Back to Journals » Journal of Inflammation Research » Volume 17
Antioxidant, Antiinflammation, and Antifibrotic Activity of Ciplukan (Physalis angulata L). Extract
Authors Wiraswati HL , Ekawardhani S , Rohmawaty E, Laelalugina A , Zuhrotun A , Hendriani R, Wardhana YW, Bestari MB , Sahirdjan EH, Dewi S
Received 30 March 2024
Accepted for publication 4 September 2024
Published 10 September 2024 Volume 2024:17 Pages 6297—6306
DOI https://doi.org/10.2147/JIR.S470318
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Hesti Lina Wiraswati,1 Savira Ekawardhani,1 Enny Rohmawaty,1 Amila Laelalugina,2 Ade Zuhrotun,3 Rini Hendriani,4 Yoga Windhu Wardhana,5 Muhammad Begawan Bestari,6 Emmy Hermiyanti Sahirdjan,7 Sumartini Dewi7
1Department of Biomedical Sciences, Universitas Padjadjaran, Sumedang, West Java, Indonesia; 2Oncology and Stem Cell Working Group, Universitas Padjadjaran, Bandung, West Java, Indonesia; 3Department of Biological Pharmacy, Universitas Padjadjaran, Sumedang, West Java, Indonesia; 4Department of Pharmacology and Clinical Pharmacy, Universitas Padjadjaran, Sumedang, West Java, Indonesia; 5Study Center of Pharmaceutical Dosage Development, Department of Pharmaceutics and Pharmaceuticals Technology, Universitas Padjadjaran, Sumedang, West Java, Indonesia; 6Division Gastro Entero Hepatology, Department of Internal Medicine, Universitas Padjadjaran, Bandung, West Java, Indonesia; 7Department of Internal Medicine, Universitas Padjadjaran, Bandung, West Java, Indonesia
Correspondence: Hesti Lina Wiraswati, Department of Biomedical Sciences, Faculty of Medicine, Universitas Padjadjaran, Sumedang, West Java, 45363, Indonesia, Tel +62-22-84288888, Fax +022-84288888, Email [email protected]
Purpose: Physalis angulata Linn. (Ciplukan) is a plant widely used in traditional medicine in subtropical and tropical regions. Most studies focus on its antioxidant and anti-inflammatory activity. Many studies also reported its therapeutic potential for treating cancer, malaria, hepatitis, rheumatism, liver problems, and tumors, but few studies have reported its anti-fibrosis activity. Here, we aimed to investigate the potential of P. angulata as an antioxidant and anti-inflammatory that may be correlated with its anti-fibrosis action.
Methods: In our study, we treated 3T3-L1 and TGF-β-induced 3T3-L1 cells with an ethanol extract of P. angulata. We then monitored the cell’s response, evaluated the antioxidant activity using an MTT assay, and observed the cells’ migration using the cell scratch assay. We used RT-PCR to determine the expression of HIF-1α and IL-6 on TGF-β-induced 3T3-L1 cells.
Results: The ethanol extract of P. angulata showed antioxidant activity and promoted cell proliferation on 3T3-L1 cells. Interestingly, the extract inhibited the migration of TGF-β-induced 3T3-L1 cells. Further analysis revealed that the extract could inhibit HIF-1α expression and suppress IL-6 expression on TGF-β-induced 3T3-L1 cells.
Conclusion: The ethanol extract of P. angulata showed antioxidant and anti-inflammation activities in 3T3-L1 cells. Both activities are associated with the antifibrotic activity of P. angulata’s ethanol extract.
Keywords: TGF-β, ethanol extract, wound healing, fibroblast cell line
Introduction
Ciplukan has a Latin name Physalis angulata Linn. and belongs to the Solanaceae family.1 Physalis is the fifth-largest Solanaceae genus, containing at least 70 species.2 P. angulata is extensive growth in tropical regions such as Australia, Pacific America, and Asia. Although originally wild and weeds, it has recently been widely cultivated in tropical and subtropical regions.3 This annual plant has a minuscule stature compared to other Physalis species. The plant has a height of between 15–60 cm. The leaves are oval with long petioles. P. angulata’s flowers are bell-shaped and covered by petals, which develop into fruit covers. The fruit is round, juicy, light green (unripe), and turns yellow (ripe).3
P. angulata is one of the natural products widely used in traditional medicine. People commonly use this plant for antibacterial, antiparasitic, anti-inflammatory, anti-analgesic, antidiabetic, and antioxidant due to its active properties.4,5 They benefit from all parts of the plant: roots, flowers, fruits, leaves, and stems. For instance, P. angulata has been used for treating malaria and liver dysfunction in Brazil.6 In the Peru and Indonesia regions, the leaves and fruit of P. angulata are also used for itching and postpartum infection.7,8 Aerial parts of Ciplukan were used for chronic inflammation treatment in Northern Nigeria.9 People in Nigeria and Ivory Coast have used tea leaves and all parts of it for the treatment of malaria.10,11 Several other diseases such as anti-dermatitis, intestinal worms, abdominal pain, wounds, hepatitis, anemia, urinary tract infections, and tumors are also treated with P. angulata as traditional medicine in several countries in the world.12,13
In addition, because of its phytochemical content, such as flavonoids, steroids, alkaloids, saponins, and tannins, the development of P. angulata for modern medicine is also being carried out.14 For example, the ethanol extract of Ciplukan leaves reported has high antioxidant activity due to its flavonoid content.15 Pillai et al also revealed cytotoxic, antimicrobial, and antioxidant activities of ethanolic extract P. angulata, which contains alkaloids, glycosides, flavonoids, tannins, and phenolics.16 This plant’s active fractions and compounds are also widely reported to have anti-cancer, immunomodulatory, immunosuppressive, and anti-inflammatory actions.17 Physalin, Withangulatin, Physagulin, Oleanolic acid, and Myricetin 3-O-neohesperidoside are active compounds successfully isolated from P. angulate.13 Specifically, Physalins B, F, and G were reported as anti-inflammation by inhibiting pro-inflammatory cytokine production.18 Isolated physalin F from ethanolic extract showed anti-cancer activity on human or animal cell lines.19 Withangulatin had strong immunosuppressive activity by eliminating T lymphocyte expression and modulating T helper balance.20
Among the many potentials of P. angulata, only a few studies have reported its activity as an anti-fibrotic agent. Given that inflammation and oxidative stress are essential in the pathogenesis of fibrosis, and it has been established that this plant has both antioxidant and anti-inflammatory activity,21,22 we investigated P. angulata potential as anti-fibrosis. In experimental models of cells or tissues, TGF-β is widely used to promote fibrosis.23,24 Many studies have used the 3T3-L1 cells induced by a profibrotic cytokine, TGF-β, as fibrosis models in vitro.25,26 Our previous study showed that adjuvant from ethanol extract of P. angulata reduced skin fibrosis in scleroderma patients.27 Previous in vivo studies also revealed the antifibrotic activity of P. angulata’s ethyl acetate fraction in rat liver fibrosis induced by CCl4.28 Our in vitro study with ethanol extract also demonstrated the in-line result that showed their antifibrotic activity through inhibition in α-SMA-expressing myofibroblasts.29 Promising effects of Ciplukan extract on fibrosis-related gene expressions are also shown in the Bleomycin-induced mouse model [ref]30 Therefore, this study focuses on the antioxidant and anti-inflammatory properties that may be correlated with P. angulata’s anti-fibrosis action.
Material and Methods
Plant Collection and Extraction
All plant parts, except the roots, were collected from several locations in West Java, Indonesia (Figure 1). The plant was identified using literature by a taxonomist, Joko Kursmoro.31–33 The Plant Taxonomy Laboratory, the Faculty of Mathematics and Natural Sciences of Universitas Padjadjaran confirmed the accuracy of the taxonomic identification with reference no.106/HB 10112020. The Herbarium of the Plant Taxonomy Laboratory prepared and deposited voucher specimens. The extraction technique is cold maceration. This technique is an extraction process using a solvent with several times stirring at room temperature. We macerated the P. angulata herb in 50% ethanol for 3×24 hours. Then, we evaporated the filtrate with a rotary vacuum evaporator (CCA-1100, EYELA, Japan). Furthermore, the condensed extract is freeze-dried into a dry extract.
 |
Figure 1 Photographs from the field of Physalis angulata Linn. |
Materials
3T3-L1 fibroblasts were purchased from the American Type Culture Collection (ATCC). Roswell Park Memorial Institute (RPMI) 1640 Medium, Fetal Bovine Serum (FBS), Penicillin-Streptomycin (PS), and Phosphate Buffer Saline (PBS) solution were supplied by PAN-Biotech. Human transforming growth factor beta-1 (TGF-β), Menadione, N-acetyl-cysteine (NAC), glutathione (GSH), and Dimethylsulfoxide (DMSO) were supplied by Sigma-Aldrich. 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT) assay kit and DMSO was obtained from Sigma-Aldrich.
Cell Culture and TGF-β-Treated 3T3-L1 Cells
3T3-L1 fibroblasts were cultured in a rich medium of RPMI 1640 supplemented with 10% FBS and 1% PS. Cells were maintained at 37°C and 5% CO2 atmosphere. We used 50 ng/mL TGF-β to stimulate 3T3-L1 fibrosis cells for 24 hours of incubation. The medium for fibrosis cells is a starvation medium comprising RPMI 1640 supplemented with 0.1% FBS and 1% PS.
Proliferation Assay
Cell proliferation and antioxidant activity were conducted using an MTT assay. First, 7.5×104 cells were plated in each well of a 96-well plate, then treated with ethanol extracts at different concentrations (10, 100, 500, 750, 1000, 1250, 1500, 2000 µg/mL) and incubated for 24h. To evaluate antioxidant activity, 7.5×104 cells in each well of a 96-well plate were treated with menadione (6 µM) and 500 µg/mL extract (or 5 mM GSH or 5 mM NAC) for 24 hours of incubation. Next, MTT reagent was added to each well. Incubation was continued for 4h. Once the formazan crystals are formed, they are dissolved in DMSO. The quantity of formazan crystals was determined at 550 nm using a plate reader (Thermo Scientific® Multiscan EX, Singapore). The graph of absorbance was plotted to determine the 3T3-L1 cell proliferation according to the kit manufacturer’s instructions. Experiments were performed in triplicates.
In Vitro Scratch Wound Healing Assay
Cells were grown in 96-well plates up to 90% confluency. Then, we scratched the monolayer of cells using a p20 pipette tip. After washing the cells with PBS, we incubated cells with a transition medium containing the extract (100 µg/mL or 500 µg/mL) for 24h or 48h. The medium with no extract was used as a negative control. We evaluated scratch closure using an inverted microscope (40x magnification), and the scratch area was analyzed using the Image J 1.38 software (NIH, Bethesda, MD, USA).
Cell Migration (Scratch) Assay
Cells were grown in 96-well plates up to 90% confluency. After the culture medium was discarded, a scratch was made on the monolayer of cells using a p20 pipette tip. The plates were rinsed with PBS and then incubated with a medium containing TGF-β (10 ng/mL) and P. angulata extracts (100 and 500 µg/mL). Medium with TGF-β was used as a control. Cells were incubated for 24 h and 48 h. The cell migration was observed using a light microscope (40x magnification), and the scratch area was analyzed using the Image J 1.38 software (NIH, Bethesda, MD, USA).
RT-PCR Analysis
After treatment with TGF-β and Physalis angulata extract, total RNA was isolated from the cells using Qiagen RNeasy Mini Kit (Qiagen, Valencia, CA, USA). Then, mRNA expression was determined by real-time PCR using SensiFAST™ SYBR® No-ROX One-Step Kit (Meridian Biosciences) and specific sequence primer in Agilent AriaMX PCR System (Agilent Technologies, USA). The primers were synthesized by Integrated DNA Technologies (IDT, Singapore). The nucleotide sequences (5′ to 3′) of the forward primers and reverse primers are GAPDH, 5′-CAAGATCATCAGCAATGCCTCC −3′ (sense), and 5′-GCCATCACGCCACAGTTTCC −3′ (antisense); HIF1α, 5′- GTA ATG CTC CCC TCA CCC AAC −3′ (sense) and 5′- GTG CAG GGT CAG CAC TAC TTC −3′ (antisense),34 IL-6, 5′-AGTGG CTAAG GACCA AGACC-3′ (sense) and 5′-TCTGA CCACA GTGAG GAATG-3′ (antisense).35 The PCR conditions were as follows: 45°C for 10 min for reverse transcription, 95°C for 2 min, 40 cycles of 95°C for 5 s, and 60°C for 20s. Data were analyzed by the 2-ΔΔCT method, using the housekeeping gene GAPDH as the internal control.
Statistical Analysis
Data were expressed as mean ± standard error of the mean (SEM). Statistical significance was determined using Microsoft Excel Office 2010 software. Differences between the two groups were analyzed by unpaired two-tailed Student’s t-tests. A p-value less than 0.05 was considered significant. Levels of significance are indicated in the Figures and text as appropriate.
Results
P.Angulata‘s Ethanol Extract Inhibits Oxidative Stress and Accelerates Wound Healing on 3T3-L1 Cells
In this study, we first treated 3T3-L1 fibroblast cells with various extract concentrations to ensure that the concentration did not become toxic, which could affect cell migration. The result showed that the extract did not disturb cell viability until 500 µg/mL of treatment. We monitored starting at 750 µg/mL of extract, and cells lost the viability of 45% (Figure 2). Then, we evaluate the antioxidant activity of 500 µg/mL extract on fibroblast 3T3-L1 cells. Here, we induced cells with menadione, widely used to induce cellular stress oxidative36,37 In parallel, we co-treated cells with the extract or other extracellular antioxidants, NAC and GSH. The results showed that the extract has been able to provide protection against menadione toxicity and reduce oxidative stress. Treatment with 500 µg/mL ethanol extract for 24h significantly increased cell viability. The extract had a similar antioxidant effect to N-acetyl-cysteine (NAC) and glutathione (GSH) as oxidizing radical scavenger agents (Figure 3).
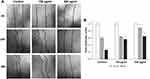
Then, we monitored the wound healing response of 3T3-L1 fibroblast cells using a cell scratch test in the absence or presence of the extract. An in vitro scratch assay has often been used in previous studies to measure the progression of wound closure.38,39 Fibroblast migration is a crucial step in the wound-healing process.40 Therefore, we evaluate the effect of the P. angulata extract on cell migration (Figure 4A). Here, we calculated the percentages of area closure for comparison. After 24h, treatment with 500 µg/mL of the extract can reduce the wound area (0.23 fold) compared to the control (0.38 fold). Moreover, the effect of the extract on scratch closure was statistically significant (Figure 4B).
P.Angulata‘s Ethanol Extract Inhibited the Migration of TGF-β-Induced 3T3-L1 Fibrosis Cells
After showing the wound-healing activity of P. angulata to 3T3-L1 cells and our knowledge of their antifibrotic effect on TGF-B-induced fibrosis in 3T3-L1 cells,25 we investigated the wound-healing activity of the extract to fibrosis cells (Figure 5A). Then, we scratched TGF-β induced-3T3-L1 fibrosis cells and treated them with non-toxic concentrations of P. angulata to cells (100 or 500 µg/mL), for 24h or 48h incubation. The result demonstrated mild migration of cells observed in treated cells and progressive migration in control. ImageJ software analysis showed a significant decrease in migration cells on 48h incubation of 100 µg/mL extract (0,56-fold) and 500 ug/mL (0,65-fold) compared to the control (0,26-fold). We observed similar results on 24h treatment of 500 µg/mL extract (0,88-fold) compared to the control (0,60-fold) (Figure 5B).
P.Angulata‘s Ethanol Extract Suppresses IL-6 and HIF-1α Genes Expression on TGF-β-Induced 3T3-L1 Fibrosis Cells
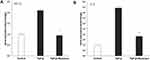
Interleukin-6 (IL-6) regulates the inflammatory phase and wound healing. In response to injury such as fibrosis, IL-6 signaling governs the immune response and switches to a reparative environment.41,42 To determine the anti-inflammatory effects of the P. angulata extract, we evaluated the expression level of the IL-6 gene in cells. RT-PCR analysis showed an elevated level of IL-6 after inducing TGF-β on 3T3-L1 fibroblast cells. However, the IL-6 level was decreased significantly after we treated fibrosis cells with 500 μg/mL extract (Figure 6A). These results demonstrated the anti-inflammatory properties of P. angulata.
Furthermore, we investigated HIF-1α gene expression, a hypoxia regulatory protein that plays an essential role in regulating the process of inflammatory and oxidative stress in hypoxia. While confirming that the extracts showed their good ability as an antioxidant, we investigated whether the oxidative stress involved in the antifibrotic activity of P. angulata’s extracts through the expression of the HIF-1α gene. We used 500 µg/mL extract to treat TGF-β-induced-3T3-L1 fibrosis cells. The result showed that the extract significantly reduced the HIF-1α level in fibrosis cells induced by TGF-β (Figure 6B). It indicated that the oxidative stress pathway is associated with the antifibrotic activity of P. angulata.
Discussion
Fibrosis is the accumulation of excess extracellular matrix (ECM) components, eventually leading to organ malfunction and death if it is highly progressive. Idiopathic pulmonary and hepatic fibrosis (IPF) is the most common and lethal form. Meanwhile, skin fibrosis causes disabilities, including hair loss, subcutaneous atrophy, and irreversible structural and functional impairment. The mechanism of fibrosis is believed to be inflammation and vascular injury at an early stage, followed by fibroblast activation and myofibroblast formation such as α-SMA, which leads to excessive ECM components such as tenascin-c, fibronectin, and collagens in fibrotic organs, which in turn disrupts homeostasis and architecture of cells and tissues.43–45
Therapies to treat fibrosis still pose challenges, including side effects, high costs, and resistance, which result in disease progression and higher morbidity.46,47 Many studies have been conducted to find new antifibrosis agents that suppress cell proliferation and ECM synthesis, thereby improving patients’ quality of life.48 Some antioxidants derived from plants have been evaluated for their antifibrotic activity, considering that plants have been an important source of medicine for years.49,50 To our knowledge, our study is the first study to report the antifibrosis activity of the plant P. angulata. We previously reported that this plant can alleviate skin fibrosis in scleroderma patients, able to improve fibrosis in rat livers, CCl4-induced fibrosis, and significantly reduce α-SMA gene in TGF-β induced-3T3-L1 fibrosis cells, an indicator gene for fibrotic active myofibroblasts.27–29 Many reports also show the activity of antifibrosis candidates through the α-SMA pathway, even being used as an important target in drug development.48,51,52
Antioxidant agents, especially from plants, are often reported to provide benefits in treating fibrosis related to their flavonoid content.49 Researchers monitored that the antioxidants derived from medicinal plants could suppress reactive oxygen species (ROS), activate antioxidant defense, inhibit ECM gene expression, and protect fibrosis in an animal model.53–55 In 3T3-L1 cells, P. angulata also showed action as an antioxidant. The antioxidant activity affects the accelerated wound healing of fibroblast cells. This result is in line with other studies that reported P. angulata leaf extract to have wound-healing activity in vivo, which supports using this plant as a wound-healing drug.56 Another important finding is that its antioxidant activity also applies in fibrosis cells by reducing HIF-1α gene expression, a key role in response to hypoxic conditions and associated with oxidative stress and inflammation. Interestingly, this ability effectively inhibited the proliferation and migration of fibrosis cells. Inhibition of cell migration of other antifibrotic agents has been reported by other groups, which also induce fibrosis in cells with TGF-β.48,57,58 These results agree with recent studies suggesting that fibrosis results from abnormal wound healing in response to the alveolar epithelium microinjury.59–61 Thus, P. angulata plays a role in deactivating cells, inhibiting cell migration, and decreasing expression of α-SMA in TGF-β-induced-3T3-L1 fibrosis cells. It also explains the clinical improvement of skin fibrosis in patients receiving P. angulata extract, which we reported previously.27
In many organs, the inflammatory state is reported to play an essential role in triggering fibrosis.44 Hence, controlling inflammation is crucial to antifibrosis drug discovery. In this present study, we showed the protective effect of P. angulata on inflammation in fibrosis cells. Our data indicated that TGF-β activated proinflammatory factor IL-6 gene expression in 3T3 cells. Meanwhile, the extract suppressed IL-6 gene expression on TGF-β-induced 3T3-L1 fibrosis cells. These results demonstrated that P. angulata plays a role in alleviating inflammation that protects cells against fibrosis. Thus, agents that inhibit ROS, inflammation, or myofibroblast formation would be promising as antifibrosis candidates, as demonstrated by P. angulata. Evaluation of the levels of ECM proteins, including tenascin-c, fibronectin, or collagen, could be performed in further studies to understand the factors contributing to the inhibition of fibrosis by P. angulata.
Conclusion
In this study, we presented that P. angulata reduced oxidative stress and accelerated wound healing in 3T3-L1 cells. Interestingly, the extract could inhibit HIF-1α and IL-6 gene expression in TGF-β-induced fibrosis cell models. Such activities decrease fibrosis cell migration, possibly benefiting patients’ skin repair. In summary, our results suggested that the antioxidant and antiinflammation activities of P. angulata are involved in its anti-fibrosis mechanism.
Acknowledgments
The authors would like to acknowledge the Indonesian Endowment Fund for Education/Lembaga Pengelola Dana Pendidikan (LPDP) and the Directorate of Research, Community Service, and Innovation Universitas Padjadjaran (DRPMI-UNPAD) for their financial support of this study.
Author Contributions
HL, SE, ER, and SD initiated the conception and design of the study. HL and SE developed the design and analyzed the results. SD, HL, and SE supervised the work. HL and AL prepared the first draft of the manuscript. HL, SE, ER, AL, AZ, RH, YW, EH, MB, and SD reviewed and edited the manuscript. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The Indonesian Ministry of Finance funded this research through the Indonesian Endowment Fund for Education/Lembaga Pengelola Dana Pendidikan (LPDP), grant number PRJ-23/LPDP/2023.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Fadhli H, Ruska S, Furi M, Suhery W, Susanti E, Nasution M. Ciplukan (Physalis angulata L.): Review Tanaman Liar yang Berpotensi Sebagai Tanaman Obat. JFIOnline. 2023;15:134–141. doi:10.35617/jfionline.v15i2.144
2. PéRez-Herrera A, Martínez-Gutiérrez GA, Morales I, Sánchez-Medina MA, Escamirosa-Tinoco C. Physicochemical characterization and antioxidant activity of wild Physalis spp. genotypes. Emir J Food Agric. 2021.
3. Sultana N, Hassan M, Begum M, Sultana MPAL. (Solanaceae) - A new angiospermic record for Bangladesh. Bangladesh J Bot. 2008. doi:10.3329/bjb.v37i2.1731
4. Luthfiyanti R, Iwansyah A, Rahayu Y, Achyadi N Study of antioxidant activities, acceptability, and shelf life prediction of Ciplukan (Physalis angulata L.) juice drinks.
5. Novitasari A, Rohmawaty E, Rosdianto AM. Physalis angulata Linn. as a medicinal plant (Review). Biomed Rep. 2024;20(3):47. doi:10.3892/br.2024.1735
6. Rodrigues E. Plants and animals utilized as medicines in the Jaú National Park (JNP), Brazilian Amazon. Phytother Res. 2006;20(5):378–391. doi:10.1002/ptr.1866
7. Jovel EM, Cabanillas J, Towers GH. An ethnobotanical study of the traditional medicine of the Mestizo people of Suni Miraño, Loreto, Peru. J Ethnopharmacol. 1996;53(3):149–156. doi:10.1016/0378-8741(96)01437-7
8. Roosita K, Kusharto C, Sekiyama M, Fachrurozi Y, Ohtsuka R. Medicinal plants used by the villagers of a Sundanese community in West Java, Indonesia. J Ethnopharmacol. 2008;115:72–81. doi:10.1016/j.jep.2007.09.010
9. Abubakar MS, Musa AM, Ahmed A, Hussaini IM. The perception and practice of traditional medicine in the treatment of cancers and inflammations by the Hausa and Fulani tribes of Northern Nigeria. J Ethnopharmacol. 2007;111(3):625–629. doi:10.1016/j.jep.2007.01.011
10. Parkash V, Aggarwal A. Traditional uses of ethnomedicinal plants of lower foot-hills of Himachal Pradesh-I. Indian J Tradit Knowl. 2010;9:519–521.
11. Zirihi G, Ng K, Etien DT, Grellier P. Ethnopharmacological study of plants used to treat malaria, in traditional medicine, by Bete Populations of Issia (Côte d’Ivoire). J Pharm Sci Res. 2010.
12. Mirzaee F, Saeed Hosseini A, Askian R. Therapeutic Activities and Phytochemistry of Physalis Species Based on Traditional and Modern Medicine. Res J Pharmacogn. 2019;6(4):79–96. doi:10.22127/rjp.2019.93529
13. Rengifo E, Vargas-Arana GPAL. (Bolsa Mullaca): a Review of its Traditional Uses, Chemistry and Pharmacology. Boletín de estudios latinoamer del Caribe. 2013;12:431.
14. Ushie AO, Neji PA, Abeng FE, Azuaga TI, Aikhoje EF, Aji DL. Phytochemical Screening and Antimicrobial Activity of the Acetone and Methanol Leaf Extracts of Physalis angulata. J Chem Socie Nige. 2020.
15. Alam T, Ekayanti M, Permana N, Hadissabil Z. The potential antioxidant activity of ethanol extract and fraction of ciplukan (Physalis angulata) on DPPH (1,1-diphenyl-2-picrylhydrazyl). J Farmasi Indonesia. 2022;19:193–199. doi:10.31001/jfi.v19i1.1490
16. Pillai J, Wali A, Menezes DG, et al. Chemical Composition Analysis, Cytotoxic, Antimicrobial and Antioxidant Activities of Physalis angulata L.: a Comparative Study of Leaves and Fruit. Molecules. 2022;27:1480. doi:10.3390/molecules27051480
17. RetnoWindya K, Noer L, Putri L. Potential of Ciplukan (Physalis Angulata L.) as Source of Functional Ingredient. Procedia Chem. 2015;14:367–372. doi:10.1016/j.proche.2015.03.050
18. Soares MB, Brustolim D, Santos LA, et al. Physalins B, F and G, seco-steroids purified from Physalis angulata L, inhibit lymphocyte function and allogeneic transplant rejection. Int Immunopharmacol. 2006;6(3):408–414. doi:10.1016/j.intimp.2005.09.007
19. Chiang HC, Jaw SM, Chen CF, Kan WS. Antitumor agent, physalin F from Physalis angulata L. Anticancer Res. 1992;12(3):837–843.
20. Lijuan S, Jianwen L, Ping L, Youjun Y, Lei M, Lihong H. Immunosuppression effect of Withangulatin A from Physalis angulata via heme oxygenase 1-dependent pathways. Process Biochem. 2011;46(2):482–488. doi:10.1016/j.procbio.2010.09.022
21. Kinnula VL, Myllärniemi M. Oxidant-antioxidant imbalance as a potential contributor to the progression of human pulmonary fibrosis. Antioxid Redox Signal. 2008;10(4):727–738. doi:10.1089/ars.2007.1942
22. Hewlett JC, Kropski JA, Blackwell TS. Idiopathic pulmonary fibrosis: epithelial-mesenchymal interactions and emerging therapeutic targets. Matrix Biol. 2018;71-72:112–127. doi:10.1016/j.matbio.2018.03.021
23. Biernacka A, Dobaczewski M, Frangogiannis NG. TGF-β signaling in fibrosis. Growth Factors. 2011;29(5):196–202. doi:10.3109/08977194.2011.595714
24. Ong CH, Tham CL, Harith HH, Firdaus N, Israf DA. TGF-β-induced fibrosis: a review on the underlying mechanism and potential therapeutic strategies. Eur J Pharmacol. 2021;911:174510. doi:10.1016/j.ejphar.2021.174510
25. Ignotz RA, Massagué J. Type beta transforming growth factor controls the adipogenic differentiation of 3T3 fibroblasts. Proc Natl Acad Sci U S A. 1985;82(24):8530–8534. doi:10.1073/pnas.82.24.8530
26. Negmadjanov U, Godic Z, Rizvi F, et al. TGF-β1-mediated differentiation of fibroblasts is associated with increased mitochondrial content and cellular respiration. PLoS One. 2015;10(4):e0123046. doi:10.1371/journal.pone.0123046
27. Dewi S, Isbagio H, Purwaningsih EH, Kertia N, Setiabudy R, Double-blind SSA. Randomized Controlled Trial of Ciplukan (Physalis angulata Linn) Extract on Skin Fibrosis, Inflammatory, Immunology, and Fibrosis Biomarkers in Scleroderma Patients. Acta Med Indones. 2019;51(4):303–310.
28. Rohmawaty E, Rosdianto A, Aminah H, et al. Antifibrotic effect of the ethyl acetate fraction of ciplukan (Physalis angulata Linn.) in rat liver fibrosis induced by CCI4. J Appl Pharm Sci. 2021. doi:10.7324/JAPS.2021.1101217
29. Ekawardani S. Physalis angulata Exerts Antifibrotic Effects on TGF-β1-Induced Fibroblasts and on Animal models. Manu Submit Publ. 2023.
30. Imaduddin UK, Berbudi A, Rohmawaty E. The Effect of Physalis angulata L. Administration on Gene Expressions Related to Lung Fibrosis Resolution in Mice-Induced Bleomycin. J Exp Pharmacol. 2024;16:49–60. doi:10.2147/jep.S439932
31. Backer CA, van den Brink RCB. Flora of Java; 1980.
32. Cronquist A. An Integrated System of Classification of Flowering Plants; 1981.
33. List. TP. Website DuniaTumbuhan. availabe from: http://www.theplantlist.org/tpl1.1/record/kew-158489.
34. Aslan C, Maralbashi S, Kahroba H, et al. Docosahexaenoic acid (DHA) inhibits pro-angiogenic effects of breast cancer cells via down-regulating cellular and exosomal expression of angiogenic genes and microRNAs. Life Sci. 2020;258:118094. doi:10.1016/j.lfs.2020.118094
35. Zhang W, Mottillo EP, Zhao J, et al. Adipocyte lipolysis-stimulated interleukin-6 production requires sphingosine kinase 1 activity. J Biol Chem. 2014;289(46):32178–32185. doi:10.1074/jbc.M114.601096
36. Wiraswati HL, Hangen E, Sanz AB, et al. Apoptosis inducing factor (AIF) mediates lethal redox stress induced by menadione. Oncotarget. 2016;7(47):76496–76507. doi:10.18632/oncotarget.12562
37. Wiraswati H, Warganegara F, Akhmaloka A, Martoprawiro M. Molecular Docking Studies of ROS Agent from Quinone Family to Reductase Enzymes: Implication in Finding Anticancer Drug Candidate. Biomed Pharmacol J. 2021;14:681–689. doi:10.13005/bpj/2170
38. Yang F, Jin S, Tang Y. Marine Collagen Peptides Promote Cell Proliferation of NIH-3T3 Fibroblasts via NF-κB Signaling Pathway. Molecules. 2019;24(22). doi:10.3390/molecules24224201
39. Che Zain MS, Lee SY, Sarian MN, Fakurazi S, Shaari K. In Vitro Wound Healing Potential of Flavonoid C-Glycosides from Oil Palm (Elaeis guineensis Jacq.) Leaves on 3T3 Fibroblast Cells. Antioxidants. 2020;9(4). doi:10.3390/antiox9040326
40. Landén NX, Li D, Ståhle M. Transition from inflammation to proliferation: a critical step during wound healing. Cell Mol Life Sci. 2016;73(20):3861–3885. doi:10.1007/s00018-016-2268-0
41. Rose-John S, Winthrop K, Calabrese L. The role of IL-6 in host defence against infections: immunobiology and clinical implications. Nat Rev Rheumatol. 2017;13(7):399–409. doi:10.1038/nrrheum.2017.83
42. Gabay C. Interleukin-6 and chronic inflammation. Arthritis Res Ther. 2006;2(Suppl 2):S3. doi:10.1186/ar1917
43. Fernandez IE, Eickelberg O. New cellular and molecular mechanisms of lung injury and fibrosis in idiopathic pulmonary fibrosis. Lancet. 2012;380(9842):680–8. doi:10.1016/s0140-6736(12)61144-1
44. Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18(7):1028–1040. doi:10.1038/nm.2807
45. Blaauboer ME, Boeijen FR, Emson CL, et al. Extracellular matrix proteins: a positive feedback loop in lung fibrosis? Matrix Biol. 2014;34:170–178. doi:10.1016/j.matbio.2013.11.002
46. Khanna D. Diagnosis and treatment of systemic and localized scleroderma. Expert Rev Dermatol. 2011;6(3):287–302. doi:10.1586/edm.11.26
47. Kowal-Bielecka O, Landewé R, Avouac J, et al. EULAR recommendations for the treatment of systemic sclerosis: a report from the EULAR Scleroderma Trials and Research group (EUSTAR). Ann Rheum Dis. 2009;68(5):620–628. doi:10.1136/ard.2008.096677
48. Molina-Molina M, Machahua-Huamani C, Vicens-Zygmunt V, et al. Anti-fibrotic effects of pirfenidone and rapamycin in primary IPF fibroblasts and human alveolar epithelial cells. BMC Pulm Med. 2018;18(1):63. doi:10.1186/s12890-018-0626-4
49. Ezhilarasan D, Sokal EM, Karthikeyan S, Najimi M, Khan S. Plant derived antioxidants and antifibrotic drugs: past, present and future. J Coastal Life Med. 2014;2:738–745.
50. Selim N, Melk M, Melek F, Saleh D, Sobeh M, El-Hawary S. Phytochemical profiling and anti-fibrotic activities of Plumbago indica L. and Plumbago auriculata Lam. in thioacetamide-induced liver fibrosis in rats. Sci Rep. 2022;12. doi:10.1038/s41598-022-13718-9
51. Hinz B, Celetta G, Tomasek JJ, Gabbiani G, Chaponnier C. Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol Biol Cell. 2001;12(9):2730–2741. doi:10.1091/mbc.12.9.2730
52. Phaosri M, Jantrapirom S, Takuathung MN, et al. Salacia chinensis L. Stem Extract Exerts Antifibrotic Effects on Human Hepatic Stellate Cells Through the Inhibition of the TGF-β1-Induced SMAD2/3 Signaling Pathway. Int J Mol Sci. 2019;20(24). doi:10.3390/ijms20246314
53. Fu Y, Zheng S, Lin J, Ryerse J, Chen A. Curcumin protects the rat liver from CCl4-caused injury and fibrogenesis by attenuating oxidative stress and suppressing inflammation. Mol Pharmacol. 2008;73(2):399–409. doi:10.1124/mol.107.039818
54. Hong SW, Jung KH, Zheng HM, et al. The protective effect of resveratrol on dimethylnitrosamine-induced liver fibrosis in rats. Arch Pharm Res Apr. 2010;33(4):601–609. doi:10.1007/s12272-010-0415-y
55. Cui Y, Han Y, Yang X, Sun Y, Zhao Y. Protective effects of quercetin and quercetin-5’,8-disulfonate against carbon tetrachloride-caused oxidative liver injury in mice. Molecules. 2013;19(1):291–305. doi:10.3390/molecules19010291
56. Abdul-Nasir-Deen A-Y, Boakye Y, Osafo N, et al. Anti-inflammatory and wound healing properties of methanol leaf extract of Physalis angulata L. S Afr J Bot. 2020;133:124–131. doi:10.1016/j.sajb.2020.06.030
57. Lin X, Yu M, Wu K, Yuan H, Zhong H. Effects of pirfenidone on proliferation, migration, and collagen contraction of human Tenon’s fibroblasts in vitro. Invest Ophthalmol Vis Sci. 2009;50(8):3763–3770. doi:10.1167/iovs.08-2815
58. Stahnke T, Kowtharapu BS, Stachs O, et al. Suppression of TGF-β pathway by pirfenidone decreases extracellular matrix deposition in ocular fibroblasts in vitro. PLoS One. 2017;12(2):e0172592. doi:10.1371/journal.pone.0172592
59. King TE Jr, Pardo A, Selman M. Idiopathic pulmonary fibrosis. Lancet. 2011;378(9807):1949. doi:10.1016/s0140-6736(11)60052-4
60. Taflinski L, Demir E, Kauczok J, et al. Blue light inhibits transforming growth factor-β1-induced myofibroblast differentiation of human dermal fibroblasts. Exp Dermatol Apr. 2014;23(4):240–246. doi:10.1111/exd.12353
61. Garrett SM, Baker Frost D, Feghali-Bostwick C. The mighty fibroblast and its utility in scleroderma research. J Scleroderma Relat Disord. 2017;2(2):69–134. doi:10.5301/jsrd.5000240
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.