Back to Journals » Journal of Multidisciplinary Healthcare » Volume 18
Association Between Caffeine Intake and Bowel Habits and Inflammatory Bowel Disease: A Population-Based Study
Authors Yang X, Yan H, Chen Y, Guo R
Received 23 January 2025
Accepted for publication 8 June 2025
Published 27 June 2025 Volume 2025:18 Pages 3717—3726
DOI https://doi.org/10.2147/JMDH.S512855
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Pavani Rangachari
Xiaoxian Yang,1,2 Haiyi Yan,1 Yan Chen,1 Rui Guo1
1Department of Hepatology, Xiyuan Hospital of China Academy of Chinese Medical Sciences, Beijing, People’s Republic of China; 2First Clinical Medical College, Nanjing University of Chinese Medicine, Nanjing, People’s Republic of China
Correspondence: Rui Guo, Xiyuan Hospital of China Academy of Chinese Medical Sciences, No. 1 Xiyuan Playground, Haidian District, Beijing, People’s Republic of China, Email [email protected]
Background: The effect of caffeine in coffee, a popular beverage, on gastrointestinal symptoms has been the subject of ongoing debate worldwide. The present study explored the association between caffeine intake and bowel habits and Inflammatory bowel disease (IBD).
Methods: Data from the National Health and Nutrition Examination Survey (NHANES) spanning 2005– 2010 were utilized for this cross-sectional survey. Bowel habits and IBD were defined by self-report. Logistic regression models assessed the linear relationship between caffeine intake and chronic constipation. Nonlinear associations were delineated using fitted smoothed curves and threshold effect analyses. Finally, subgroup analyses and interactions were used to test the stability of the findings.
Results: This population-based study included a total of 12,759 adults. We found that caffeine intake was negatively associated with chronic diarrhea. There was a U-shaped nonlinear relationship between caffeine intake and chronic constipation. To the left of breakpoint 2.04 (100 mg/1 unit), caffeine intake was negatively associated with chronic constipation (OR [95% CI]: 0.82 [0.74, 0.90]), however, to the right of the breakpoint, there was a positive association (OR [95% CI]: 1.06 [1.00, 1.12]). In addition, no significant association was found between caffeine intake and IBD. Subgroup analyses and interaction tests showed that caffeine intake was simply negatively associated with chronic constipation in older adults.
Conclusion: In conclusion, moderate caffeine intake may help with bowel movements, but excessive caffeine intake may cause chronic constipation. Appropriate caffeine intake in older adults may help prevent chronic constipation. This suggests that in our clinical practice, we need to strategize caffeine intake according to the population’s defecation status.
Keywords: caffeine intake, chronic constipation, chronic diarrhea, U-shaped, NHANES
Introduction
Coffee is one of the most widely consumed beverages in the world today, and caffeine is considered to be the most consumed psychoactive drug in the world, with an annual consumption of approximately 120,000 tons.1 According to a survey conducted by the National Coffee Association, about 64% of adults in the United States drink coffee every day and consume about 517 million cups of coffee per day.2 Coffee and tea are the main sources of caffeine (>80% of daily intake).3 In the United States, the prevalence of chronic diarrhea is estimated to be 11–30% of the total population, affecting 6.6% of the US population,4,5 while the global prevalence of chronic constipation is 14%. These two conditions have become some of the most common gastrointestinal disorders worldwide. IBD is an idiopathic inflammatory gastrointestinal disorder that includes Crohn’s disease (CD) and ulcerative colitis (UC). The number of people affected by IBD in the United States and Europe has exceeded 3 million and continues to rise, with chronic diarrhea being the most common symptom.6–8
The gastrointestinal tract is the first organ that coffee and its many components come into contact with after ingestion. With the popularity of coffee beverages, there is growing concern about the effects that caffeine produces on the gastrointestinal tract. Some studies have shown that caffeine stimulates the distal colonic motor response, which acts as a laxative and may reduce the risk of chronic constipation.9 However, it has also been shown that caffeine has a protective effect on mice with acute colitis and significantly reduces clinical symptoms such as diarrhea.10 It has also been found that higher doses of caffeine reduce inflammatory biomarkers and activate anti-inflammatory mechanisms,11 and that caffeine has been negatively associated with the development of CD, possibly by modulating inflammatory mediators to attenuate inflammation in the gut.12 Recent studies have shown that caffeine may affect intestinal function by modulating the Melanocortin System. Melanocortin Receptor is widely expressed in the central nervous system and peripheral intestines and is involved in the regulation of intestinal motility and inflammatory responses.13 Of particular note, caffeine, as an adenosine receptor antagonist, enhances melanocortin signaling pathway activity through inhibition of A2A receptors, which in turn promotes colonic propulsive motility.14 In the field of IBD, Melanocortin 3 and 5 receptors were found to have significantly increased expression in the colon of IBD patients, and these results suggest that the melanocortin system may be a potential target for caffeine’s association with intestinal diseases.15
Existing findings suggest that caffeine is associated with these gastrointestinal symptoms and diseases, but these findings are contradictory and controversial. The aim of this study was to further explore the association between caffeine intake and chronic diarrhea, chronic constipation, and IBD by analyzing a large database of samples to help people better prevent and manage gastrointestinal symptoms in their daily lives.
Materials and Methods
Survey Description
Data for this study were sourced from the NHANES program administered by the National Center for Health Statistics (NCHS). This initiative evaluates the health and nutritional conditions of both children and adults across the United States through various methods, including screenings, laboratory tests, and interviews. All participants in the NHANES program provided informed consent, which was reviewed and approved by the NCHS Research Ethics Review Board. Comprehensive details regarding the NHANES study designs and datasets can be accessed publicly at www.cdc.gov/nchs/nhanes/.
Study Population
The analysis utilized samples from three NHANES cross-sectional surveys conducted during the years 2005–2006, 2007–2008, and 2009–2010, focusing on “bowel health” data. Initially, the study involved 31,034 adult participants (aged 20 years and older). After removing individuals with incomplete information on caffeine consumption (n=6787) and bowel habits (n=11488), a final cohort of 12,759 eligible subjects remained. This group included those with normal bowel function (n=10,785), chronic diarrhea (n=988), and chronic constipation (n=986). As the NCHS included inquiries about IBD only in the 2009–2010 survey, our analysis focused exclusively on 4151 eligible subjects from the 10,537 surveyed in that timeframe (Figure 1).
 |
Figure 1 Flowchart of the sample selection from NHANES 2005–2010, 2009–2010. |
Definition of Caffeine Intake and Bowel Habits
Assessment of Caffeine Intake
All participants in the NHANES study had the opportunity to take part in two 24-hour dietary recall interviews. The initial recall interview was conducted in person at a mobile testing unit, while the second was carried out via telephone within a 3–10 day window. For the NHANES data collection, daily nutrient and food component totals were computed for all reported foods using the USDA Dietary Study Food and Nutrition Database, which encompasses around 50 coffee-related beverages, 30 types of tea, and both caffeinated and non-caffeinated sodas. As a result, caffeine consumption was estimated from all sources of caffeinated food and drink, including energy drinks. The analysis incorporated the average caffeine intake derived from both 24-hour recall interviews.
Assessment of Bowel Habits
Bowel habits were assessed using the Bristol Stool Form Scale (BSFS), which categorizes stools from Type 1 to Type 7. Participants were shown an illustrated card displaying the scale and were asked, “Please look at this card and tell me the number that corresponds to your usual or most common type of stool”. Based on previous research, chronic constipation was characterized by BSFS types 1 (hard lumps, similar to nuts) or 2 (sausage-shaped but lumpy). Chronic diarrhea was defined by BSFS types 6 (fluffy lumps with rough edges, pasty stool) or 7 (watery stool with no solid particles). Types 3, 4, and 5 were classified as indicating normal bowel function.4,16–18 Participants were considered to have IBD if they responded affirmatively to the question, “Has a doctor or other health professional ever told you that you had UC/CD?”
Selection of Covariates
To strengthen the link between caffeine consumption, bowel habits, and IBD, we accounted for various potential confounders, including age, gender, race, educational status, socioeconomic status, smoking status, drinking status, and BMI. Racial categories included non-Hispanic white, non-Hispanic black, Mexican American, and others. Education was categorized as less than high school, high school graduate, or higher education. Socioeconomic status was classified into three groups: low income (PIR < 1.3), middle income (1.3 ≤ PIR < 3.5), and high income (PIR ≥ 3.5). Smoking status was defined by whether the individual had smoked at least 100 cigarettes in their lifetime, while drinking status was based on having consumed at least 12 alcoholic drinks/1 year.
Statistical Analysis
Statistical analyses were conducted using EmpowerStats (2.0) and R (4.1.3), with NHANES sampling weights applied in all calculations. Descriptive statistics for baseline characteristics were presented as means and standard deviations (SD) for continuous variables, and as percentages for categorical variables, categorized by bowel habits and IBD status. Multivariate logistic regression was employed to calculate odds ratios (ORs) and 95% confidence intervals (CIs) for associations between various factors such as caffeine intake, chronic diarrhea, chronic constipation, and IBD. Three models were used for the regression analysis: Crude model, which was unadjusted for covariates; Minimally adjusted model, which adjusted for age, gender, and race; and Fully adjusted model, which adjusted for all relevant covariates. For additional sensitivity analysis, caffeine intake was divided into thirds. To account for potential nonlinear relationships, smooth curve fitting was applied, and threshold effects and breakpoints (K) were identified using a threshold effects analysis model. Subgroup analyses and interaction tests were conducted to examine potential heterogeneity in the results. Statistical significance was set at a bilateral p-value of < 0.05.
Results
Baseline Characteristics of Participants
The average age of individuals with chronic diarrhea was 50.81 ± 15.81 years, while those with chronic constipation had a mean age of 46.36 ± 17.58 years. In both the chronic diarrhea and constipation groups, the proportion of women exceeded that of men. Non-Hispanic whites were the most prevalent race in the survey. The mean caffeine intake for chronic diarrhea and constipation was 191.07 ± 231.12 and 154.22 ± 202.63, respectively. In the analysis of IBD, we included 4151 participants from the 2009–2010 cohort, of whom 51 were diagnosed with IBD. The average age of the IBD patients was 49.57 ± 14.21 years, with 57.17% identifying as female and 42.83% as male. The patients were 79.16% non-Hispanic white, and the mean caffeine intake was 170.51 ± 182.43.
Supplementary Material 1 provides a summary of the basic characteristics of the study population. We observed that gender, race, education level, socioeconomic status, smoking habits, BMI, and caffeine intake exhibited statistically significant differences (p < 0.05) between individuals with chronic diarrhea and constipation. In contrast, significant differences in age and education level (p < 0.05) were found among patients with IBD.
The Association Between Caffeine Intake and Chronic Diarrhea
Because 1 mg unit is too small, caffeine intake in this study was 100 mg as 1 unit. Table 1 shows the association between caffeine intake and chronic diarrhea. We found a positive association between caffeine intake and chronic diarrhea in the fully adjusted model. For each additional unit of caffeine intake, the risk of chronic diarrhea increased by 4% (OR [95% CI]: 1.04 [1.00, 1.08]). Sensitivity analyses were then performed by transforming caffeine intake into a categorical variable (tertiles), and no significant association with chronic diarrhea was found.
 |
Table 1 The Association Between Caffeine Intake and Chronic Diarrhea |
Furthermore, we conducted a smoothed curve-fitting analysis and found no evidence of a nonlinear association between caffeine intake and chronic diarrhea (Figure 2).
 |
Figure 2 Smoothed curve fit plot between caffeine intake and chronic diarrhea. |
The Association Between Caffeine Intake and Chronic Constipation
Table 2 presents the relationship between caffeine intake and chronic constipation. In the fully adjusted model, no statistically significant link was observed between caffeine intake and chronic constipation (OR [95% CI]: 0.97 [0.93, 1.02]). However, when caffeine intake was divided into tertiles, the highest tertile showed a negative association with chronic constipation across all three models. Specifically, in the fully adjusted model (tertile 3), individuals in the highest tertile had a 25% reduced risk of chronic constipation (OR [95% CI]: 0.75 [0.63, 0.90]) compared to those in the lowest tertile (tertile 1).
 |
Table 2 The Association Between Caffeine Intake and Chronic Constipation |
Smooth curve fitting identified a nonlinear association between caffeine intake and chronic constipation (Figure 3). This relationship was further confirmed through a threshold analysis, which pinpointed a breakpoint at 2.04. To the left of this breakpoint, each unit increase in caffeine intake was associated with an 18% reduction in the risk of chronic constipation (OR [95% CI]: 0.82 [0.74, 0.90]). Conversely, to the right of the breakpoint, each unit increase in caffeine intake led to a 6% increase in the risk of chronic constipation (OR [95% CI]: 1.06 [1.00, 1.12]) (Table 3).
 |
Table 3 Threshold Effect Analysis of Caffeine Intake on Chronic Constipation |
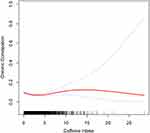 |
Figure 3 Smoothed curve fit plot between caffeine intake and Chronic constipation. |
The Association Between Caffeine Intake and IBD
We then explored the association between caffeine intake and IBD and found no statistical association between the two, either in continuous or categorical variables (Supplementary material 2).
Subgroup Analysis
To examine whether specific baseline characteristics influenced the relationship between caffeine consumption and chronic diarrhea, chronic constipation, and IBD, we performed subgroup analyses and interaction tests. These analyses were stratified by gender, age, race, socioeconomic status, drinking consumption, smoking habits, and BMI. Interaction tests showed that high-income individuals were at greater risk of developing chronic diarrhea compared with the general population, with a 12% increase in the risk of chronic diarrhea in high-income individuals for each 1-unit increase in caffeine intake (OR [95% CI]: 1.12 [1.05, 1.19]). In addition, an interaction test showed that the risk of chronic constipation in older adults aged ≥60 years was negatively associated with caffeine intake, with a 14% decrease in the risk of chronic constipation for each 1-unit increase in caffeine intake (OR [95% CI]: 0.86 [0.77, 0.95]). None of the other covariates significantly influenced the relationship between caffeine intake and chronic diarrhea, chronic constipation, or IBD (Table 4).
 |
Table 4 Subgroup Analysis |
Discussion
Based on our cross-sectional study of 12,759 subjects from 2005–2010, we found that caffeine intake was negatively associated with chronic diarrhea. There was a U-shaped nonlinear relationship between caffeine intake and chronic constipation with a breakpoint of 2.04 (100 mg/1 unit), with caffeine intake negatively associated with chronic constipation on the left side of the breakpoint and positively associated with the right side. Moreover, no notable relationship was observed between caffeine intake and IBD. This means that when caffeine intake is less than 204 mg, it may have a laxative effect, while when intake is greater than 204 mg, it may increase the risk of constipation. Subgroup analyses and interaction tests suggest that this U-shaped relationship does not apply to older adults ≥60 years of age, where high caffeine intake is associated with a low risk of chronic constipation.
There have been controversial results on the effects of caffeine on the gastrointestinal tract, which may be related to the dose of caffeine. An animal study demonstrated disparate effects on colonic tissue and clinical signs of colitis in mice administered different caffeine doses.10 A United States-based study indicated that consuming a minimum of six cups of caffeinated coffee exacerbated GERD symptoms by 23% while consuming the same quantity of decaffeinated coffee exacerbated GERD symptoms by 48%.19 Furthermore, a meta-analysis revealed varying caffeine doses to have differing effects on the incidence of gallstone disease.20 All these studies have demonstrated the varying effects of different caffeine doses on the gastrointestinal tract. Our findings revealed a U-shaped association between caffeine intake and chronic constipation, suggesting the existence of a threshold in caffeine consumption where its impact transitions from facilitating defecation to impeding it. This phenomenon may contribute to resolving the query, “Does coffee induce constipation or diarrhea?” The results of a cross-sectional study similarly found that high caffeine intake was associated with low odds of constipation, which is consistent with our findings, but this study did not find a U-shaped node, which may be related to the fact that they did not precisely analyze caffeine intake in mg, but rather categorized caffeine intake into 5 categories for statistical analysis, as well as the inclusion of different confounders.21
Due to the insufficiency of the current related research, we are unable to determine the exact node in a more authoritative and detailed way, but the results of this study can bring us new thinking perspectives and help us to better explore this possible node in the future.
The mechanism through which caffeine impacts bowel habits remains unstandardized. Several studies indicate that caffeine exerts a notable influence on intestinal flora, augmenting beneficial bacteria like bifidobacteria and influencing gastrointestinal function.22,23 Additionally, there are suggestions that caffeine could modulate defecation by eliciting motor responses in the distal colon.24,25 It has also been studied that caffeine is suspected to play an indirect role in the colon through neural mechanisms or gastrointestinal hormones.26
Additionally, we found no significant association between caffeine intake and IBD. Previous studies on this relationship have yielded conflicting results. Beygi et al showed that MSCs treated with caffeine and naloxone improved symptoms and reduced inflammation in a mouse model of ulcerative colitis.27 It has also been shown that caffeine is able to reduce the production of pro-inflammatory cytokines while increasing the levels of the anti-inflammatory cytokine IL-10, which has a protective effect on the incidence of UC.28,29 A Japanese case-control study also found that coffee and caffeine intake were associated with a reduced risk of UC.30 However, other studies have found that caffeine intake did not show a significant protective effect in some cases and that prolonged caffeine intake may be associated with increased inflammation.31,32 Our study did not find a significant relationship between the two, which may suggest the need for further validation by randomized controlled experiments with larger sample sizes.
Lastly, we conducted subgroup analyses and interaction tests, which showed that high-income individuals were at greater risk of developing chronic diarrhea, with a 12% increase in their risk of chronic diarrhea for each 1-unit increase in caffeine intake (OR [95% CI]: 1.12 [1.05, 1.19]). This may be related to higher work stress, gut dysfunction due to adverse psychological factors such as anxiety and depression, and the fact that higher-income individuals typically receive more medications, some of which (eg, antibiotics, antacids, and certain antidepressants) may lead to diarrhea as a side effect when used over a long period of time.33 In addition, caffeine intake was negatively associated with chronic constipation in older adults ≥60 years of age, suggesting that older adults may consume coffee appropriately to reduce the risk of chronic constipation. Other confounding factors did not significantly influence the association between caffeine intake and chronic constipation.
Our study has several advantages. First, it utilized a relatively large sample size, thereby increasing the reliability of the findings. Second, this study innovatively identified a U-shaped association between caffeine intake and chronic constipation, and we analyzed chronic diarrhea, chronic constipation, and IBD all separately, making the intestinal symptoms very comprehensive and allowing for a better understanding of the effects of caffeine on intestinal health. Finally, we conducted subgroup analyses and interaction tests, which revealed a relationship between caffeine intake and chronic constipation specifically among individuals aged 60 years and older. However, there are several limitations to this study. First, the cross-sectional design restricts the ability to draw causal conclusions regarding caffeine consumption and gut health, highlighting the need for more robust randomized controlled trials to confirm these findings. Second, although we accounted for a variety of key covariates, the influence of other potential confounding factors could not be fully ruled out. Third, caffeine intake was collected through dietary questionnaires, which may be affected by biases in subjects’ memories and produce bias. Fourth, bowel habits were defined by participants’ self-reports, and the information collected may be influenced by participants’ subjective awareness. Finally, due to the limited number of IBD patients in the NHANES database and the relatively small sample size included in the analysis, the IBD results should be interpreted with caution, and a larger sample size is needed for further validation.
Conclusion
In conclusion, our study found a U-shaped association between caffeine intake and chronic constipation. Moderate caffeine intake may help defecation, but excessive caffeine intake may cause chronic constipation. However, older adults were associated with lower odds of chronic constipation regardless of caffeine intake. Furthermore, caffeine intake was not significantly associated with IBD. Therefore, this suggests that caffeine use in clinical practice needs to be approached strategically, with the right amount of caffeine being consumed according to the defecation status of the population.
Data Sharing Statement
The datasets supporting the conclusions of this article are available in the NHANES repository, www.cdc.gov/nchs/nhanes/.
Ethics Approval
This is an observational study. The data used were from the NHANES database published by the National Center for Health Statistics (NCHS). The NCHS Ethics Review Board (ERB) reviews and approves NHANES survey protocols. NCHS ERB ensures that research involving human participants protects the rights and welfare of study participants and conforms to US federal regulations. The NCHS ERB, and the formal review bodies that preceded it, have approved each NHANES study protocol since the survey began running continuously in 1999. Information on the approval of the ERB is available on the public website: https://www.cdc.gov/nchs/nhanes/about/erb.html. As the data covered in this study have all been ethically approved by the NCHS ERB and did not involve data from the authors’ institutions, the authors’ institutions indicated that they did not need to go through ethical review and approval again.
Acknowledgments
Thanks to all the authors for their contributions.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Disclosure
The authors have no relevant financial or non-financial interests to disclose in this work.
References
1. Laurent C, Eddarkaoui S, Derisbourg M, et al. Beneficial effects of caffeine in a transgenic model of Alzheimer’s disease-like tau pathology. Neurobiol Aging. 2014;35:2079–2090. doi:10.1016/j.neurobiolaging.2014.03.027
2. Feng J, Wang J, Jose M, Seo Y, Feng L, Ge S. Association between caffeine intake and all-cause and cause-specific mortality: an analysis of the National Health and Nutrition Examination Survey (NHANES) 1999-2014 database. Nurs Rep. 2021;11:901–912. doi:10.3390/nursrep11040083
3. Reyes CM, Cornelis MC, Vinknes KJ. Caffeine in the diet: country-level consumption and guidelines. Nutrients. 2018;11:10. doi:10.3390/nu11010010
4. Singh P, Mitsuhashi S, Ballou S, et al. Demographic and dietary associations of chronic diarrhea in a representative sample of adults in the United States. Am J Gastroenterol. 2018;113:593–600. doi:10.1038/ajg.2018.24
5. Bharucha AE, Dunivan G, Goode PS, et al. Epidemiology, pathophysiology, and classification of fecal incontinence: state of the science summary for the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) workshop. Am J Gastroenterol. 2015;110:127–136. doi:10.1038/ajg.2014.396
6. Hodson R. Inflammatory bowel disease. Nature. 2016;540:S97. doi:10.1038/540S97a
7. Weisman MH, Oleg S, Seok Kim H, Hou JK, Miller FW, Dillon CF. Inflammatory bowel disease prevalence: surveillance data from the U.S. national health and nutrition examination survey. Prev Med Rep. 2023;33:102173. doi:10.1016/j.pmedr.2023.102173
8. Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol. 2015;12:720–727. doi:10.1038/nrgastro.2015.150
9. Murakami K, Okubo H, Sasaki S. Dietary intake in relation to self-reported constipation among Japanese women aged 18-20 years. Eur J Clin Nutr. 2006;60:650–657. doi:10.1038/sj.ejcn.1602365
10. Lee IA, Low D, Kamba A, Llado V, Mizoguchi E. Oral caffeine administration ameliorates acute colitis by suppressing chitinase 3-like 1 expression in intestinal epithelial cells. J Gastroenterol. 2014;49:1206–1216. doi:10.1007/s00535-013-0865-3
11. Dong H, Xu F, Linghu E. Unraveling the link between plasma caffeine concentrations and inflammatory bowel disease risk through Mendelian randomization. Am J Clin Nutr. 2024;119:711–715. doi:10.1016/j.ajcnut.2024.01.003
12. Mizoguchi E, Sadanaga T, Okada T, Minagawa T, Akiba J. Does caffeine have a double-edged sword role in inflammation and carcinogenesis in the colon? Intest Res. 2023;21:306–317. doi:10.5217/ir.2022.00118
13. Spana C, Taylor AW, Yee DG, Makhlina M, Yang W, Dodd J. Probing the role of melanocortin type 1 receptor agonists in diverse immunological diseases. Front Pharmacol. 2018;9:1535. doi:10.3389/fphar.2018.01535
14. Gravina AG, Pellegrino R, Durante T, et al. The melanocortin system in inflammatory bowel diseases: insights into its mechanisms and therapeutic potentials. Cells. 2023;13:12. doi:10.3390/cells13010012
15. Gravina AG, Panarese I, Trotta MC, et al. Melanocortin 3,5 receptors immunohistochemical expression in colonic mucosa of inflammatory bowel disease patients: a matter of disease activity? World J Gastroenterol. 2024;30:1132–1142. doi:10.3748/wjg.v30.i9.1132
16. Ballou S, Katon J, Singh P, et al. Chronic diarrhea and constipation are more common in depressed individuals. Clin Gastroenterol Hepatol. 2019;17:2696–2703. doi:10.1016/j.cgh.2019.03.046
17. Markland AD, Palsson O, Goode PS, Burgio KL, Busby-Whitehead J, Whitehead WE. Association of low dietary intake of fiber and liquids with constipation: evidence from the national health and nutrition examination survey. Am J Gastroenterol. 2013;108:796–803. doi:10.1038/ajg.2013.73
18. Sommers T, Mitsuhashi S, Singh P, et al. Prevalence of chronic constipation and chronic diarrhea in diabetic individuals in the United States. Am J Gastroenterol. 2019;114:135–142. doi:10.1038/s41395-018-0418-8
19. Mehta RS, Song M, Staller K, Chan AT. Association between beverage intake and incidence of gastroesophageal reflux symptoms. Clin Gastroenterol Hepatol. 2020;18:2226–2233.e2224. doi:10.1016/j.cgh.2019.11.040
20. Zhang YP, Li WQ, Sun YL, Zhu RT, Wang WJ. Systematic review with meta-analysis: coffee consumption and the risk of gallstone disease. Aliment Pharmacol Ther. 2015;42:637–648. doi:10.1111/apt.13328
21. Kang Y, Yan J. Exploring the connection between caffeine intake and constipation: a cross-sectional study using national health and nutrition examination survey data. BMC Public Health. 2024;24:3. doi:10.1186/s12889-023-17502-w
22. Jaquet M, Rochat I, Moulin J, Cavin C, Bibiloni R. Impact of coffee consumption on the gut microbiota: a human volunteer study. Int J Food Microbiol. 2009;130:117–121. doi:10.1016/j.ijfoodmicro.2009.01.011
23. Jung ES, Park JI, Park H, Holzapfel W, Hwang JS, Lee CH. Seven-day green tea supplementation revamps gut microbiome and caecum/skin metabolome in mice from stress. Sci Rep. 2019;9:18418. doi:10.1038/s41598-019-54808-5
24. Lohsiriwat S, Kongmuang P, Leelakusolvong S. Effects of caffeine on anorectal manometric findings. Dis Colon Rectum. 2008;51:928–931. doi:10.1007/s10350-008-9271-y
25. Rao SS, Welcher K, Zimmerman B, Stumbo P. Is coffee a colonic stimulant? Eur J Gastroenterol Hepatol. 1998;10:113–118. doi:10.1097/00042737-199802000-00003
26. Scheperjans F, Pekkonen E, Kaakkola S, Auvinen P. Linking smoking, coffee, urate, and parkinson’s disease - a role for gut microbiota? J Parkinsons Dis. 2015;5:255–262. doi:10.3233/JPD-150557
27. Beygi M, Shayegh J, Esmaeili Gouvarchin Ghaleh H. Caffeine and naloxone treated mesenchymal stem cells improve symptoms and reduce inflammation in a mouse model of ulcerative colitis. Transpl Immunol. 2024;82:101986. doi:10.1016/j.trim.2024.101986
28. Saygili S, Hegde S, Shi XZ. Effects of coffee on gut microbiota and bowel functions in health and diseases: a literature review. Nutrients. 2024;17:16. doi:10.3390/nu17010016
29. Niewiadomski O, Studd C, Wilson J, et al. Influence of food and lifestyle on the risk of developing inflammatory bowel disease. Intern Med J. 2016;46:669–676. doi:10.1111/imj.13094
30. Tanaka K, Okubo H, Miyake Y, et al. Coffee and caffeine intake reduces risk of ulcerative colitis: a case-control study in Japan. J Gastroenterol Hepatol. 2024;39:512–518. doi:10.1111/jgh.16439
31. Ohta A, Lukashev D, Jackson EK, Fredholm BB, Sitkovsky M. 1,3,7-trimethylxanthine (caffeine) may exacerbate acute inflammatory liver injury by weakening the physiological immunosuppressive mechanism. J Immunol. 2007;179:7431–7438. doi:10.4049/jimmunol.179.11.7431
32. Barcelos RP, Lima FD, Carvalho NR, Bresciani G, Royes LF. Caffeine effects on systemic metabolism, oxidative-inflammatory pathways, and exercise performance. Nutr Res. 2020;80:1–17. doi:10.1016/j.nutres.2020.05.005
33. Chang JY, Locke GR 3rd, Schleck CD, Zinsmeister AR, Talley NJ. Risk factors for chronic diarrhoea in the community in the absence of irritable bowel syndrome. Neurogastroenterol Motil. 2009;21:1060–e1087. doi:10.1111/j.1365-2982.2009.01328.x
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

