.
Plant Glycosylation of Worm Proteins
Glycosylation, particularly N-glycosylation, is a critical post-translational modification of worm proteins, essential for proper protein folding. It plays a pivotal role in glycosylation-dependent quality control, protein localization, resistance to proteolytic degradation, solubility, and biological activity,221 significantly influencing immune interactions between the worm and its host.91,222–225 However, this complexity makes the large-scale purification of immunoregulatory glycoproteins from worms technically challenging.
Fortunately, plants can have complex glycosylation processes, including N-glycosylation and O-glycosylation. Over the past two decades, plants have evolved into a multifunctional platform for producing recombinant proteins.226 Recently, plants have successfully produced recombinant proteins from parasites, utilizing their relatively simple glycome to generate heterologous proteins with more consistent glycosylation profiles. Compared to other production systems like E. coli,227 insect cells,228 and TE671 cells,229 which often introduce unintended alterations in glycan compositions, plant-produced glycoproteins feature remarkably uniform N-glycan profiles.230,231 Certain characteristic features of the limited plant glycome align with those of worms, including non-mammalian N-glycan core modifications and a lack of sialylation. Plants’ tolerance for engineered exogenous N- and O-glycans and their capacity to modify their glycosylation strategies through glycoengineering render them an optimal platform for glycoprotein production232 (Figure 4). Additionally, the plant expression systems offer scalability from minor cell suspensions to GMP bioreactors, ensuring no mammalian pathogens or carcinogenic sequences are present, thereby facilitating safe, rapid, and flexible biopharmaceutical production.233 Research has identified enzymes (HEXO2 and HEXO3) that specifically cleave N-glycans on the κ-5 glycoproteins of worms. By precisely editing these enzymes to minimize undesirable N-glycan processing, it is possible to manufacture therapeutically relevant glycoproteins with tailored worm N-glycans in plants,234 mainly since plants are highly adaptable to engineering modifications of their intrinsic N-glycosylation pathways.230 Experiments with plant-produced recombinant ω1 in obese mice have demonstrated significant increases in CTLA-4 levels, pointing to potential of ω1 to boost Treg functions, reduce body fat, and improve overall insulin sensitivity and glucose tolerance in a time- and dose-dependent manners.57,171 Utilizing plants as a production system reduces pathogen contamination risks and enhances the safety of the final product due to their non-host status for human or animal pathogens.
 |
Figure 4 Comparison of protein glycosylation patterns in different biological systems. In the plant section, a specific glycosylation pattern can be seen by transferring T-DNA containing the Bacillus thuringiensis (Bt) gene into plant cells and regenerating plants. The types of glycosylation expressed by proteins are also shown in yeast, insects, and mammals (such as CHO and HEK293 cells). Created in BioRender. Yunhuan Zhu (2025) https://BioRender.com/l22b252. |
Combination Therapy Strategy
Researchers can either deliver individual parasitic factors or co-deliver multiple parasite ES molecules to investigate their synergistic effects or administer them in conjunction with other immunomodulators such as rapamycin, cytokines, and bacterial antigens, which individually exhibit considerable variability in circulation duration and biodistribution.235,236 Immunomodulators, encompassing a broad spectrum of physicochemical properties from small molecules to nucleic acids, proteins, lipids, and fatty acids,237,238 require a co-delivery system to facilitate synergistic interactions, and a topic extensively reviewed in prior literature.239
Helminth-derived molecules can be combined with anti-diabetic drugs to enhance therapeutic outcomes by modulating immune and metabolic pathways. Current anti-diabetic drugs, including metformin and thiazolidinediones, have demonstrated certain anti-inflammatory properties, which contribute to their effectiveness in managing DM.240,241 The combination of these drugs with worm-derived molecules could augment their anti-inflammatory effects, potentially improving blood glucose control and reducing the risk of diabetes-related complications,241 as well as mitigating side effects associated with oral hypoglycemic agents.242 Research has indicated that Dipeptidyl Peptidase-IV inhibitors, commonly used in DM treatment, can be co-administered with worm ESPs to enhance glucose-lowering effects.243,244 However, comprehensive clinical trials are still necessary to thoroughly assess the safety and efficacy of combining worm-derived molecules with conventional medications, ensuring that the benefits outweigh any potential risks.245
Challenges in Translating Helminthic Therapy to Clinical Use
Challenges in Clinical Efficacy
Translating these findings from mouse models into clinical applications is a challenging endeavor. One major issue arises from the diversity of parasitic worm species and the complexity of their life cycles. This raises concerns about safety and tolerance, as the introduction of live worms or their components could provoke unexpected side effects, such as increased susceptibility to other infections.246,247 Furthermore, little is understood about the optimal dose or duration of parasitic infections necessary to confer protection in humans, and concerns regarding the potential harmful effects of these infections have hindered clinical trials. The interpretation of clinical data on experimental worm infections is also complicated by high levels of immune variability across different genetic backgrounds. In fact, not all studies have demonstrated an impact of worm infections or deworming on allergic inflammation,248,249 which is consistent with the lack of therapeutic effect of worm infections in human autoimmune allergic inflammation.250–252
Therefore, selecting the appropriate species of worms and determining the optimal treatment dose and duration remain critical unresolved challenges in both scientific research and clinical practice. Although worm-derived proteins have shown promise in modulating immune responses and alleviating inflammation in diseases like inflammatory bowel disease and asthma, the precise mechanisms underlying their effects are not yet fully understood.253,254 While regulation of pro-inflammatory cytokines and induction of anti-inflammatory responses have been observed, these effects tend to be localized and cannot be consistently replicated at the systemic level.254,255 Additionally, factors such as host genetics, diet, and environmental conditions contribute to variability in treatment responses, complicating the standardization of therapeutic protocols.256 Species-specific differences among worms and the formulation of appropriate dosing regimens are also key factors that require further investigation to optimize therapeutic outcomes. As with other biological therapies, worm-based treatments also carry the risk of developing resistance, as the host immune system may gradually build tolerance, reducing the efficacy of treatment over time.257 Finally, the clinical effectiveness of worm therapy may be significantly affected by the formulation and storage conditions of the therapeutic worms. Experimental studies have shown that improper pH during storage can impair the clinical efficacy of Trichuris suis eggs.258 The development of worm-based biopharmaceuticals faces further challenges related to the pharmacokinetics and chemical properties of these compounds.259
Public Awareness and Acceptance
The concept of using parasites as a therapeutic approach is often met with skepticism and fear by the public, with these attitudes representing a major barrier to the acceptance and adoption of worm therapy.260 Furthermore, perceptions of worms differ significantly across cultures and societies. In Western societies, where personal hygiene and environmental sanitation are prioritized, the idea of introducing worms into the human body is particularly hard to accept. This cultural resistance may impede the acceptance of worm therapy and its integration into mainstream medical practice.261,262
Regulatory and Economic Challenges
Due to the complex and stringent approval processes for biological therapies, worm therapy faces significant regulatory challenges. Additionally, extensive clinical trials are required to demonstrate both safety and efficacy, which can be expensive and time-intensive.263 Furthermore, the economic challenges associated with developing and commercializing worm-based therapies add another layer of difficulty. The costs related to research, development, and regulatory approval may be prohibitively high, particularly for small companies or research teams.263
Conclusion
In recent decades, the global prevalence of DM has surged dramatically, positioning it as a significant global public health challenge. This article investigates the therapeutic potential of parasitic interventions for DM, particularly in the context of the “hygiene hypothesis”, which posits that parasitic infections may provide a novel therapeutic approach by modulating immune responses, exerting anti-inflammatory effects, and improving insulin sensitivity. A growing body of evidence indicates that certain helminthic infections can enhance insulin sensitivity by modulating host immune responses and suppressing chronic inflammation, offering promise for the treatment of DM. ESPs derived from parasites have been identified as an emerging therapeutic strategy, demonstrating significant immunoregulatory potential in various inflammatory conditions, with a relatively lower incidence of adverse effects.21
Although parasitic derivatives may help alleviate the chronic inflammation associated with DM, their potential risks and challenges must not be overlooked. Therefore, the need to balance the therapeutic benefits and potential risks of parasitic interventions is a crucial focus for future research. Further exploration of the immunoregulatory mechanisms of worm-derived products,264 optimization of their drug development processes,265 and undertaking large-scale clinical trials are essential steps toward providing safer and more effective treatment alternatives for diabetic patients.266
Future research should focus on verifying the long-term clinical efficacy and safety of these therapies and investigating their potential for combination with existing DM medications, such as insulin or metformin. Additionally, enhancing the acceptance and adoption of parasitic therapies through educational initiatives and public awareness campaigns will facilitate their broader application in DM management.267–269 Parasitic derivatives, as an emerging therapeutic approach, may offer an economically viable treatment option for diabetic patients worldwide, particularly in resource-limited regions. This therapy holds the potential not only to reduce the global healthcare burden of DM but also to provide new treatment opportunities for both healthcare providers and patients.
Funding
This research was funded by the National Natural Science Foundation of China (Grant No: 32000293); Guangxi Natural Science Foundation (Grant Nos: 2020JJA130077 and 2018JJB140423); the University Level Scientific Research Project of Zhejiang Shuren University (Grant No: 2022R064); Zhejiang Shuren University Basic Scientific Research Special Funds (Grant No: 2024XZ014); and the National Innovation and Entrepreneurship Training Program for College Students in 2023 (Grant No: 202311842052X).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Balooch Hasankhani M, Mirzaei H, Karamoozian A. Global trend analysis of diabetes mellitus incidence, mortality, and mortality-to-incidence ratio from 1990 to 2019. Sci Rep. 2023;13(1):21908. doi:10.1038/s41598-023-49249-0
2. Sun J, Hu W, Ye S, et al. The description and prediction of incidence, prevalence, mortality, disability-adjusted life years cases, and corresponding age-standardized rates for global diabetes. J Epidemiol Glob Health. 2023;13(3):566–576. doi:10.1007/s44197-023-00138-9
3. International Diabetes Federation. IDF diabetes atlas. 10th ed. Brussels, Belgium; 2021. Available from: https://www.diabetesatlas.org.
4. Haller MJ, Schatz DA. Cytokines and Type 1 Diabetes Complications: Casual or Causal Association? Wiley Online Library; 2008:1–2.
5. Padgett LE, Broniowska KA, Hansen PA, et al. The role of reactive oxygen species and proinflammatory cytokines in type 1 diabetes pathogenesis. Ann N Y Acad Sci. 2013;1281(1):16–35. doi:10.1111/j.1749-6632.2012.06826.x
6. Enns JE, Taylor CG, Zahradka P. The role of inflammation in type 2 diabetes-driven atherosclerosis. Diabetic Cardiomyopathy. 2014:213–237.
7. Kamal MA, Priyamvada S, Anbazhagan AN, et al. Linking Alzheimer’s disease and type 2 diabetes mellitus via aberrant insulin signaling and inflammation. CNS Neurol Disord Drug Targets. 2014;13(2):338–346.
8. Stulnig TM. Inflammation as a trigger for insulin resistance and cardiometabolic disease. In: Morbid Obesity in Adolescents: Conservative Treatment and Surgical Approaches. Springer; 2014:15–20.
9. Shin JJ, Lee EK, Park TJ, et al. Damage-associated molecular patterns and their pathological relevance in diabetes mellitus. Ageing Res Rev. 2015;24:66–76. doi:10.1016/j.arr.2015.06.004
10. Ballak DB, Stienstra R, Tack CJ, et al. IL-1 family members in the pathogenesis and treatment of metabolic disease: focus on adipose tissue inflammation and insulin resistance. Cytokine. 2015;75(2):280–290. doi:10.1016/j.cyto.2015.05.005
11. Schön M, Herder C. Inflammation in the insulin-resistant endotype of diabetes: consequences for prevention and treatment. Die Diabetologie. 2024:1–3.
12. Lontchi-Yimagou E, Sobngwi E, Matsha TE, et al. Diabetes mellitus and inflammation. Curr Diab Rep. 2013;13(3):435–444. doi:10.1007/s11892-013-0375-y
13. Arif M, Nigoskar S, Verma MK, et al. Screening type 2 diabetes mellitus among Indians using inflammatory biomarkers. Bioinformation. 2024;20(5):515–519. doi:10.6026/973206300200515
14. Du Y, Zhang Q, Zhang X, et al. Correlation between inflammatory biomarkers, cognitive function and glycemic and lipid profiles in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Clin Biochem. 2023;121-122:110683. doi:10.1016/j.clinbiochem.2023.110683
15. Nolan MS, Murray KO, Mejia R, et al. Elevated pediatric Chagas disease burden complicated by concomitant intestinal parasites and malnutrition in El Salvador. Trop Med Infect Dis. 2021;6(2):72. doi:10.3390/tropicalmed6020072
16. Apaza C, Cuna W, Brañez F, et al. Frequency of gastrointestinal parasites, anemia, and nutritional status among children from different geographical regions of Bolivia. J Trop Med. 2023;2023:5020490. doi:10.1155/2023/5020490
17. Ali AY, Mohamed Abdi A, Mambet E. Small bowel obstruction caused by massive ascariasis: two case reports. Ann Med Surg. 2023;85(3):486–489. doi:10.1097/MS9.0000000000000224
18. Yeshi K, Ruscher R, Loukas A, et al. Immunomodulatory and biological properties of helminth-derived small molecules: potential applications in diagnostics and therapeutics. Front Parasitol. 2022;1:984152. doi:10.3389/fpara.2022.984152
19. Abdoli A, Badirzadeh A, Mojtabavi N, et al. Immunomodulatory effects of parasites on autoimmunity. In: Translational Autoimmunity. Elsevier; 2022:395–424.
20. Elsawey AM, Nabih N, Etewa S, et al. Parasitic infections’ immunomodulatory effects and autoimmune diseases. Egypt Acad J Biol Sci E Med Entomol Parasitol. 2022;14(2):27–37. doi:10.21608/eajbse.2022.259101
21. Mu Y, McManus DP, Hou N, et al. Schistosome infection and schistosome-derived products as modulators for the prevention and alleviation of immunological disorders. Front Immunol. 2021;12:619776. doi:10.3389/fimmu.2021.619776
22. Camaya I, O’Brien B, Donnelly S. How do parasitic worms prevent diabetes? An exploration of their influence on macrophage and β-cell crosstalk. Front Endocrinol. 2023;14:1205219. doi:10.3389/fendo.2023.1205219
23. Berbudi A, Ajendra J, Wardani APF, et al. Parasitic helminths and their beneficial impact on type 1 and type 2 diabetes. Diabetes/Metab Res Rev. 2016;32(3):238–250. doi:10.1002/dmrr.2673
24. Cabalén ME, Cabral MF, Sanmarco LM, et al. Chronic Trypanosoma cruzi infection potentiates adipose tissue macrophage polarization toward an anti-inflammatory M2 phenotype and contributes to diabetes progression in a diet-induced obesity model. Oncotarget. 2016;7(12):13400. doi:10.18632/oncotarget.7630
25. Shen SW, Lu Y, Li F, et al. The potential long-term effect of previous schistosome infection reduces the risk of metabolic syndrome among Chinese men. Parasite Immunol. 2015;37(7):333–339. doi:10.1111/pim.12187
26. Chen Y, Lu J, Huang Y, et al. Association of previous schistosome infection with diabetes and metabolic syndrome: a cross-sectional study in rural China. J Clin Endocrinol Metab. 2013;98(2):E283–7. doi:10.1210/jc.2012-2517
27. Shen SW, Lu Y, Li F, et al. Potential long-term effects of previous schistosome infection may reduce the atherogenic index of plasma in Chinese men. Int J Parasitol. 2015;45(5):289–294. doi:10.1016/j.ijpara.2015.01.001
28. Zaccone P, Hall SW. Helminth infection and type 1 diabetes. Rev Diabetic Stud. 2012;9(4):272. doi:10.1900/RDS.2012.9.272
29. Tahapary DL, de Ruiter K, Martin I, et al. Helminth infections and type 2 diabetes: a cluster-randomized placebo controlled SUGARSPIN trial in Nangapanda, Flores, Indonesia. BMC Infect Dis. 2015;15:133.
30. Hays RJ. Helminth Infection and metabolic Disease: Strongyloides Stercoralis Infection and Type 2 Diabetes Mellitus in an Aboriginal Community. James Cook University; 2018.
31. Sanya RE, Webb EL, Zziwa C, et al. The effect of helminth infections and their treatment on metabolic outcomes: results of a cluster-randomized trial. Clinl Infect Dis. 2019;71:601–613. doi:10.1093/cid/ciz859
32. Croese J, Miller GC, Marquart L, et al. Randomized, placebo controlled trial of experimental hookworm infection for improving gluten tolerance in celiac disease. Clin Transl Gastroenterol. 2020;11(12):e00274. doi:10.14309/ctg.0000000000000274
33. Croese J, Giacomin P, Navarro S, et al. Experimental hookworm infection and gluten microchallenge promote tolerance in celiac disease. J Allergy Clin Immunol. 2015;135(2):508–516. doi:10.1016/j.jaci.2014.07.022
34. Tanasescu R, Tench CR, Constantinescu CS, et al. Hookworm treatment for relapsing multiple sclerosis: a randomized double-blinded placebo-controlled trial. JAMA neurol. 2020;77(9):1089–1098. doi:10.1001/jamaneurol.2020.1118
35. Pierce D, Merone L, Lewis C, et al. Safety and tolerability of experimental hookworm infection in humans with metabolic disease: study protocol for a phase 1b randomised controlled clinical trial. BMC Endocr Disord. 2019;19(1):136. doi:10.1186/s12902-019-0461-5
36. Cantacessi C, Giacomin P, Croese J, et al. Impact of experimental hookworm infection on the human gut microbiota. J Infect Dis. 2014;210(9):1431–1434. doi:10.1093/infdis/jiu256
37. Croese J, Gaze ST, Loukas A. Changed gluten immunity in celiac disease by Necator americanus provides new insights into autoimmunity. Int J Parasitol. 2013;43(3–4):275–282. doi:10.1016/j.ijpara.2012.12.005
38. Pierce DR, McDonald M, Merone L, et al. Effect of experimental hookworm infection on insulin resistance in people at risk of type 2 diabetes. Nat Commun. 2023;14(1):4503. doi:10.1038/s41467-023-40263-4
39. Alimpolos JA, Martinez CR, Morris NM, et al. Potential association of strongyloides stercoralis and its effectivity against type 2 diabetes mellitus: a systematic review of scientific attestation. Int J Multidiscip. 2023;4(12):4496–4503.
40. Dasan B, Rajamanickam A, Munisankar S, et al. Hookworm infection induces glycometabolic modulation in South Indian individuals with type 2 diabetes. IJID Regions. 2023;9:18–24. doi:10.1016/j.ijregi.2023.08.009
41. Rajamanickam A, Munisankar S, Dolla C, et al. Helminth infection modulates systemic pro-inflammatory cytokines and chemokines implicated in type 2 diabetes mellitus pathogenesis. PLoS Negl Trop Dis. 2020;14:e0008101.
42. Mgbere O, Khuwaja S, Bell TK, et al. System and patient barriers to care among people living with HIV/AIDS in Houston/Harris County, Texas: HIV medical care providers’ perspectives. J Int Assoc Provid AIDS Care. 2015;14(6):505–515. doi:10.1177/2325957414539045
43. Rajamanickam A, Dasan B, Munisankar S, et al. Impact of Strongyloides stercoralis infection on complement activation in Type 2 diabetes mellitus: insights from a clinical and anthelmintic intervention study. PLoS Negl Trop Dis. 2024;18(4):e0012048. doi:10.1371/journal.pntd.0012048
44. Taghipour A, Javanmard E, Rahimi HM, et al. Prevalence of intestinal parasitic infections in patients with diabetes: a systematic review and meta-analysis. Int Health. 2024;16(1):23–34. doi:10.1093/inthealth/ihad027
45. El-Kady AM, Alzahrani AM, Elshazly H, et al. Pancreatic pathological changes in murine toxoplasmosis and possible association with diabetes mellitus. Biomedicines. 2022;11(1):18. doi:10.3390/biomedicines11010018
46. Catchpole A, Zabriskie BN, Bassett P, et al. Association between toxoplasma gondii infection and type-1 diabetes mellitus: a systematic review and meta-analysis. Int J Environ Res Public Health. 2023;20(5):4436. doi:10.3390/ijerph20054436
47. Rodríguez-Pérez EG, Arce-Mendoza AY, Saldívar-Palacios R, et al. Fatal Strongyloides stercoralis hyperinfection syndrome in an alcoholic diabetic patient from México. Biomedica. 2020;40(Supl. 1):32–36. doi:10.7705/biomedica.5071
48. Gökçe C, Aycan-Kaya Ö, Yula E, et al. The effect of blood glucose regulation on the presence of opportunistic Demodex folliculorum mites in patients with type 2 diabetes mellitus. J Int Med Res. 2013;41(5):1752–1758. doi:10.1177/0300060513494730
49. Zhang N, Wen K, Liu Y, et al. High prevalence of demodex infestation is associated with poor blood glucose control in type 2 diabetes mellitus: a cross-sectional study in the Guangzhou diabetic eye study. Cornea. 2023;42(6):670–674. doi:10.1097/ICO.0000000000003116
50. Abidha CA, Amoako YA, Nyamekye RK, et al. Fasting blood glucose in a Ghanaian adult is causally affected by malaria parasite load: a mechanistic case study using convergent cross mapping. Malaria j. 2022;21(1):93. doi:10.1186/s12936-022-04076-y
51. Ch’ng JH, Moll K, Wyss K, et al. Enhanced virulence of Plasmodium falciparum in blood of diabetic patients. PLoS One. 2021;16(6):e0249666. doi:10.1371/journal.pone.0249666
52. Amdare N, Khatri VK, Yadav RSP, et al. Therapeutic potential of the immunomodulatory proteins Wuchereria bancrofti L2 and Brugia malayi abundant larval transcript 2 against streptozotocin-induced type 1 diabetes in mice. J Helminthol. 2016;91:539–548. doi:10.1017/S0022149X1600064X
53. Amer AS, Othman AA, Dawood LM, et al. The interaction of Schistosoma mansoni infection with diabetes mellitus and obesity in mice. Sci Rep. 2023;13(1):9417. doi:10.1038/s41598-023-36112-5
54. Ajendra J, Berbudi A, Hoerauf A, et al. Combination of worm antigen and proinsulin prevents type 1 diabetes in NOD mice after the onset of insulitis. Clin Immunol. 2016;164:119–122. doi:10.1016/j.clim.2016.02.005
55. Lund ME, O’Brien BA, Hutchinson AT, et al. Secreted proteins from the helminth fasciola hepatica inhibit the initiation of autoreactive T cell responses and prevent diabetes in the NOD mouse. PLoS One. 2014;9:e86289.
56. Khudhair Z, Trybała W, Chaumont-Dubel S, et al. Administration of hookworm excretory/secretory proteins improves glucose tolerance in a mouse model of type 2 diabetes. Biomolecules. 2022;13:12. doi:10.3390/biom13010012
57. Hams E, Bermingham R, Wurlod FA, et al. The helminth T2 RNase ω1 promotes metabolic homeostasis in an IL-33–and group 2 innate lymphoid cell–dependent mechanism. FASEB J. 2016;30(2):824. doi:10.1096/fj.15-277822
58. van Der Zande HJ, Gonzalez MA, de Ruiter K, et al. The helminth glycoprotein omega‐1 improves metabolic homeostasis in obese mice through type 2 immunity‐independent inhibition of food intake. FASEB J. 2021;35(2).
59. Whitehead B, Boysen AT, Mardahl M, et al. Unique glycan and lipid composition of helminth-derived extracellular vesicles may reveal novel roles in host-parasite interactions. Int J Parasitol. 2020;50(9):647–654. doi:10.1016/j.ijpara.2020.03.012
60. Aprahamian TR, Zhong X, Amir S, et al. The immunomodulatory parasitic worm product ES-62 reduces lupus-associated accelerated atherosclerosis in a mouse model. Int J Parasitol. 2015;45(4):203–207. doi:10.1016/j.ijpara.2014.12.006
61. Queiroz-Glauss CP, Vieira MS, Gonçalves-Pereira MH, et al. Helminth infection modulates number and function of adipose tissue Tregs in high fat diet-induced obesity. PLoS Negl Trop Dis. 2022;16(5):e0010105. doi:10.1371/journal.pntd.0010105
62. Su CW, Chen C-Y, Li Y, et al. Helminth infection protects against high fat diet-induced obesity via induction of alternatively activated macrophages. Sci Rep. 2018;8(1):4607. doi:10.1038/s41598-018-22920-7
63. Kang SA, Yu HS. Anti-obesity effects by parasitic nematode (Trichinella spiralis) total lysates. Front Cell Infect Microbiol. 2024;13:1285584. doi:10.3389/fcimb.2023.1285584
64. Hussaarts L, García‐Tardón N, Beek L, et al. Chronic helminth infection and helminth‐derived egg antigens promote adipose tissue M2 macrophages and improve insulin sensitivity in obese mice. FASEB J. 2015;29(7):3027–3039. doi:10.1096/fj.14-266239
65. Jeerawattanawart S, Hansakon A, Roytrakul S, et al. Regulation and function of adiponectin in the intestinal epithelial cells in response to Trichinella spiralis infection. Sci Rep. 2023;13(1):14004. doi:10.1038/s41598-023-41377-x
66. Andersen D, Moll JM, Arora P, et al. Oral administration of helminth fluid modulates distinct tuft cell and immune‐metabolic cues linked to reduced body fat. Parasite Immunol. 2023;45(7):e12998. doi:10.1111/pim.12998
67. Parks SC, Nguyen S, Nasrolahi S, et al. Parasitic nematode fatty acid-and retinol-binding proteins compromise host immunity by interfering with host lipid signaling pathways. PLoS Pathog. 2021;17(10):e1010027. doi:10.1371/journal.ppat.1010027
68. Crowe J, Lumb FE, Harnett MM, et al. Parasite excretory‐secretory products and their effects on metabolic syndrome. Parasite Immunol. 2017;39(5):e12410. doi:10.1111/pim.12410
69. da Silva VJ, Dias SRC, Alves WP, et al. Hookworm infection aggravates metabolic disorder in obesity. Mol Biochem Parasitol. 2019;232:111200. doi:10.1016/j.molbiopara.2019.111200
70. Zavala G, García OP, Campos‐Ponce M, et al. Children with moderate-high infection with Entamoeba coli have higher percentage of body and abdominal fat than non-infected children. Pediatr Obes. 2016;11(6):443–449. doi:10.1111/ijpo.12085
71. Rajamanickam A, Munisankar S, Thiruvengadam K, et al. Impact of helminth infection on metabolic and immune homeostasis in non-diabetic obesity. Front Immunol. 2020;11:2195. doi:10.3389/fimmu.2020.02195
72. Kuipers ME, Koning RI, Bos E, et al. Optimized protocol for the isolation of extracellular vesicles from the parasitic worm schistosoma mansoni with improved purity, concentration, and yield. J Immunol Res. 2022;2022:5473763. doi:10.1155/2022/5473763
73. Eichenberger RM, Ryan S, Jones L, et al. Hookworm secreted extracellular vesicles interact with host cells and prevent inducible colitis in mice. Front Immunol. 2018;9:850. doi:10.3389/fimmu.2018.00850
74. Yuan Y, Nian F, Li H, et al. [Protective effect of excretory-secretory proteins from Trichinella spiralis muscle larvae against myocardial injury in septic mice]. Nan Fang Yi Ke Da Xue Xue Bao. 2022;42(6):824–831. Sesotho. doi:10.12122/j.issn.1673-4254.2022.06.05
75. Kidd P. Th1/Th2 balance: the hypothesis, its limitations, and implications for health and disease. Altern Med Review. 2003;8(3):223–246.
76. Alghanmi M, Minshawi F, Altorki TA, et al. Helminth-derived proteins as immune system regulators: a systematic review of their promise in alleviating colitis. BMC Immunol. 2024;25(1):21. doi:10.1186/s12865-024-00614-2
77. White MPJ, Smyth DJ, Cook L, et al. The parasite cytokine mimic Hp-TGM potently replicates the regulatory effects of TGF-β on murine CD4+ T cells. Immunol cell biol. 2021;99(8):848–864. doi:10.1111/imcb.12479
78. Cook L, Reid KT, Häkkinen E, et al. Induction of stable human FOXP3+ Tregs by a parasite-derived TGF-β mimic. Immunol cell biol. 2021;99(8):833–847. doi:10.1111/imcb.12475
79. Stachyra A, Wesołowska A. Immunomodulatory in vitro effects of Trichinella cystatin-like protein on mouse splenocytes. Exp Parasitol. 2023;252:108585. doi:10.1016/j.exppara.2023.108585
80. Khatri V, Amdare N, Tarnekar A, et al. Brugia malayi cystatin therapeutically ameliorates dextran sulfate sodium-induced colitis in mice. J Dig Dis. 2015;16(10):585–594. doi:10.1111/1751-2980.12290
81. Bisht N, Khatri V, Chauhan N, et al. Cystatin from filarial parasites suppress the clinical symptoms and pathology of experimentally induced colitis in mice by inducing T-regulatory cells, B1-cells, and alternatively activated macrophages. Biomedicines. 2019;7(4):85. doi:10.3390/biomedicines7040085
82. Voldsgaard A, Bager P, Garde E, et al. Trichuris suis ova therapy in relapsing multiple sclerosis is safe but without signals of beneficial effect. Mult Scler. 2015;21(13):1723–1729. doi:10.1177/1352458514568173
83. Yang Q, Li J, Zhang L, et al. Type I cystatin derived from cysticercus pisiformis-stefins, suppresses LPS-mediated inflammatory response in RAW264.7 cells. Microorganisms. 2024;12(5):850. doi:10.3390/microorganisms12050850
84. Martínez-Colón GJ, Moore BB. Prostaglandin E(2) as a regulator of immunity to pathogens. Pharmacol Ther. 2018;185:135–146. doi:10.1016/j.pharmthera.2017.12.008
85. Robb CT, McSorley HJ, Lee J, et al. Prostaglandin E(2) stimulates adaptive IL-22 production and promotes allergic contact dermatitis. J Allergy Clin Immunol. 2018;141(1):152–162. doi:10.1016/j.jaci.2017.04.045
86. Tang CL, Yu X-H, Li Y, et al. Schistosoma japonicum soluble egg antigen protects against type 2 diabetes in Lepr (db/db) mice by enhancing regulatory T cells and Th2 cytokines. Front Immunol. 2019;10:1471. doi:10.3389/fimmu.2019.01471
87. Nono JK, Lutz MB, Brehm K. Expansion of host regulatory T cells by secreted products of the tapeworm echinococcus multilocularis. Front Immunol. 2020;11:798. doi:10.3389/fimmu.2020.00798
88. Pineda MA, Lumb F, Harnett MM, et al. ES-62, a therapeutic anti-inflammatory agent evolved by the filarial nematode Acanthocheilonema viteae. Mol Biochem Parasitol. 2014;194(1–2):1–8. doi:10.1016/j.molbiopara.2014.03.003
89. Harnett MM, Doonan J, Lumb FE, et al. The parasitic worm product ES-62 protects the osteoimmunology axis in a mouse model of obesity-accelerated ageing. Front Immunol. 2022;13:953053. doi:10.3389/fimmu.2022.953053
90. Ke XD, Shen S, Song L-J, et al. Characterization of Schistosoma japonicum CP1412 protein as a novel member of the ribonuclease T2 molecule family with immune regulatory function. Parasit Vectors. 2017;10(1):89. doi:10.1186/s13071-016-1962-y
91. Acharya S, Da’dara AA, Skelly PJ. Schistosome immunomodulators. PLoS Pathog. 2021;17(12):e1010064. doi:10.1371/journal.ppat.1010064
92. Creusot RJ, Biswas J, Thomsen L, et al. Instruction of naive CD4+ T cells by polarized CD4+ T cells within dendritic cell clusters. Eur J Immunol. 2003;33(6):1686–1696. doi:10.1002/eji.200323811
93. Gerner MY, Wu Y, Huang JY. Dendritic cells regulate innate cell trafficking into lymph nodes during inflammation. J Immunol. 2022;208(1_Supplement):
94. Wang X, Zhao C, Zhang G, et al. Molecular characterization of a novel GSTO2 of Fasciola hepatica and its roles in modulating murine macrophages. Parasite. 2022;29:16. doi:10.1051/parasite/2022016
95. Suckling CJ, Alam S, Olson MA, et al. Small molecule analogues of the parasitic worm product ES-62 interact with the TIR domain of MyD88 to inhibit pro-inflammatory signalling. Sci Rep. 2018;8(1):2123. doi:10.1038/s41598-018-20388-z
96. Ball DH, Al-Riyami L, Harnett W, et al. IL-33/ST2 signalling and crosstalk with FcεRI and TLR4 is targeted by the parasitic worm product, ES-62. Sci Rep. 2018;8(1):4497. doi:10.1038/s41598-018-22716-9
97. Alanazi S, Doonan J, Lumb FE, et al. Reduction in creatine metabolites in macrophages exposed to small molecule analogues of the anti-inflammatory parasitic worm product ES-62. Parasite Immunol. 2024;46(2):e13026. doi:10.1111/pim.13026
98. Camaya I, Mok TY, Lund M, et al. The parasite-derived peptide FhHDM-1 activates the PI3K/Akt pathway to prevent cytokine-induced apoptosis of β-cells.. J Mol Med. 2021;99(11):1605–1621. doi:10.1007/s00109-021-02122-x
99. Quinteros SL, Von Krusenstiern E, Snyder NW, et al. The helminth derived peptide FhHDM-1 redirects macrophage metabolism towards glutaminolysis to regulate the pro-inflammatory response. Front Immunol. 2023;14:1018076. doi:10.3389/fimmu.2023.1018076
100. Cortes‐Selva D, Fairfax K. Schistosome and intestinal helminth modulation of macrophage immunometabolism. Immunology. 2021;162(2):123–134. doi:10.1111/imm.13231
101. Togarsimalemath SK, Pushpamithran G, Schön T, et al. Helminth antigen exposure enhances early immune control of mycobacterium tuberculosis in monocytes and macrophages. J Innate Immun. 2021;13(3):148–163. doi:10.1159/000512279
102. Hallowell R, Collins SL, Craig JM, et al. mTORC2 signalling regulates M2 macrophage differentiation in response to helminth infection and adaptive thermogenesis. Nat Commun. 2017;8(1):14208. doi:10.1038/ncomms14208
103. Li H, Qiu D, Yang H, et al. Therapeutic efficacy of excretory-secretory products of trichinella spiralis adult worms on sepsis-induced acute lung injury in a mouse model. Front Cell Infect Microbiol. 2021;11:653843. doi:10.3389/fcimb.2021.653843
104. Harnett MM, Harnett W. Can parasitic worms cure the modern world’s ills? Trends Parasitol. 2017;33(9):694–705. doi:10.1016/j.pt.2017.05.007
105. Doolan R, Putananickal N, Tritten L, et al. How to train your myeloid cells: a way forward for helminth vaccines? Front Immunol. 2023;14:1163364. doi:10.3389/fimmu.2023.1163364
106. Bunte MJM, Schots A, Kammenga JE, et al. Helminth glycans at the host-parasite interface and their potential for developing novel therapeutics. Front Mol Biosci. 2021;8:807821. doi:10.3389/fmolb.2021.807821
107. Narasimhan PB, Bennuru S, Meng Z, et al. Microfilariae of brugia malayi inhibit the mTOR pathway and induce autophagy in human dendritic cells. Infect Immun. 2016;84(9):2463–2472. doi:10.1128/IAI.00174-16
108. Sanku G, Ricciardi A, Redekar NR, et al. Brugia malayi filarial helminth-derived extracellular vesicles suppress antigen presenting cell function and antigen-specific CD4+ T cell responses. Front Immunol. 2024;15:1436818. doi:10.3389/fimmu.2024.1436818
109. Li H, Qiu D, Yuan Y, et al. Trichinella spiralis cystatin alleviates polymicrobial sepsis through activating regulatory macrophages. Int Immunopharmacol. 2022;109:108907. doi:10.1016/j.intimp.2022.108907
110. Zhang T, Zhang Y, Yang Z, et al. Echinococcus multilocularis protoscoleces enhance glycolysis to promote M2 Macrophages through PI3K/Akt/mTOR Signaling Pathway. Pathog Global Health. 2023;117(4):409–416. doi:10.1080/20477724.2022.2104055
111. Pineda MA, Eason RJ, Harnett MM, et al. From the worm to the pill, the parasitic worm product ES-62 raises new horizons in the treatment of rheumatoid arthritis. Lupus. 2015;24(4–5):400–411. doi:10.1177/0961203314560004
112. Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11(5):373–384. doi:10.1038/ni.1863
113. Pattanaik KP, Sengupta S, Jit BP, et al. Host-mycobacteria conflict: immune responses of the host vs. the mycobacteria TLR2 and TLR4 ligands and concomitant host-directed therapy. Microbiol Res. 2022;264:127153. doi:10.1016/j.micres.2022.127153
114. Bhoj P, Togre N, Khatri V, et al. Harnessing immune evasion strategy of lymphatic filariae: a therapeutic approach against inflammatory and infective pathology. Vaccines. 2022;10(8). doi:10.3390/vaccines10081235
115. Khatri V, Chauhan N, Kalyanasundaram R. Parasite cystatin: immunomodulatory molecule with therapeutic activity against immune mediated disorders. Pathogens. 2020;9(6):431. doi:10.3390/pathogens9060431
116. Mukherjee S, Karmakar S, Babu SP. TLR2 and TLR4 mediated host immune responses in major infectious diseases: a review. Braz J Infect Dis. 2016;20(2):193–204. doi:10.1016/j.bjid.2015.10.011
117. Goodridge HS, Marshall FA, Else KJ, et al. Immunomodulation via novel use of TLR4 by the filarial nematode phosphorylcholine-containing secreted product, ES-62. J Iimmunol. 2005;174(1):284–293. doi:10.4049/jimmunol.174.1.284
118. Manoury B, Gregory WF, Maizels RM, et al. Bm-CPI-2, a cystatin homolog secreted by the filarial parasite Brugia malayi, inhibits class II MHC-restricted antigen processing. Curr Biol. 2001;11(6):447–451. doi:10.1016/S0960-9822(01)00118-X
119. Kaisar MMM, Ritter M, Del Fresno C, et al. Dectin-1/2-induced autocrine PGE2 signaling licenses dendritic cells to prime Th2 responses. PLoS biol. 2018;16(4):e2005504. doi:10.1371/journal.pbio.2005504
120. Satoh T, Takeuchi O, Vandenbon A, et al. The Jmjd3-Irf4 axis regulates M2 macrophage polarization and host responses against helminth infection. Nat Immunol. 2010;11(10):936–944. doi:10.1038/ni.1920
121. Alvarado R, O’Brien B, Tanaka A, et al. A parasitic helminth-derived peptide that targets the macrophage lysosome is a novel therapeutic option for autoimmune disease. Immunobiology. 2015;220(2):262–269. doi:10.1016/j.imbio.2014.11.008
122. Fleming JO, Weinstock JV. Clinical trials of helminth therapy in autoimmune diseases: rationale and findings. Parasite Immunol. 2015;37(6):277–292. doi:10.1111/pim.12175
123. Fleming J, Hernandez G, Hartman L, et al. Safety and efficacy of helminth treatment in relapsing-remitting multiple sclerosis: results of the HINT 2 clinical trial. Mult Scler. 2019;25(1):81–91. doi:10.1177/1352458517736377
124. Sobotková K, Parker W, Levá J, et al. Helminth therapy - from the parasite perspective. Trends Parasitol. 2019;35(7):501–515. doi:10.1016/j.pt.2019.04.009
125. Morais SB, Figueiredo BC, Assis NRG, et al. Schistosoma mansoni SmKI-1 serine protease inhibitor binds to elastase and impairs neutrophil function and inflammation. PLoS Pathog. 2018;14(2):e1006870. doi:10.1371/journal.ppat.1006870
126. Díaz-Godínez C, Carrero JC. The state of art of neutrophil extracellular traps in protozoan and helminthic infections. Biosci Rep. 2019;39(1):BSR20180916. doi:10.1042/BSR20180916
127. Mendez J, Sun D, Tuo W, et al. Bovine neutrophils form extracellular traps in response to the gastrointestinal parasite Ostertagia ostertagi. Sci Rep. 2018;8(1):17598. doi:10.1038/s41598-018-36070-3
128. Garza JJ, Greiner SP, Bowdridge SA. Ovine vital neutrophil extracellular traps bind and impair Haemonchus contortus L3 in a breed‐dependent manner. Parasite Immunol. 2018;40(9):e12572. doi:10.1111/pim.12572
129. Tamarozzi F, Turner JD, Pionnier N, et al. Wolbachia endosymbionts induce neutrophil extracellular trap formation in human onchocerciasis. Sci Rep. 2016;6(1):35559. doi:10.1038/srep35559
130. Debrah LB, Gyasi C, Ahiadorme M, et al. Association of haemato-biochemical indices and blood composite ratios with microfilaridermia in Onchocerciasis patients. BMC Infect Dis. 2024;24(1):384. doi:10.1186/s12879-024-09278-0
131. Akkus GN, Yildiz K. Extracellular traps development in canine neutrophils induced by infective stage Toxocara canis larvae. Vet Parasitol. 2024;328:110186. doi:10.1016/j.vetpar.2024.110186
132. Chauhan A, Sharma A, Tripathi JK, et al. Helminth derived factors inhibit neutrophil extracellular trap formation and inflammation in bacterial peritonitis. Sci Rep. 2021;11(1):12718. doi:10.1038/s41598-021-92001-9
133. Bonne-Année S, Kerepesi LA, Hess JA, et al. Extracellular traps are associated with human and mouse neutrophil and macrophage mediated killing of larval Strongyloides stercoralis. Microb Infect. 2014;16(6):502–511. doi:10.1016/j.micinf.2014.02.012
134. Wang J, Tang B, You X, et al. Trichinella spiralis excretory/secretory products from adult worms inhibit NETosis and regulate the production of cytokines from neutrophils. Parasit Vectors. 2023;16(1):374. doi:10.1186/s13071-023-05979-8
135. Jo YR, Park HT, Yu HS, et al. Trichinella infection ameliorated vincristine-induced neuroinflammation in mice. Korean J Parasitol. 2022;60(4):247–254. doi:10.3347/kjp.2022.60.4.247
136. Gazzinelli-Guimaraes PH, Jones SM, Voehringer D, et al. Eosinophils as modulators of host defense during parasitic, fungal, bacterial, and viral infections. J Leukoc Biol. 2024:qiae173.
137. Cadman ET, Thysse KA, Bearder S, et al. Eosinophils are important for protection, immunoregulation and pathology during infection with nematode microfilariae. PLoS Pathog. 2014;10(3):e1003988. doi:10.1371/journal.ppat.1003988
138. Huang L, Appleton JA. Eosinophils in helminth infection: defenders and dupes. Trends Parasitol. 2016;32(10):798–807. doi:10.1016/j.pt.2016.05.004
139. Santiago HDC, Ribeiro-Gomes FL, Bennuru S, et al. Helminth infection alters IgE responses to allergens structurally related to parasite proteins. J Immunol. 2015;194(1):93–100. doi:10.4049/jimmunol.1401638
140. Driss V, El Nady M, Delbeke M, et al. The schistosome glutathione S-transferase P28GST, a unique helminth protein, prevents intestinal inflammation in experimental colitis through a Th2-type response with mucosal eosinophils. Mucosal Immunol. 2016;9(2):322–335. doi:10.1038/mi.2015.62
141. Voehringer D. Basophils in immune responses against helminths. Microb Infect. 2011;13(11):881–887. doi:10.1016/j.micinf.2011.05.001
142. Aumüller E, Schramm G, Gronow A, et al. Echinococcus multilocularis metacestode extract triggers human basophils to release interleukin-4. Parasite Immunol. 2004;26(10):387–395. doi:10.1111/j.0141-9838.2004.00724.x
143. Nakanishi K. Basophils as APC in Th2 response in allergic inflammation and parasite infection. Curr Opin Immunol. 2010;22(6):814–820. doi:10.1016/j.coi.2010.10.018
144. Maddur MS, Kaveri SV, Bayry J. Basophils as antigen presenting cells. Trends Immunol. 2010;31(2):45–48. doi:10.1016/j.it.2009.12.004
145. Webb LM, Oyesola OO, Früh SP, et al. The Notch signaling pathway promotes basophil responses during helminth-induced type 2 inflammation. J Exp Med. 2019;216(6):1268–1279. doi:10.1084/jem.20180131
146. Schworer SA, Olbrich CL, Larsen LD, et al. Notch 2 signaling contributes to intestinal eosinophil adaptations in steady state and tissue burden following oral allergen challenge. J Leukoc Biol. 2024;116(2):379–391. doi:10.1093/jleuko/qiae122
147. Smita S, Webb LM, Mooney B, et al. Basophil responses in susceptible AKR mice upon infection with the intestinal helminth parasite Trichuris muris. Parasite Immunol. 2023;45(8):e12999. doi:10.1111/pim.12999
148. Marcilla A, Trelis M, Cortés A, et al. Extracellular vesicles from parasitic helminths contain specific excretory/secretory proteins and are internalized in intestinal host cells. PLoS One. 2012;7(9):e45974. doi:10.1371/journal.pone.0045974
149. Buck AH, Coakley G, Simbari F, et al. Exosomes secreted by nematode parasites transfer small RNAs to mammalian cells and modulate innate immunity. Nat Commun. 2014;5:5488. doi:10.1038/ncomms6488
150. Quinn SM, Cunningham K, Raverdeau M, et al. Anti-inflammatory trained immunity mediated by helminth products attenuates the induction of T cell-mediated autoimmune disease. Front Immunol. 2019;10:1109. doi:10.3389/fimmu.2019.01109
151. Cunningham KT, Finlay CM, Mills KHG. Helminth imprinting of hematopoietic stem cells sustains anti-inflammatory trained innate immunity that attenuates autoimmune disease. J Iimmunol. 2021;206(7):1618–1630. doi:10.4049/jimmunol.2001225
152. Goodridge HS, Marshall FA, Wilson EH, et al. In vivo exposure of murine dendritic cell and macrophage bone marrow progenitors to the phosphorylcholine-containing filarial nematode glycoprotein ES-62 polarizes their differentiation to an anti-inflammatory phenotype. Immunology. 2004;113(4):491–498. doi:10.1111/j.1365-2567.2004.01993.x
153. Rzepecka J, Siebeke I, Coltherd JC, et al. The helminth product, ES-62, protects against airway inflammation by resetting the Th cell phenotype. Int J Parasitol. 2013;43(3–4):211–223. doi:10.1016/j.ijpara.2012.12.001
154. Ebner F, Lindner K, Janek K, et al. A helminth-derived chitinase structurally similar to mammalian chitinase displays immunomodulatory properties in inflammatory lung disease. J Immunol Res. 2021;2021:6234836. doi:10.1155/2021/6234836
155. Buitrago G, Harnett MM, Harnett W. Conquering rheumatic diseases: are parasitic worms the answer? Trends Parasitol. 2023;39(9):739–748. doi:10.1016/j.pt.2023.06.010
156. Corbet M, Pineda MA, Yang K, et al. Suppression of inflammatory arthritis by the parasitic worm product ES-62 is associated with epigenetic changes in synovial fibroblasts. PLoS Pathog. 2021;17(11):e1010069. doi:10.1371/journal.ppat.1010069
157. Doonan J, Lumb FE, Pineda MA, et al. Protection against arthritis by the parasitic worm product ES-62, and its drug-like small molecule analogues, is associated with inhibition of osteoclastogenesis. Front Immunol. 2018;9:1016. doi:10.3389/fimmu.2018.01016
158. Acevedo N, Lozano A, Zakzuk J, et al. Cystatin from the helminth Ascaris lumbricoides upregulates mevalonate and cholesterol biosynthesis pathways and immunomodulatory genes in human monocyte-derived dendritic cells. Front Immunol. 2024;15:1328401. doi:10.3389/fimmu.2024.1328401
159. Pace F, Carvalho BM, Zanotto TM, et al. Helminth infection in mice improves insulin sensitivity via modulation of gut microbiota and fatty acid metabolism. Pharmacol Res. 2018;132:33–46. doi:10.1016/j.phrs.2018.04.008
160. Ying W, Lee YS, Dong Y, et al. Expansion of islet-resident macrophages leads to inflammation affecting β cell proliferation and function in obesity. Cell Metab. 2019;29(2):457–474.e5. doi:10.1016/j.cmet.2018.12.003
161. Alvarado R, To J, Lund ME, et al. The immune modulatory peptide FhHDM-1 secreted by the helminth Fasciola hepatica prevents NLRP3 inflammasome activation by inhibiting endolysosomal acidification in macrophages. FASEB J. 2017;31(1):85–95. doi:10.1096/fj.201500093r
162. Sun XM, Guo K, Hao C-Y, et al. Trichinella spiralis excretory-secretory products stimulate host regulatory T cell differentiation through activating dendritic cells. Cells. 2019;8(11):1404. doi:10.3390/cells8111404
163. Cvetkovic J, Ilic N, Gruden-Movsesijan A, et al. DC-SIGN signalling induced by Trichinella spiralis products contributes to the tolerogenic signatures of human dendritic cells. Sci Rep. 2020;10(1):20283. doi:10.1038/s41598-020-77497-x
164. Coronado S, Zakzuk J, Regino R, et al. Ascaris lumbricoides cystatin prevents development of allergic airway inflammation in a mouse model. Front Immunol. 2019;10:2280. doi:10.3389/fimmu.2019.02280
165. Li Y, Li Y, Yang T, et al. Dioscin attenuates oxLDL uptake and the inflammatory reaction of dendritic cells under high glucose conditions by blocking p38 MAPK. Mol Med Rep. 2020;21(1):304–310. doi:10.3892/mmr.2019.10806
166. Zawistowska-Deniziak A, Lambooij JM, Kalinowska A, et al. Fasciola hepatica fatty acid binding protein 1 modulates T cell polarization by promoting dendritic cell thrombospondin-1 secretion without affecting metabolic homeostasis in obese mice. Front Immunol. 2022;13:884663. doi:10.3389/fimmu.2022.884663
167. Harnett W, Harnett MM. Epigenetic changes induced by parasitic worms and their excretory-secretory products. Biochem Soc Trans. 2024;52(1):55–63. doi:10.1042/BST20230087
168. Corbet M. Exploiting the Helminth-Derived Immunomodulator, ES-62 and Its Small Molecule Analogues to Dissect the Mechanisms Underpinning the Development of the Pathogenic Phenotype of Synovial Fibroblasts in Autoimmune Arthritis. University of Glasgow; 2017.
169. van Riet P. Helminth Infections Induce Immunomodulation: Consequences and Mechanisms. Leiden University; 2008.
170. Khudhair Z, Alhallaf R, Eichenberger RM, et al. Administration of hookworm excretory/secretory proteins improves glucose tolerance in a mouse model of type 2 diabetes. Biomolecules. 2022;12(5):637. doi:10.3390/biom12050637
171. van der Zande HJP, Gonzalez MA, de Ruiter K, et al. The helminth glycoprotein omega-1 improves metabolic homeostasis in obese mice through type 2 immunity-independent inhibition of food intake. FASEB J. 2021;35(2):e21331. doi:10.1096/fj.202001973R
172. Berbudi A, Surendar J, Ajendra J, et al. Filarial infection or antigen administration improves glucose tolerance in diet-induced obese mice. J Innate Immun. 2016;8(6):601–616. doi:10.1159/000448401
173. Surendar J, Frohberger SJ, Karunakaran I, et al. Adiponectin limits IFN-γ and IL-17 producing CD4 T cells in obesity by restraining cell intrinsic glycolysis. Front Immunol. 2019;10:2555. doi:10.3389/fimmu.2019.02555
174. Bhargava P, Li C, Stanya KJ, et al. Immunomodulatory glycan LNFPIII alleviates hepatosteatosis and insulin resistance through direct and indirect control of metabolic pathways. Nat Med. 2012;18(11):1665–1672. doi:10.1038/nm.2962
175. Crowe J, Lumb FE, Doonan J, et al. The parasitic worm product ES-62 promotes health-and life-span in a high calorie diet-accelerated mouse model of ageing. PLoS Pathog. 2020;16(3):e1008391. doi:10.1371/journal.ppat.1008391
176. Crowe J, Lumb FE, Doonan J, et al. Parasitic worm-derived ES-62 promotes health-and life-span in high calorie diet-fed mice. BioRxiv. 2019:622753.
177. Doonan J, Thomas D, Wong MH, et al. Failure of the anti-inflammatory parasitic worm product ES-62 to provide protection in mouse models of type I diabetes, multiple sclerosis, and inflammatory bowel disease. Molecules. 2018;23(10):2669. doi:10.3390/molecules23102669
178. Mulyani WRW, Sanjiwani MID, Sandra S, et al. Chaperone-based therapeutic target innovation: heat shock protein 70 (HSP70) for Type 2 diabetes mellitus. Diabetes Metab Syndr Obesity. 2020;13:559–568. doi:10.2147/DMSO.S232133
179. de Lemos Muller CH, Schroeder HT, Rodrigues-Krause J, et al. Extra and intra cellular HSP70 levels in adults with and without metabolic disorders: a systematic review and meta-analysis. Cell Stress Chaperones. 2023;28(6):761–771. doi:10.1007/s12192-023-01368-3
180. De Oliveira A, Priviero F, Webb RC, et al. Disruption of the eHSP70-to-iHSP70 ratio impairs vascular function in diabetic rats. Physiology. 2023;38(S1):5673125. doi:10.1152/physiol.2023.38.S1.5673125
181. Nagai M, Kaji H. Thermal effect on heat shock protein 70 family to prevent atherosclerotic cardiovascular disease. Biomolecules. 2023;13(5):867. doi:10.3390/biom13050867
182. Das K, Upadhyay S, Mahindroo N. Helminths derived immune-modulatory molecules: implications in host-parasite interaction. In: Parasitic Helminths and Zoonoses-From Basic to Applied Research. IntechOpen; 2022.
183. Ryan S, Shiels J, Taggart CC, et al. Fasciola hepatica-derived molecules as regulators of the host immune response. Front Immunol. 2020;11:2182. doi:10.3389/fimmu.2020.02182
184. Tanaka A. Characterisation of an immune-modulating peptide secreted by a helminth worm. 2018.
185. Bonam VR, Srinivasan AR, DanielDevaprasad M, Kudikala RB. Genetic polymorphism of Pro12Ala in type 2 diabetes mellitus: role in inflammation linked to insulin resistance. Bioinformation. 2023;19(9):946. doi:10.6026/97320630019946
186. Yahia SH, Badawey MS, Abdalla AE, et al. The immunological effects of Schistosoma mansoni-derived soluble egg antigen on murine streptozotocin-induced autoimmune type 1 diabetes. Zagazig Univ Med J. 2024. doi:10.21608/zumj.2024.297774.3445
187. El-Gebaly NS, Rehan MK, Abdelfattah DS. Immunomodulating effect of Schistosoma mansoni soluble egg antigen on course of induced diabetes mellitus in experimental mice. Parasitol United J. 2019;12(1):45–52. doi:10.21608/puj.10929.1036
188. Zaccone P, Burton OT, Gibbs S, et al. Immune modulation by Schistosoma mansoni antigens in NOD mice: effects on both innate and adaptive immune systems. Biomed Res Int. 2010;2010(1):795210.
189. Hartmann S, Schönemeyer A, Sonnenburg B, et al. Cystatins of filarial nematodes up‐regulate the nitric oxide production of interferon‐γ‐activated murine macrophages. Parasite Immunol. 2002;24(5):253–262. doi:10.1046/j.1365-3024.2002.00459.x
190. Colomb F, Jamwal A, Ogunkanbi A, et al. Heparan sulphate binding controls in vivo half-life of the HpARI protein family. BioRxiv. 2024:
191. Chauché C, Vacca F, Chia SL, et al. A truncated form of HpARI stabilizes IL-33, amplifying responses to the cytokine. Front Immunol. 2020;11:1363. doi:10.3389/fimmu.2020.01363
192. Halder J, Gupta S, Kumari R, et al. Microneedle array: applications, recent advances, and clinical pertinence in transdermal drug delivery. J Pharm Innovation. 2021;16:558–565. doi:10.1007/s12247-020-09460-2
193. Waghule T, Singhvi G, Dubey SK, et al. Microneedles: a smart approach and increasing potential for transdermal drug delivery system. Biomed Pharmacother. 2019;109:1249–1258. doi:10.1016/j.biopha.2018.10.078
194. Liu T, Chen M, Fu J, et al. Recent advances in microneedles-mediated transdermal delivery of protein and peptide drugs. Acta Pharmaceutica Sinica B. 2021;11(8):2326–2343. doi:10.1016/j.apsb.2021.03.003
195. Chakraborty R, Afrose N, Kuotsu K. Novel synergistic approaches of protein delivery through physical enhancement for transdermal microneedle drug delivery: a review. J Drug Deliv Sci Technol. 2023;84:104467. doi:10.1016/j.jddst.2023.104467
196. Bhatnagar S, Gadeela PR, Thathireddy P, et al. Microneedle-based drug delivery: materials of construction. J Chem Sci. 2019;131:1–28. doi:10.1007/s12039-019-1666-x
197. Huang H, Hu D, Chen Z, et al. Immunotherapy for type 1 diabetes mellitus by adjuvant-free Schistosoma japonicum-egg tip-loaded asymmetric microneedle patch (STAMP). J Nanobiotechnol. 2022;20(1):377. doi:10.1186/s12951-022-01581-9
198. Phillips MA, Gran ML, Peppas NA. Targeted nanodelivery of drugs and diagnostics. Nano Today. 2010;5(2):143–159. doi:10.1016/j.nantod.2010.03.003
199. Kim KT, Lee HS, Lee -J-J, et al. Nanodelivery systems for overcoming limited transportation of therapeutic molecules through the blood-brain barrier. Future Med Chem. 2018;10(22):2659–2674. doi:10.4155/fmc-2018-0208
200. Kerry RG, Mahapatra GP, Maurya GK, et al. Molecular prospect of type-2 diabetes: nanotechnology based diagnostics and therapeutic intervention. Rev Endocr Metab Disord. 2021;22(2):421–451. doi:10.1007/s11154-020-09606-0
201. Dubey S, Mody N, Sharma R, Agrawal U, Vyas SP. Nanobiomaterials: novel nanoplatforms for protein and peptide delivery. In: Nanobiomaterials in Drug Delivery. Elsevier; 2016:111–146.
202. Perry SL, McClements DJ. Recent advances in encapsulation, protection, and oral delivery of bioactive proteins and peptides using colloidal systems. Molecules. 2020;25(5):1161. doi:10.3390/molecules25051161
203. La Manna S, Di Natale C, Onesto V, et al. Self-assembling peptides: from design to biomedical applications. Int J Mol Sci. 2021;22(23):12662. doi:10.3390/ijms222312662
204. Barik SR, Mohapatra RK, Mohapatra PK, et al. Recent developments in biopolymeric nanoparticles for drug delivery systems: an overview. Micro Nanosyst. 2022;14(2):92–100. doi:10.2174/1876402913666210405155127
205. Siles-Lucas M, Morchon R, Simon F, et al. Exosome-transported microRNAs of helminth origin: new tools for allergic and autoimmune diseases therapy? Parasite Immunol. 2015;37(4):208–214. doi:10.1111/pim.12182
206. Qadeer A, Wajid A, Rafey HA, et al. Exploring extracellular vesicles in zoonotic helminth biology: implications for diagnosis, therapeutic and delivery. Front Cell Infect Microbiol. 2024;14:1424838. doi:10.3389/fcimb.2024.1424838
207. Matsuzaka Y, Yashiro R. Extracellular vesicles as novel drug-delivery systems through intracellular communications. Membranes. 2022;12(6):550. doi:10.3390/membranes12060550
208. Uddin MJ, Mohite P, Munde S, et al. Extracellular vesicles: the future of therapeutics and drug delivery systems. Intell Pharm. 2024;2:312–328. doi:10.1016/j.ipha.2024.02.004
209. Meng W, He C, Hao Y, et al. Prospects and challenges of extracellular vesicle-based drug delivery system: considering cell source. Drug Deliv. 2020;27(1):585–598. doi:10.1080/10717544.2020.1748758
210. Rodríguez DA, Vader P. Extracellular vesicle-based hybrid systems for advanced drug delivery. Pharmaceutics. 2022;14(2):267. doi:10.3390/pharmaceutics14020267
211. Ilahibaks NF. Engineering Extracellular Vesicles for Protein-Based Drug Delivery. Utrecht University; 2023.
212. Erana-Perez Z, Igartua M, Santos-Vizcaino E, et al. Genetically engineered loaded extracellular vesicles for drug delivery. Trends Pharmacol Sci. 2024;45:350–365. doi:10.1016/j.tips.2024.02.006
213. N’Diaye E-R, Orefice NS, Ghezzi C, et al. Chemically modified extracellular vesicles and applications in radiolabeling and drug delivery. Pharmaceutics. 2022;14(3):653. doi:10.3390/pharmaceutics14030653
214. Hawdon JM, Nixon D, Suhao HA, et al. Engineered Human Hookworms as a Novel Biodelivery System for Vaccines and Biologicals. Google Patents; 2019.
215. Pomatto MAC, Negro F, Camussi G. Optimized protocol for plasma-derived extracellular vesicles loading with synthetic miRNA mimic using electroporation. Methods Mol Biol. 2022;2504:219–230.
216. Yoshikawa FSY, Teixeira FME, Sato MN, et al. Delivery of microRNAs by extracellular vesicles in viral infections: could the news be packaged? Cells. 2019;8(6):611. doi:10.3390/cells8060611
217. Fu P, Guo Y, Luo Y, et al. Visualization of microRNA therapy in cancers delivered by small extracellular vesicles. J Nanobiotechnol. 2023;21(1):457. doi:10.1186/s12951-023-02187-5
218. Tapparo M, Pomatto MAC, Deregibus MC, et al. Serum derived extracellular vesicles mediated delivery of synthetic miRNAs in human endothelial cells. Front Mol Biosci. 2021;8:636587. doi:10.3389/fmolb.2021.636587
219. Pottash AE, Levy D, Powsner EH, et al. Enhanced extracellular vesicle cargo loading via microRNA biogenesis pathway modulation. ACS Biomater Sci Eng. 2024;10(10):6286–6298. doi:10.1021/acsbiomaterials.4c00821
220. de Abreu RC, Ramos CV, Becher C, et al. Exogenous loading of miRNAs into small extracellular vesicles. J Extracell Vesicles. 2021;10(10):e12111. doi:10.1002/jev2.12111
221. Strasser R, Seifert G, Doblin MS, et al. Cracking the “Sugar Code”: a snapshot of N- and O-glycosylation pathways and functions in plants cells. Front Plant Sci. 2021;12:640919. doi:10.3389/fpls.2021.640919
222. Mersha FB, McClung CM, Chen M, et al. Defining the filarial N-glycoproteome by glycosite mapping in the human parasitic nematode Brugia malayi. Sci Rep. 2023;13(1):7951. doi:10.1038/s41598-023-34936-9
223. Walsh G. Biopharmaceutical benchmarks 2018. Nature Biotechnol. 2018;36(12):1136–1145. doi:10.1038/nbt.4305
224. Skelly PJ, Da’dara AA. Schistosome secretomes. Acta Trop. 2022;236:106676. doi:10.1016/j.actatropica.2022.106676
225. Yakubu RR, Weiss LM, Silmon de Monerri NC. Post-translational modifications as key regulators of apicomplexan biology: insights from proteome-wide studies. Mol Microbiol. 2018;107(1):1–23. doi:10.1111/mmi.13867
226. Stoger E, Fischer R, Moloney M, et al. Plant molecular pharming for the treatment of chronic and infectious diseases. Annu Rev Plant Biol. 2014;65:743–768. doi:10.1146/annurev-arplant-050213-035850
227. Zhang L, Li Y, R L, et al. Glycoprotein in vitro N-glycan processing using enzymes expressed in E. coli. Molecules. 2023;28(6):2753.
228. Geisler C, Mabashi-Asazuma H, Jarvis DL. An overview and history of glyco-engineering in insect expression systems. Methods Mol Biol. 2015;1321:131–152.
229. Rosenlöcher J, Weilandt C, Sandig G, et al. The human rhabdomyosarcoma cell line TE671--Towards an innovative production platform for glycosylated biopharmaceuticals. Protein Expr Purif. 2015;115:83–94. doi:10.1016/j.pep.2015.08.008
230. Bosch D, Castilho A, Loos A, et al. N-glycosylation of plant-produced recombinant proteins. Curr Pharm Des. 2013;19(31):5503–5512. doi:10.2174/1381612811319310006
231. Wilbers RH, Westerhof LB, Reuter LJ, et al. The N-glycan on Asn54 affects the atypical N-glycan composition of plant-produced interleukin-22, but does not influence its activity. Plant Biotechnol J. 2016;14(2):670–681. doi:10.1111/pbi.12414
232. van der Kaaij A, van Noort K, Nibbering P, et al. Glyco-engineering plants to produce helminth glycoproteins as prospective biopharmaceuticals: recent advances, challenges and future prospects. Front Plant Sci. 2022;13:882835. doi:10.3389/fpls.2022.882835
233. Lobato Gómez M, Huang X, Alvarez D, et al. Contributions of the international plant science community to the fight against human infectious diseases - part 1: epidemic and pandemic diseases. Plant Biotechnol J. 2021;19(10):1901–1920. doi:10.1111/pbi.13657
234. Alvisi N, van Noort K, Dwiani S, et al. β-hexosaminidases along the secretory pathway of nicotiana benthamiana have distinct specificities toward engineered helminth N-glycans on recombinant glycoproteins. Front Plant Sci. 2021;12:638454. doi:10.3389/fpls.2021.638454
235. Haque A, Akcesme B, Mujić J, et al. Helminth-derived product (s): source for potential therapeutic. Period Eng Nat Sci. 2013;1(1):1–7.
236. Al-Riyami L, Harnett W. The clinical potential of worms and their products in treating inflammatory diseases. Current Immunol Rev. 2009;5(1):35–41. doi:10.2174/157339509787314431
237. Pearson RM, Casey LM, Hughes KR, et al. In vivo reprogramming of immune cells: technologies for induction of antigen-specific tolerance. Adv Drug Delivery Rev. 2017;114:240–255. doi:10.1016/j.addr.2017.04.005
238. Rad LM, Arellano G, Podojil JR, et al. Engineering nanoparticle therapeutics for food allergy. J Allergy Clin Immunol. 2024;153(3):549–559. doi:10.1016/j.jaci.2023.10.013
239. Maldonado RA, LaMothe RA, Ferrari JD, et al. Polymeric synthetic nanoparticles for the induction of antigen-specific immunological tolerance. Proc Natl Acad Sci USA. 2015;112(2):E156–65. doi:10.1073/pnas.1408686111
240. Kothari V, Galdo JA, Mathews ST. Hypoglycemic agents and potential anti-inflammatory activity. J Inflamm Res. 2016;9:27–38. doi:10.2147/JIR.S86917
241. Pollack RM, Donath MY, LeRoith D, et al. Anti-inflammatory agents in the treatment of diabetes and its vascular complications. Diabetes Care. 2016;39(Supplement_2):S244–S252. doi:10.2337/dcS15-3015
242. Ghadge AA, Kuvalekar AA. Controversy of oral hypoglycemic agents in type 2 diabetes mellitus: novel move towards combination therapies. Diabetes Metab Syndr. 2017;11:S5–S13.
243. Mera Y, Kuroki Y, Combination therapy for use in treating diabetes. 2011.
244. Matsumoto K, et al. Combined usage of diabetes therapeutic agents. 2017.
245. Ruyssers NE, De Winter BY, De Man JG, et al. Worms and the treatment of inflammatory bowel disease: are molecules the answer? J Immunol Res. 2008;2008(1):567314.
246. Bohnacker S, Troisi F, de Los Reyes Jiménez M, et al. What can parasites tell us about the pathogenesis and treatment of asthma and allergic diseases. Front Immunol. 2020;11:2106. doi:10.3389/fimmu.2020.02106
247. Sipahi AM, Baptista DM. Helminths as an alternative therapy for intestinal diseases. World J Gastroenterol. 2017;23(33):6009. doi:10.3748/wjg.v23.i33.6009
248. Alcantara-Neves NM, Veiga RV, Dattoli VCC, et al. The effect of single and multiple infections on atopy and wheezing in children. J Allergy Clin Immunol. 2012;129(2):359–367.e3. doi:10.1016/j.jaci.2011.09.015
249. Cooper PJ, Chico ME, Vaca MG, et al. Effect of albendazole treatments on the prevalence of atopy in children living in communities endemic for geohelminth parasites: a cluster-randomised trial. Lancet. 2006;367(9522):1598–1603. doi:10.1016/S0140-6736(06)68697-2
250. Bager P, Arnved J, Rønborg S, et al. Trichuris suis ova therapy for allergic rhinitis: a randomized, double-blind, placebo-controlled clinical trial. J Allergy Clin Immunol. 2010;125(1):123–130.e3. doi:10.1016/j.jaci.2009.08.006
251. Feary J, Venn A, Brown A, et al. Safety of hookworm infection in individuals with measurable airway responsiveness: a randomized placebo‐controlled feasibility study. Clin Exp Immunol. 2009;39(7):1060–1068. doi:10.1111/j.1365-2222.2009.03187.x
252. Feary J, Venn AJ, Mortimer K, et al. Experimental hookworm infection: a randomized placebo‐controlled trial in asthma. Clin Exp Immunol. 2010;40(2):299–306. doi:10.1111/j.1365-2222.2009.03433.x
253. Elliott DE, Weinstock JV. Helminthic therapy: using worms to treat immune-mediated disease. Pathogen-Derived Immunomodulatory Mol. 2009:157–166.
254. Heylen M, Ruyssers NE, De Man JG, et al. Worm proteins of Schistosoma mansoni reduce the severity of experimental chronic colitis in mice by suppressing colonic proinflammatory immune responses. PLoS One. 2014;9(10):e110002. doi:10.1371/journal.pone.0110002
255. Maruszewska-Cheruiyot M, Donskow-łysoniewska K, Doligalska M. Helminth therapy: advances in the use of parasitic worms against inflammatory bowel diseases and its challenges. Helminthologia. 2018;55(1):1–11. doi:10.1515/helm-2017-0048
256. Venkatakrishnan A, Sarafian JT, Jirků-Pomajbíková K, et al. Socio-medical studies of individuals self-treating with helminths provide insight into clinical trial design for assessing helminth therapy. Parasitol Int. 2022;87:102488. doi:10.1016/j.parint.2021.102488
257. Sutherland I, Moen I, Leathwick D. Increased burdens of drug-resistant nematodes due to anthelmintic treatment. Parasitology. 2002;125(4):375–381. doi:10.1017/S0031182002002184
258. Parker W. Not infection with parasitic worms, but rather colonization with therapeutic helminths. Immunol Lett. 2017;192:104–105. doi:10.1016/j.imlet.2017.07.008
259. Coghlan A, Partridge FA, Duque-Correa MA, et al. A drug repurposing screen for whipworms informed by comparative genomics. PLoS Negl Trop Dis. 2023;17(9):e0011205. doi:10.1371/journal.pntd.0011205
260. Bizhani N. Herbal therapy and treatment of worm infections, emphasizing taenia solium. Iran J Public Health. 2015;44(11):1555–1556.
261. Finkelman FD. Worming their way into the pharmacy: use of worms and worm products to treat inflammatory diseases. Arthritis Rheum. 2012;64(10):3068–3071. doi:10.1002/art.34635
262. Liu X, Jiang Y, Ye J, et al. Helminth infection and helminth-derived products: a novel therapeutic option for non-alcoholic fatty liver disease. Front Immunol. 2022;13:999412. doi:10.3389/fimmu.2022.999412
263. Skurnik M, Pajunen M, Kiljunen S. Biotechnological challenges of phage therapy. Biotechnol Lett. 2007;29:995–1003. doi:10.1007/s10529-007-9346-1
264. Sotillo J, Toledo R, Mulvenna J, et al. Exploiting helminth-host interactomes through big data. Trends Parasitol. 2017;33(11):875–888. doi:10.1016/j.pt.2017.06.011
265. Navarro S, Pickering DA, Ferreira IB, et al. Hookworm recombinant protein promotes regulatory T cell responses that suppress experimental asthma. Sci Transl Med. 2016;8(362):362ra143. doi:10.1126/scitranslmed.aaf8807
266. Zhao H, Xing C, Zhang J, et al. Comparative efficacy of oral insulin sensitizers metformin, thiazolidinediones, inositol, and berberine in improving endocrine and metabolic profiles in women with PCOS: a network meta-analysis. Reprod Health. 2021;18(1):171. doi:10.1186/s12978-021-01207-7
267. Morimoto M, Azuma N, Kadowaki H, et al. Regulation of type 2 diabetes by helminth-induced Th2 immune response. J Vet Med Sci. 2017;78(12):1855–1864. doi:10.1292/jvms.16-0183
268. Mohammed I, Hollenberg MD, Ding H, et al. A critical review of the evidence that metformin is a putative anti-aging drug that enhances healthspan and extends lifespan. Front Endocrinol. 2021;12:718942. doi:10.3389/fendo.2021.718942
269. Cardel MI, Ross KM, Butryn M, et al. Acceptance-based therapy: the potential to augment behavioral interventions in the treatment of type 2 diabetes. Nut Diabetes. 2020;10(1):3. doi:10.1038/s41387-020-0106-9
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

