Back to Journals » Journal of Inflammation Research » Volume 17
Association Between Serum Interleukin-32 Level and Disease Status in Cases with Neuromyelitis Optica Spectrum Disorders
Authors Yu HF, Xu J, Fang Y, Xiao LC
Received 10 July 2024
Accepted for publication 19 August 2024
Published 26 August 2024 Volume 2024:17 Pages 5645—5652
DOI https://doi.org/10.2147/JIR.S476435
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Hong-Fei Yu,* Jin Xu,* Yi Fang, Lian-Chen Xiao
Department of Neurology, Xiangyang Central Hospital, Affiliated Hospital of Hubei University of Arts and Science, Xiangyang, Hubei, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Lian-Chen Xiao; Yi Fang, Department of Neurology, Xiangyang Central Hospital, Affiliated Hospital of Hubei University of Arts and Science, No. 136, Jingzhou Road, Xiangyang, People’s Republic of China, Email [email protected]; [email protected]
Background: Various cytokines are involved in the pathogenesis of neuromyelitis optica spectrum disorders (NMOSD), but whether serum interleukin-32 (IL-32) level is related to disease activity in cases with NMOSD remains poorly understood. Thus, we investigated the underlying role of IL-32 in NMOSD cases.
Methods: Our observation recruited 32 cases with acute NMOSD, 36 NMOSD cases in remission, and 60 healthy individuals in this study. Serum concentrations of IL-32 were detected using ELISA. The associations among IL-32 levels and clinical characteristics were assessed by Spearman correlation coefficient and logistic regression analysis.
Results: IL-32 concentrations were strongly increased in cases with acute NMOSD [(52.06 ± 16.56) pg/mL] and NMOSD in remission [(25.78 ± 8.31) pg/mL] compared with healthy controls [(10.83 ± 6.94) pg/mL] (all p < 0.001). ROC analysis suggested that the AUC for IL-32 and the combined diagnosis of acute NMOSD was 0.811 (P = 0.026, 95% CI 0.673– 0.949), with a sensitivity of 0.800 and a specificity of 0.806. The level of IL-32 was positively correlated with EDSS scores in patients with acute NMOSD (r = 0.620, p < 0.001). EDSS score was independently associated with increased serum levels of LI-32 (B = 1.529, p < 0.001).
Conclusion: Higher level of IL-32 is related to disease severity in NMOSD. Therefore, serum IL-32 may be a novel biomarker for acute NMOSD.
Keywords: IL-32, neuromyelitis optica spectrum disorders, biomarker
Introduction
Neuromyelitis optica spectrum disorder (NMOSD) is a chronic inflammatory disorder of CNS characterized by optic neuritis (ON) and longitudinally extensive transverse myelitis (LETM).1 It has been reported that 80% NMOSD cases are positive for aquaporin-4 (AQP4) immunoglobulin G antibodies, which leads to astrocyte damage, inflammatory cell infiltration, and myelin loss.2 In 2015, the Wingerchuk group reported the first diagnostic criteria for NMOSD.3 Thus, increasing number of patients with NMOSD are being diagnosed according to the latest diagnostic criteria. Nevertheless, the complex pathogenesis of NMOSD has not been fully uncovered. The main pathology of NMOSD involves in AQP4-IgG and lymphocyte function. AQP4-Ig penetrates the blood-brain barrier (BBB) and reacts with AQP4 in astrocyte, followed by recruiting and activating complement to trigger complement-dependent-cytotoxicity. Moreover, the AQP4-specific T-cells and B cell immuno-regulatory dysfunction may destroy the BBB and recruit sustained levels of pathogenic effectors in NMOSD.4 Many studies have revealed that various inflammatory factors (IL-17A, IL-6, IL-22, or IL-35) and chemokines (CXCL8 or CXCL10) participate in the autoimmune response and CNS injury.4,5 However, few researches have reported the association among IL-32 and clinical characteristics of cases with NMOSD.
IL-32 plays an important role in the pathogenesis of immune-associated chronic inflammatory disorder.6 It stimulates the production of various pro-inflammatory factors, including IL-1β and IL-6. IL-32 overexpression has been investigated in many autoimmune disorders, including arthritis,7 asthma,8 and myasthenia gravis.9 IL-32 induces the maturation and activation of dendritic cells (DCs), thereby mediating Th1 and Th17 cell-associated immune response by secreting IL-12 and IL-6 through the NF-κB pathway.10 Meanwhile, some researchers reported increased plasma levels of IL-32 in patients with NMOSD.11 Nevertheless, the correlations among IL-32 levels and clinical variables in NMOSD cases have not been investigated.
We assessed IL-32 levels in cases with acute and remitted NMOSD. We also investigated whether IL-32 level is related to disease severity in cases and whether it is a sensitive biomarker for NMOSD.
Materials and Methods
Subjects
This was a cross-section study conducted at Xiangyang Central Hospital, Affiliated Hospital of Hubei University of Arts and Science. In total, 68 patients, 32 with acute NMOSD and 36 with remitted NMOSD, were consecutively enrolled from July 2020 to Mar 2023. The inclusion criteria were adopted according to previous report.3 Patients included in the acute NMOSD group were drug-naive before sampling, including steroids. Patients with other autoimmune diseases were excluded from this study. Additionally, 60 healthy subjects who had no chronic or acute diseases were recruited as the control group in our study. The included subjects voluntarily agreed to their participation and signed the written informed consent. The present study was approved by the ethics committee of the Xiangyang Central Hospital, Affiliated Hospital of Hubei University of Arts and Science (No.2020–080).
High-Throughput Screening Analysis
In order to preliminary explore the potential biomarkers for NMOSD, we collected 8 serum samples, including four healthy individuals and four patients with NMOSD, to screen the differentially expressed genes. Then, the differentially expressed genes were subsequently identified by RNA-sequencing and high-throughput screening analysis (Baikede Biotechnology Co. Ltd., Wuhan, China).
Collection of Clinical Data
The clinical characteristics of patients with NMOSD were obtained via the electronic recording system. Sex, age, current disease duration, serum autoimmune antibodies (anti-DNA, anti-Sm, anti-SSA, anti-SSB, and rheumatoid factor), expanded disability status scale (EDSS) score, and cerebrospinal fluid (CSF) protein levels were collected. Moreover, the involvement of the optic nerve, brain, cervical spinal cord, thoracic spinal cord, and lumbar spinal cord was assessed by MRI. The interval from the time of the current attack to the time of sampling was deemed current disorder duration (CDD). CDD < 30 days was regarded as the acute phase, and CDD ≥ 30 days was considered the remission phase. EDSS scores were assessed at the time of sampling. Patients with acute NMOSD who received immunomodulatory drugs before sampling were excluded due to the effects of these drugs on outcomes.
Measurement of IL-32 Levels
Blood samples were obtained from peripheral veins and left of patients from six to eight in the morning after an overnight fast. After coagulation, the samples were centrifuged at 1200 g for 5 min. Then, serum samples were collected and stored at −70 °C. Serum concentrations of IL-32 in all samples were detected using ELISA at the same time point. Serum levels of IL-32 were measured by an ELISA kit (R&D Systems; USA) with intra-assay Coefficients of Variability (CV) < 10% and Inter-assay CV < 8%. The results in all samples were high above LLOQ. IL-32 level was quantified by a microplate reader (BioTek Instruments, Inc., Winooski, USA).
Statistics Analysis
Data were analyzed using SPSS version 22.0 (SPSS Inc., USA). The normality of data was assessed using the Kolmogorov–Smirnov Z test. Count data are shown as a percentage (%). The chi-square test or Fisher’s test was used to compare differences between the two groups. Continued variables with a normal distribution are shown as mean ± SD. Such data were analyzed using Student’s t test. Data with non-normal distribution are shown as median and interquartile range (IQR). Such data were analyzed using the Mann–Whitney U-test. Receiver operating characteristic (ROC) curve was used to assess the diagnostic significance of IL-32 for NMOSD. Associations of IL-32 level with CSF protein level and EDSS score were assessed using Spearman’s rank correlation coefficient. Univariate and multiple linear regression analyses were used to assess the correlation of IL-32 levels with clinical characteristics of patients with NMOSD. p < 0.05 was regarded statistically significant.
Results
Baseline Characteristics of Subjects
In total, 68 patients with NMOSD were recruited, including 32 drug-naive cases in the acute phase and 36 cases in the remission phase. The average age in the acute phase cases [(50.59 ± 9.26) years] was higher than that in the remission cases [(40.78 ± 8.90) years] (p = 0.031). Current disease duration was 9 (IQR = 9) days in the acute cases and 37 (IQR = 29) days in the remission cases (p = 0.006). CSF protein level [(401.06±66.62) mg/L] and EDSS score (6, IQR = 3) in the acute group were significantly higher compared with the remission group [(303.97±72.52) mg/L; 2, IQR = 2] (all p < 0.01). Moreover, gender, anti-AQP4 level, serum levels of autoimmune antibodies, and the involvement of optic nerve, brain, cervical spinal cord, thoracic spinal cord, and lumbar spinal cord were not significantly different between the two groups (all p > 0.05). In addition, we recruited 60 healthy individuals as controls (31 males and 29 females) whose mean age was [(44.95±10.41) years] (Table 1).
 |
Table 1 Baseline Characteristics of Individuals with NMOSD and Healthy Controls |
Measurement of IL-32 Concentrations in Cases with NMOSD and Healthy Individuals
Our study collected 4 serum samples from patients and 4 samples from healthy controls to perform high-throughput sequencing and identify potential diagnostic biomarkers for acute NMOSD. The volcano plot and heat map suggested that IL-32 was significantly and differentially expressed between the two groups (Figure 1A-B). The results in Figure 1C indicated that antigen processing and presentation and cytokine-cytokine receptor interaction were mostly associated with NMOSD. In addition, the data in Figure 1D demonstrated that the biologic process (immune response) was closely correlated with NMOSD (Figure 1D).
Moreover, we compared the serum IL-32 levels between 68 patients with NMOSD and 60 healthy controls. The serum concentrations of IL-32 were significantly higher in patients with acute NMOSD [(52.06 ± 16.56) pg/mL] and remitted NMOSD [(25.78 ± 8.31) pg/mL] compared with healthy controls [(10.83 ± 6.94) pg/mL] (all p <0.001, Figure 2). Additionally, the difference in IL-32 levels between patients with acute and remitted NMOSD was also significant (p <0.001).
 |
Figure 2 Serum IL-32 levels were compared between patients with NMOSD and healthy controls. |
Next, we explored the diagnostic efficacy of serum IL-32 for NMOSD using ROC curve analysis. AUC was 0.811 (P = 0.026, 95% CI 0.673–0.949), with a sensitivity of 0.800 and a specificity of 0.806 (Figure 3).
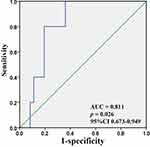 |
Figure 3 ROC curve analysis of serum IL-32 levels in patients with NMOSD. |
Association Between the Serum Levels of IL-32, CSF Protein Levels, and EDSS Scores Among Patients with NMOSD
There was a significant correlation between serum IL-32 levels and CSF protein levels among all patients with NMOSD (r = 0.524, p < 0.001) and patients with remitted NMOSD (r = 0.580, p < 0.001), but there was no significant correlation among patients with acute NMOSD (r = 0.093, p = 0.612). Concerning serum IL-32 levels and EDSS score, there was a significant correlation among all patients with NMOSD (r = 0.653, p < 0.001) and patients with acute NMOSD (r = 0.620, p < 0.001). However, there was no significant difference among patients with remitted NMOSD (r = 0.039, p = 0.822, Figure 4).
 |
Figure 4 Spearman’s rank correlation coefficient showing the association between serum IL-32 levels with CSF protein levels and EDSS scores. |
Correlations Between Serum Levels of IL-32 and Clinical Characteristics Among Patients with Acute NMOSD
The logistic regression analysis was performed to assess the association between IL-32 and clinical data among patients with acute NMOSD. In univariate linear regression analysis, anti-AQP4 antibody level, CSF protein level, involvement of cervical spinal cord and lumbar spinal cord, and EDSS score were associated with increased serum levels of IL-32. In multivariate linear regression analysis, however, only anti-AQP4 antibody and EDSS score were independently correlated with serum levels of IL-32 after adjusting for the other confounders (Table 2).
 |
Table 2 Association of IL-32 Levels with Clinical Characteristics of Patients with NMOSD |
Discussion
It is well known that the roles of IL-32 in the development of NMOSD have not previously been observed so far. We found that IL-32 concentrations are strongly higher in cases with acute and remitted NMOSD compared with healthy individuals, suggesting that IL-32 is correlated with disease status. Moreover, IL-32 concentrations are positively related to EDSS score and CSF protein levels in patients with NMOSD, suggesting that IL-32 is correlated with disease severity. Additionally, IL-32 showed a significant diagnostic value for acute NMOSD with a sensitivity of 0.800 and a specificity of 0.806.
IL-32 is closely associated with innate and adaptive immune responses and participates in some inflammatory diseases via different mechanism.6,12–14 Previous observations revealed that the CSF levels of IL-6 are strongly higher in cases with NMOSD compared with cases with multiple sclerosis, suggesting their different pathogenesis.15 Moreover, IL-6 can induce the secretion of AQP4-Ab from plasmablasts in NMOSD.16 Thus, higher levels of IL-32 can induce IL-6 overproduction and induce AQP4-Ab levels. Therefore, IL-32 may be a biomarker for NMOSD. A previous study reported that the anti-IL-6 receptor monoclonal antibody tocilizumab is highly effective for cases with refractory NMOSD.17 However, the effects of IL-32, an upstream molecule of IL-6, on NMOSD have not been fully elucidated.
IL-32 concentration was detected in NMOSD cases and in healthy controls to explore whether IL-32 is involved in the development of NMOSD. We revealed that the IL-32 concentrations are strongly higher in cases with acute and remitted NMOSD than in healthy individuals. These findings are consistent with a previous study which indicates that increased IL-32 levels were found in cases with neuromyelitis optica.11 Moreover, our results suggested that IL-32 levels were positively related to CSF protein levels and EDSS scores, indicating that IL-32 is associated with higher physical disability scores in NMOSD. Some researchers revealed that IL-32 is associated with IL-17A in NMOSD and correlated with EDSS score, suggesting that IL-32 may be involved in B cell-mediated autoimmune disorders, such as NMOSD [11]. In encephalomyelitis cases, IL-17A is found to be significantly increased in blood and CSF, particularly during relapses.18 Moreover, IL-17A is upregulated in NMOSD and associated with disease severity.19,20 In addition, IL-32 and IL-17A exert similar biological functions dependent on TNF-receptor 1 different action mechanisms.21 Thus, these findings support our conclusion that IL-32 is related to higher physical disability scores in NMOSD. IL-32 involves in the pathogenesis of NMOSD by upregulating the levels of IL-6 and IL-7A in NMOSD. IL-32 has found to induce the maturation and activation of dendritic cells (DCs) which could trigger Th1 and Th17 cell-mediated immune responses by secreting IL-12 and IL-6 via the NF-κB signaling pathway and further promote NMOSD progression.11 Although extensive studies have observed the roles of IL-32 in the pathogenesis of NMOSD, there are some limitations to this study. First, the sample size was relatively small, and larger multi-center studies are needed. Second, follow-up data before and after treatment were not collected and analyzed in this study. Third, because the sample is not so big and consequently, there exists bias, thence more statistical analyses should be performed to validate our conclusion. Finally, the levels of IL-32 in CSF have not been measured.
Conclusion
In summary, we reported that the IL-32 levels were significantly higher in NMOSD cases than in healthy individuals, and IL-32 levels were positively related to CSF protein levels and EDSS scores among all patients with NMOSD, suggesting that IL-32 is related to higher physical disability scores in NMOSD. Thus, our study may suggest that IL-32 may be a new target for treating NMOSD. More studies should be conducted to uncover the precise mechanism involved in NMOSD.
Data Sharing Statement
The dataset used in the preparation of this study will be available from the corresponding author upon reasonable request.
Ethical Statement
Our study complies with the Declaration of Helsinki.
Acknowledgments
The authors would like to express their gratitude to EditSprings (https://www.editsprings.cn) for the expert linguistic services provided. Additionally, we thanked Qi-Rong Wang for helping to revise the manuscript.
Disclosure
The authors declare that they have no conflicts of interests in this work.
References
1. Siriratnam P, Huda S, Butzkueven H, van der Walt A, Jokubaitis V, Monif M. A comprehensive review of the advances in neuromyelitis optica spectrum disorder. Autoimmun Rev. 2023;22(12):103465. doi:10.1016/j.autrev.2023.103465
2. Wang L, Du L, Li Q, et al. Neuromyelitis optica spectrum disorder with anti-aquaporin-4 antibody: outcome prediction models. Front Immunol. 2022;13:873576. doi:10.3389/fimmu.2022.873576
3. Wingerchuk DM, Banwell B, Bennett JL, et al. International Panel for NMO Diagnosis. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85(2):
4. Arellano G, Loda E, Chen Y, et al. Interferon-gamma controls aquaporin 4-specific Th17 and B cells in neuromyelitis optica spectrum disorder. Brain. 2024;147(4):1344–1361. doi:10.1093/brain/awad373
5. Mireles-Ramírez MA, Pacheco-Moises FP, González-Usigli HA, et al. Neuromyelitis optica spectrum disorder: pathophysiological approach. Int J Neurosci. 2024;134(8):826–838. doi:10.1080/00207454.2022.2153046
6. Mohammed Jasim E, Khudhur Jameel S, Ihsan Awadh N. Estimation of interleukin 32 and interleukin 37 serum levels in Iraqi patients with rheumatoid arthritis. Arch Razi Inst. 2023;78(2):743–750. doi:10.22092/ARI.2022.359861.2489
7. Kwon OC, Kim S, Hong S, et al. Role of IL-32 gamma on bone metabolism in autoimmune arthritis. Immune Netw. 2018;18(3):e20. doi:10.4110/in.2018.18.e20
8. Boreika R, Sitkauskiene B. Interleukin-32 in pathogenesis of atopic diseases: proinflammatory or anti-Inflammatory role? J Interferon Cytokine Res. 2021;41(7):235–243. doi:10.1089/jir.2020.0230
9. Na SJ, So SH, Lee KO, Choi YC. Elevated serum level of interleukin-32α in the patients with myasthenia gravis. J Neurol. 2011;258(10):1865–1870. doi:10.1007/s00415-011-6036-7
10. Jung MY, Son MH, Kim SH, Cho D, Kim TS. IL-32gamma induces the maturation of dendritic cells with Th1- and Th17-polarizing ability through enhanced IL-12 and IL-6 production. J Immunol. 2011;186(12):6848–6859. doi:10.4049/jimmunol.1003996
11. Wang H, Wang K, Wang C, Xu F, Qiu W, Hu X. Increased plasma interleukin-32 expression in patients with neuromyelitis optica. J Clin Immunol. 2013;33(3):666–670. doi:10.1007/s10875-012-9837-2
12. Xu Q, Pan X, Shu X, et al. Increased interleukin-32 expression in chronic hepatitis B virus-infected liver. J Infect. 2012;65(4):336–342. doi:10.1016/j.jinf.2012.05.009
13. de Albuquerque R, Komsi E, Starskaia I, Ullah U, Lahesmaa R. The role of Interleukin-32 in autoimmunity. Scand J Immunol. 2021;93(2):e13012. doi:10.1111/sji.13012
14. Netea MG, Azam T, Ferwerda G, et al. IL-32 synergizes with nucleotide oligomerization domain (NOD) 1 and NOD2 ligands for IL-1beta and IL-6 production through a caspase 1-dependent mechanism. Proc Natl Acad Sci. 2005;102(45):16309–16314. doi:10.1073/pnas.0508237102
15. Akatani R, Chihara N, Hara A, et al. Interleukin-6 signaling blockade induces regulatory plasmablasts in neuromyelitis optica spectrum disorder. Neurol Neuroimmunol Neuroinflamm. 2024;11(4):e200266. doi:10.1212/NXI.0000000000200266
16. Fung S, Shirley M. Satralizumab: a review in neuromyelitis optica spectrum disorder. CNS Drugs. 2023;37(4):363–370. doi:10.1007/s40263-023-00995-9
17. Rosso M, Saxena S, Chitnis T. Targeting IL-6 receptor in the treatment of neuromyelitis optica spectrum: a review of emerging treatment options. Expert Rev Neurother. 2020;20(5):509–516. doi:10.1080/14737175.2020.1757434
18. Aktas O, Hartung HP, Smith MA, et al. Serum neurofilament light chain levels at attack predict post-attack disability worsening and are mitigated by inebilizumab: analysis of four potential biomarkers in neuromyelitis optica spectrum disorder. J Neurol Neurosurg Psychiatry. 2023;94(9):757–768. doi:10.1136/jnnp-2022-330412
19. Wang H, Dai Y, Qiu W, et al. Interleukin-17-secreting T cells in neuromyelitis optica and multiple sclerosis during relapse. J Clin Neurosci. 2011;18(10):1313–1317. doi:10.1016/j.jocn.2011.01.031
20. Li Y, Wang H, Long Y, Lu Z, Hu X. Increased memory Th17 cells in patients with neuromyelitis optica and multiple sclerosis. J Neuroimmunol. 2011;234(1–2):155–160. doi:10.1016/j.jneuroim.2011.03.009
21. Moreira Gabriel E, Wiche Salinas TR, Gosselin A, et al. Overt IL-32 isoform expression at intestinal level during HIV-1 infection is negatively regulated by IL-17A. AIDS. 2021;35(12):1881–1894. doi:10.1097/QAD.0000000000002972
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


