Back to Journals » Journal of Inflammation Research » Volume 18
Association Between Thymosin β4 and Coronary Arterial Lesions in Children with Kawasaki Disease
Authors Wu J, Yang P, Zhang J, Chen Z, Wei Y , Su Y, Yi Q
Received 26 January 2025
Accepted for publication 26 June 2025
Published 3 July 2025 Volume 2025:18 Pages 8755—8765
DOI https://doi.org/10.2147/JIR.S519589
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Jinhui Wu, Penghui Yang, Jing Zhang, Zhuo Chen, Yi Wei, Ya Su, Qijian Yi
Department of Cardiovascular Medicine, Ministry of Education Key Laboratory of Child Development and Disorders, Chongqing Key Laboratory of Pediatric Metabolism and Inflammatory Diseases, Key Laboratory of Children’s Important Organ Development and Diseases of Chongqing Municipal Health Commission, National Clinical Research Center for Child Health and Disorders, National Clinical Key Cardiovascular Specialty, Children’s Hospital of Chongqing Medical University, Chongqing, 400014, People’s Republic of China
Correspondence: Ya Su, Department of Cardiovascular Medicine, Children’s Hospital of Chongqing Medical University, Chongqing, 400014, People’s Republic of China, Tel/Fax +86 02363624344, Email [email protected] Qijian Yi, Department of Cardiovascular Medicine, Children’s Hospital of Chongqing Medical University, Chongqing, 400014, People’s Republic of China, Tel/Fax +86 02363624344, Email [email protected]
Background: Kawasaki disease (KD) is an acute systemic vasculitis primarily affecting children and is a leading cause of acquired heart disease in developed countries. Recently, an increasing number of studies have demonstrated the close correlations between inflammation and KD. Thymosin β 4 (Tβ 4) has been reported to play a role in cardiovascular protection and repair by modulating inflammation, angiogenesis, and endothelial function. However, its role in KD still remains poorly understood. This study aims to explore the potential involvement of Tβ 4 in the pathogenesis of KD, with a particular focus on its relationship to inflammation and coronary artery lesions (CALs).
Methods: Serum Tβ 4 levels were measured using enzyme-linked immunosorbent assay (ELISA) in children with KD and age-matched healthy controls. The KD group was further categorized into patients with and without CALs. Correlation analyses were performed between Tβ 4 levels and clinical or laboratory parameters.
Results: Serum Tβ 4 levels were significantly lower in patients with KD compared to healthy controls and were further reduced in patients with CALs. After intravenous immunoglobulin (IVIG) treatment, Tβ 4 levels significantly increased. Tβ 4 levels were negatively correlated with several pro-inflammatory (eg, TNF-α, IL-1β) and anti-inflammatory cytokines (eg, IL-4, IL-10).
Conclusion: Tβ 4 levels were significantly lower in children with KD, particularly in those with CALs. These findings suggest that Tβ 4 may be involved in the inflammatory pathogenesis of KD and the progression of CALs, thus could represent a potential target for future diagnostic or therapeutic interventions.
Keywords: cytokine, anti-inflammatory, intravenous immunoglobulin, angiogenesis
Introduction
Kawasaki disease (KD) is an acute, self-limiting systemic vasculitis primarily affecting children under five years of age.1 Its defining pathological hallmark is inflammation of medium-sized blood vessels, with a marked predilection for the coronary arteries. The most severe complication of KD is the development of coronary artery lesions (CALs), which can progress to coronary artery aneurysms, significantly elevating the risk of myocardial infarction, ischemic heart disease, and arterial rupture.2 Despite extensive research, the precise pathogenesis of KD remains poorly understood.3 However, emerging evidence highlights immune system dysfunction as a central mechanism that leads to the overproduction of inflammatory cytokines, and result in vasculitis and the formation of CALs.4 Studies have consistently demonstrated elevated serum pro-inflammatory cytokine levels, such as interleukin-17 (IL-17),5 IL-1β,6 IL-6,7,8 and tumor necrosis factor- α (TNF-α), in patients with KD. Conversely, anti-inflammatory cytokine IL-35 levels are significantly reduced9 These imbalances in pro- and anti-inflammatory mediators are closely associated with vasculitis and the development of CALs observed in KD.
Thymosin β4 (Tβ4) is a 43-amino acid lymphopoietic peptide widely distributed across various tissues, with prominent expression in the myocardium and vascular smooth muscle.10 It serves as a key regulator of actin dynamics, mediating a wide range of biological functions, including actin regulation,11 inhibition of apoptosis,12 modulation of inflammatory responses,13 and promotion of angiogenesis.14 The anti-inflammatory properties of Tβ4 have been extensively demonstrated in preclinical studies. For instance, in mouse models of ethanol- and lipopolysaccharide-induced liver injury, as well as neonatal models of fetal alcohol spectrum disorder, Tβ4 can attenuate the inflammation response by suppressing the production of pro-inflammatory cytokines such as TNF-α and IL-1β.13,15 Additionally, Tβ4 plays a pivotal role in angiogenesis. In a mouse model of hindlimb ischemia, Tβ4 enhanced blood vessel formation by upregulating vascular endothelial growth factor, thereby increasing the proliferation and migratory capacity of endothelial progenitor cells.14 Recent evidence further underscores the essential role of Tβ4 in all three stages of cardiac vessel development—vasculogenesis, angiogenesis, and arteriogenesis in animal models. Remarkably, Tβ4 facilitates coronary vascularization during both childhood and adulthood by supporting cardiomyocyte survival and exerting protective effects on the heart.10
Despite the well-documented roles of Tβ4 in inflammation and angiogenesis, whether its involvement in the pathogenesis of KD remains unexplored. This study aimed to address this gap by evaluating serum Tβ4 levels in children with acute KD and exploring their correlation with clinical parameters and CALs.
Methods
General Characteristics of Participants
This study included children diagnosed with KD at the Children’s Hospital of Chongqing Medical University between April 2023 and October 2024. Diagnosis was made in accordance with the American Heart Association’s diagnostic and treatment guidelines.16 An age-matched healthy control (HC) group consisting of children without any medical conditions, was also recruited. All participants underwent screening to exclude underlying inflammatory, immunological, metabolic, hematological, or cardiac disorders. Ethical approval for the study was obtained from the Ethics Committee of the Children’s Hospital of Chongqing Medical University (Approval Number: ID245-2024). Written informed consent was obtained from the parents or legal guardians of all participants.
Echocardiographic evaluations were conducted on patients with KD before the initiation of treatment with intravenous immunoglobulin (IVIG) and anticoagulants. Based on coronary artery z-scores, patients with KD were categorized into two groups: those with z-scores ≥2.0 were classified as the KD-CALs group, while those with z-scores <2.0 were designated as the KD-NCALs.17 All patients with KD received standard treatment consisting of intravenous immunoglobulin (2 g/kg as a single infusion) and oral aspirin (initially 30–50 mg/kg/day in divided doses during the acute phase, followed by 3–5 mg/kg/day as a single daily dose during the subacute phase), according to the AHA guidelines.16
Blood Sample Collection and Processing
Blood samples were collected from patients with KD during the acute phase of the disease, prior to the administration of IVIG or anticoagulant therapy, as well as from healthy children in the HC group. The samples were centrifuged at 3000 rpm for 10 minutes to separate the serum, which was subsequently stored at −80°C for further analysis.
Serum Tβ4, Cytokine Levels, and Laboratory Variables
Serum Tβ4 levels were measured using an enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions (Cusabio, China). A comprehensive panel of laboratory parameters was also evaluated, including white blood cell (WBC) count, red blood cell (RBC) count, hemoglobin (Hb), platelet (PLT) count, neutrophil percentage (N%), lymphocyte percentage (L%), C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), procalcitonin (PCT), and creatine kinase isoenzymes (CK-MB). Coagulation markers such as prothrombin time (PT), activated partial thromboplastin time (APTT), and thrombin time (TT) were also assessed. Additionally, liver function tests were performed, measuring aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels. Serum cytokine levels were quantified for IL-2, IL-12p70, interferon-alpha (IFN-α), IL-8, IL-4, IL-5, IL-10, TNF-α, IFN-γ, IL-17A, IL-1β, and IL-6.
Statistical Analysis
Statistical analyses were conducted using IBM SPSS Statistics for Windows (version 25.0; IBM SPSS Corp., Armonk, NY, USA). Normally distributed data were compared using Student’s t-test, while non-normally distributed data were analyzed with the Mann–Whitney U-test. Categorical variables were evaluated using the chi-square test. The relationship between serum Tβ4 levels and clinical parameters was examined through Spearman’s rank correlation analysis. Results are reported as mean ± standard deviation (SD) for normally distributed data, median (P25, P75) for non-normally distributed data, or counts and percentages (n, %) for categorical variables. A P-value of <0.05 was considered statistically significant.
Results
Participant Demographics
The study included 90 children diagnosed with KD, comprising 54 males and 36 females, mean age of 2.50 ± 1.59 years. These patients were further classified into two subgroups: 43 in the KD-CALs group and 47 in the KD-NCALs group. Additionally, 55 children (32 males and 23 females, mean age of 2.96 ± 1.77 years) in the HC group were included. There were no statistically significant differences in age or sex between the KD and HC groups (P >0.05).
Serum Tβ4 Levels in All Participants
As shown in Figure 1, the serum Tβ4 levels in the KD group (0.903 mg/mL [0.575, 1.155], n = 90) were significantly lower compared to the HC group (1.190 mg/mL [0.840, 1.441], n = 55) (P <0.05).
 |
Figure 1 Serum Tβ4 levels in the KD group and the HC group. **P<0.01. |
Laboratory Variables and Serum Tβ4 Levels in the KD-CAL and KD-NCAL Groups
As shown in Table 1, there were no significant differences in WBC, RBC, Hb, PLT, N%, L%, CRP, ESR, PCT, AST, ALT, CK-MB, PT, APTT, TT or IL-2, IL-12p70, IFN-α, IL-8, IL-4, IL-5, TNF-α, IFN-γ, IL-17A, IL-1β, IL-6 between the KD-CALs and KD-NCALs groups (P >0.05). However, compared to the KD-NCALs group, the KD-CALs group showed significantly higher serum IL-10 levels and significantly lower Tβ4 levels (P < 0.05).
 |
Table 1 Laboratory Variables and Serum Tβ4 Levels in the KD-CALs and KD-NCALs Groups |
Relationship Between Serum Tβ4 Levels and IVIG Treatment in Patients with KD
As shown in Figure 2, serum Tβ4 levels increased significantly following IVIG treatment. The median Tβ4 level before IVIG treatment was 0.783 ng/mL (0.531, 1.142), while the level after treatment was 1.572 ng/mL (0.717, 2.277) (P < 0.05).
 |
Figure 2 Serum Tβ4 levels increase after IVIG treatment. **P<0.01. |
Correlations Between Tβ4 Levels and Laboratory Variables in Patients with KD
Serum Tβ4 levels were positively correlated with RBC and Hb levels while they showed negative correlations with PCT, TT, IL-2, IL-12p70, IFN-α, IL-4, IL-10, TNF-α, and IL-1β (P <0.05; Table 2). No significant correlations were observed between serum Tβ4 levels and other variables, including WBC, PLT, N%, L%, CRP, PT, APTT, ESR, AST, ALT, CK-MB, IL-8, IL-5, IFN-γ, IL-17A, or IL-6 in the KD group (P >0.05).
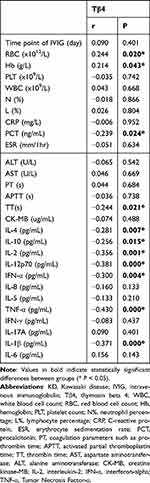 |
Table 2 Correlations Between Tβ4 Levels and Laboratory Variables in Patients with KD |
Correlations Between Tβ4 Levels and Laboratory Variables in the KD-CALs and KD-NCALs Groups
There were no significant correlations between serum Tβ4 levels with WBC, RBC, Hb, PLT, N%, L%, PCT, ESR, CRP, AST, ALT, CK-MB, APTT, PT, IL-2, IL-12p70, IFN-α, IL-8, IL-5, IL-10, IFN-γ, IL-17A, or IL-6 (P >0.05), negative correlations were observed with TT, IL-4, TNF-α, and IL-1β in the KD-CALs group (P <0.05). However, There was a positive correlation between serum Tβ4 levels and RBC and negatively correlated with PCT, IL-2, IL-12p70, IFN-α, TNF-α, and IL-1β in the KD-NCALs group (P <0.05; Table 3).
 |
Table 3 Correlations Between Tβ4 Levels and Laboratory Variables in the KD-CALs and KD-NCALs Groups |
Discussion
KD is an acute systemic vasculitis, CALs being its most severe complication. While previous research has suggested that acute inflammation, coagulation abnormalities, and endothelial dysfunction contribute to the pathogenesis of KD, the precise mechanisms remain unclear.18,19 Tβ4 is known for its anti-inflammatory properties11 and ability to promote angiogenesis14 in cardiovascular disease, but whether it is involved in the pathogenesis of KD remains unclear. This study aimed to evaluate serum Tβ4 levels in patients with KD and their relationship with CALs. Key findings include: (1) Serum Tβ4 levels in the KD group were significantly lower than those in the HC group; (2) Tβ4 levels were further reduced in the KD-CALs group compared to the KD-NCALs group; (3) serum Tβ4 levels increased significantly post-IVIG treatment in children with KD; (4) There were positively correlated between Tβ4 levels and RBC and Hb levels, while negatively correlating with TT, PCT, IL-2, IL-12p70, IFN-α, IL-4, TNF-α, and IL-1β in the KD group. Notably, negative correlations between Tβ4 levels and TT, IL-4, TNF-α, and IL-1β were specifically observed in the KD-CALs group.
Tβ4 is an endogenous peptide with protective and regenerative properties, demonstrated in models of cellular and organ injury.13,15 It is increasingly recognized as a potential biomarker in a range of conditions, including cardiovascular disease,10 hepatic disorders,20 infectious,21 and autoimmune diseases.22 Tβ4 is a key regulator of actin dynamics, promoting angiogenesis,14 inhibiting apoptosis,12 and modulating inflammation.13 While its role has been explored in cardiovascular diseases,23 rheumatoid arthritis,24 sepsis,25 and liver disorders,15 but its involvement in acute inflammatory diseases like KD remains less documented. We found that serum Tβ4 levels were significantly lower in the KD group compared to healthy controls, with even lower levels observed in patients with CALs. These findings suggest that Tβ4 may be involved in the inflammatory pathogenesis of KD and the progression of CALs. Tβ4 is known to be actively secreted by endothelial and immune cells under physiological and reparative conditions and plays an important role in promoting endothelial cell migration, angiogenesis, and tissue repair.26,27 IVIG treatment in KD has been shown to reduce systemic inflammation and coronary artery damage by downregulating proinflammatory cytokines such as IL-6, TNF-α, and MMP-9,28,29 while also decreasing markers of cellular injury and necrosis.30 Consistent with these effects, we observed a significant increase in serum Tβ4 levels following IVIG administration. Given the reduction in tissue damage and inflammation, this increase is unlikely to be due to passive leakage due to cell death. Instead, it may reflect an upregulation of active Tβ4 production in response to vascular repair and immune modulation. However, due to the observational nature of the study and the partial overlap in Tβ4 levels across groups, further mechanistic investigations are needed to elucidate the underlying pathways and to determine whether Tβ4 could serve as a meaningful indicator of disease activity or vascular involvement in KD.
IL-2, IL-12p70, IFN-α, TNF-α, and IL-1β are classic pro-inflammatory cytokines that play distinct roles in the activation and progression of inflammation through various mechanisms. IL-231 and IL-12p7032 promote the differentiation of Th1 cells, thereby enhancing immune responses and exacerbating inflammation. Additionally, Tβ4 exhibited protective effects by reducing TNF-α and IL-1β levels in a colitis mouse model,33 However, no studies have directly linked Tβ4 with IL-2, IFN-α or IL-12p70. Our study is the first to show that Tβ4 levels are negatively correlated with IL-2, IL-12p70, IFN-α, TNF-α, and IL-1β levels in patients with KD. These associations suggest a potential interaction between Tβ4 and the cytokine milieu during the acute phase of KD. Lower Tβ4 levels were correlated with elevated concentrations of both pro- and anti-inflammatory cytokines, indicating that Tβ4 may be involved in complex immunomodulatory pathways. Whether Tβ4 is regulated by inflammatory cytokines, contributes to their expression, or both, remains to be elucidated through further mechanistic studies.
In contrast, IL-10 and IL-4, classical anti-inflammatory cytokines, are significantly elevated during the acute phase of KD.34 IL-4 promotes Th2 immune responses while suppressing Th1-mediated inflammation, potentially mitigating disease progression.35 However, the precise role of IL-4 in KD remains unclear. Previous studies suggest that Tβ4 may protect against elevated IL-10 levels,31 but no direct evidence has linked Tβ4 with IL-4. Our study is the first to demonstrate that Tβ4 levels are negatively correlated with both IL-10 and IL-4 in patients with KD. These findings may suggest a compensatory mechanism: IL-4 and IL-10 may be upregulated following Tβ4 downregulation to mitigate inflammation. Their expression may not directly reflect Tβ4 levels but instead be mediated by broader immunoregulatory pathways that underlie Tβ4’s protective effect on the endothelium and the promotion of tissue remodeling. Notably, IL-4 can induce endothelial activation and monocyte recruitment via MCP-1,36 whereas IL-10 is linked to IVIG resistance and coronary complications,37 underscoring their context-dependent pathogenic potential. Furthermore, Wang et al indicated that serum IL-4 serves as an independent predictor of coronary artery disease.38 Our research further revealed that Tβ4 levels are negatively correlated with IL-4 in patients with KD-CALs, suggesting that Tβ4 may play a crucial regulatory role in the pathogenesis of KD-CALs.
Furthermore, Tβ4 levels were negatively correlated with TNF-α and IL-1β in the KD group. Both of these pro-inflammatory cytokines play a crucial role in the pathogenesis of coronary artery damage in KD. TNF-α inhibitors are already being used to prevent coronary aneurysms,39 and IL-1β has been shown to be closely linked to inflammation and vascular damage in KD cell models.40 Tβ4 may be associated with reduced levels of TNF-α and IL-1β, potentially contributing to its observed protective role, although the underlying mechanisms remain to be clarified.
Prolonged TT is commonly indicative of coagulation abnormalities, reflecting impairments in fibrin generation and stabilization.41 Previous studies have demonstrated that Tβ4 enhances the stability of the fibrin network and supports coagulation by facilitating the attachment of factor XIIIa (transglutaminase) to fibrin and collagen.42 Coagulation dysfunction has been closely associated with the development of CALs42 in patients with KD. Our study revealed that there were negative correlation between Tβ4 levels and TT, suggesting that reduced Tβ4 levels may prolong TT, compromise fibrin formation and stability, and promote a hypercoagulable state. This, in turn, may contribute to coronary artery thrombogenesis and further result in myocardial ischemia in patients with KD.
Conclusions
Our study demonstrated that serum Tβ4 levels were significantly lower in patients with KD, particularly in those with CALs. Tβ4 levels were negatively correlated with several pro-inflammatory cytokines (such as IL-2, IL-12p70, IFN-α, TNF-α, and IL-1β) and anti-inflammatory cytokines (such as IL-10 and IL-4). While these associations suggest that Tβ4 may be involved in the inflammatory milieu of KD and the development of CALs, the specific mechanism remains unclear. Moreover, although serum Tβ4 levels increased following IVIG treatment, the underlying mechanism remains unclear. Due to the observed overlap of Tβ4 levels between groups, its potential diagnostic or protective value should be further investigated in larger, mechanistic studies. Furthermore, this study has several limitations. First, the sample size was relatively small and recruited from a single center, which may limit the generalizability of the results. Second, the observational design of the study precludes conclusions about causality between Tβ4 levels and cytokine expression. Third, we did not perform mechanistic experiments to directly explore the functional roles of Tβ4 in Kawasaki disease. Further studies incorporating in vitro and in vivo functional analyses as well as larger, multicenter cohorts are warranted to validate and expand upon our findings.
Abbreviations
KD, Kawasaki disease; Tβ4, thymosin beta 4; CALs, coronary artery lesions; NCALs, non-CALs; ELISA, enzyme-linked immunosorbent assay; IVIG, intravenous immunoglobulin; WBC, white blood cell count; RBC, red blood cell count; Hb, hemoglobin; PLT, platelet count; N%, neutrophil percentage; L%, lymphocyte percentage; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; PCT, procalcitonin; CK-MB, creatine kinase isoenzymes; PT, coagulation parameters such as prothrombin time; APTT, activated partial thromboplastin time; TT, thrombin time; AST, aspartate aminotransferase; ALT, alanine aminotransferase; CK-MB, creatine kinase-MB; IL-4, interleukin-4; IFN-α, interferon-alpha; TNF-α, Tumor Necrosis Factor-α; IFN-γ, interferon-gamma; HC, healthy control; SD, standard deviation.
Ethics Approval and Informed Consent
The study was approved by the Ethics Committee of the Children’s Hospital of Chongqing Medical University (ethics committee approval number:ID245-2024). Informed consent was obtained from the parents or legal guardians of all participants. This study conforms with the principles outlined in the Declaration of Helsinki.
Author Contributions
All authors made a significant contribution to the work reported, whether in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Chongqing Municipal Science and Technology Bureau Natural Science Foundation project (CSTB2023NSCQ-MSX0144).
Disclosure
The authors declare that they have no competing interests.
References
1. Nakamura Y. Kawasaki disease: epidemiology and the lessons from it. Int J Rheum Dis. 2018;21(1):16–19. doi:10.1111/1756-185X.13211
2. Liang CD, Kuo HC, Yang KD, Wang CL, Ko SF. Coronary artery fistula associated with Kawasaki disease. Am Heart J. 2009;157(3):584–588. doi:10.1016/j.ahj.2008.11.020
3. Kuo HC, Yang KD, Chang WC, Ger LP, Hsieh KS. Kawasaki disease: an update on diagnosis and treatment. Pediatr Neonatol. 2012;53(1):4–11. doi:10.1016/j.pedneo.2011.11.003
4. Mahmoudinezhad Dezfouli SM, Salehi S, Khosravi S. Pathogenic and therapeutic roles of cytokines in Kawasaki diseases. Clin Chim Acta Int J Clin Chem. 2022;532:21–28. doi:10.1016/j.cca.2022.05.015
5. Brodeur KE, Liu M, Ibanez D, et al. Elevation of IL-17 cytokines distinguishes Kawasaki disease from other pediatric inflammatory disorders. Arthritis Rheumatol Hoboken NJ. 2024;76(2):285–292. doi:10.1002/art.42680
6. Barranco C. Vasculitis syndromes: Kawasaki disease is IL-1β-mediated. Nat Rev Rheumatol. 2016;12(12):693. doi:10.1038/nrrheum.2016.177
7. Kaneko S, Shimizu M, Shimbo A, et al. Clinical significance of serum cytokine profiles for differentiating between Kawasaki disease and its mimickers. Cytokine. 2023;169:156280. doi:10.1016/j.cyto.2023.156280
8. Tan Z, Yuan Y, Chen S, Chen Y, Chen TX. Plasma endothelial microparticles, TNF-a and IL-6 in Kawasaki disease. Indian Pediatr. 2013;50(5):501–503. doi:10.1007/s13312-013-0152-7
9. Su Y, Feng S, Luo L, Liu R, Yi Q. Association between IL-35 and coronary arterial lesions in children with Kawasaki disease. Clin Exp Med. 2019;19(1):87–92. doi:10.1007/s10238-018-0513-6
10. Smart N, Risebro CA, Melville AAD, et al. Thymosin beta4 induces adult epicardial progenitor mobilization and neovascularization. Nature. 2007;445(7124):177–182. doi:10.1038/nature05383
11. Belsky JB, Rivers EP, Filbin MR, Lee PJ, Morris DC. Thymosin beta 4 regulation of actin in sepsis. Expert Opin Biol Ther. 2018;18(sup1):193–197. doi:10.1080/14712598.2018.1448381
12. Zhao Y, Qiu F, Xu S, Yu L, Fu G. Thymosin β4 activates integrin-linked kinase and decreases endothelial progenitor cells apoptosis under serum deprivation. J Cell Physiol. 2011;226(11):2798–2806. doi:10.1002/jcp.22624
13. Zhang J, Wu J, Zeng W, Yao K, Zu H, Zhao Y. Function of thymosin beta-4 in ethanol-induced microglial activation. Cell Physiol Biochem Int J Exp Cell Physiol Biochem Pharmacol. 2016;38(6):2230–2238. doi:10.1159/000445578
14. Zhao Y, Song J, Bi X, et al. Thymosin β4 promotes endothelial progenitor cell angiogenesis via a vascular endothelial growth factor‑dependent mechanism. Mol Med Rep. 2018;18(2):2314–2320. doi:10.3892/mmr.2018.9199
15. Shah R, Reyes-Gordillo K, Cheng Y, Varatharajalu R, Ibrahim J, Lakshman MR. Thymosin β4 prevents oxidative stress, inflammation, and fibrosis in ethanol- and LPS-induced liver injury in mice. Oxid Med Cell Longev. 2018;2018:9630175. doi:10.1155/2018/9630175
16. McCrindle BW, Rowley AH, Newburger JW, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. 2017;135(17):e927–e999. doi:10.1161/CIR.0000000000000484
17. Manlhiot C, Millar K, Golding F, McCrindle BW. Improved classification of coronary artery abnormalities based only on coronary artery z-scores after Kawasaki disease. Pediatr Cardiol. 2010;31(2):242–249. doi:10.1007/s00246-009-9599-7
18. Laurito M, Stazi A, Delogu AB, et al. Endothelial and platelet function in children with previous Kawasaki disease. Angiology. 2014;65(8):716–722. doi:10.1177/0003319713502392
19. Burns JC, Glode MP, Clarke SH, Wiggins J, Hathaway WE. Coagulopathy and platelet activation in Kawasaki syndrome: identification of patients at high risk for development of coronary artery aneurysms. J Pediatr. 1984;105(2):206–211. doi:10.1016/s0022-3476(84)80114-6
20. Henkel C, Schwamborn K, Zimmermann HW, et al. From proteomic multimarker profiling to interesting proteins: thymosin-β(4) and kininogen-1 as new potential biomarkers for inflammatory hepatic lesions. J Cell Mol Med. 2011;15(10):2176–2188. doi:10.1111/j.1582-4934.2010.01204.x
21. Wang Y, Carion TW, Ebrahim AS, Sosne G, Berger EA. Adjunctive thymosin beta-4 treatment influences PMN effector cell function during pseudomonas aeruginosa-induced corneal infection. Cells. 2021;10(12):3579. doi:10.3390/cells10123579
22. Zhao X, Li N, Yang N, et al. Thymosin β4 alleviates autoimmune dacryoadenitis via suppressing Th17 cell response. Invest Ophthalmol Vis Sci. 2023;64(11):3. doi:10.1167/iovs.64.11.3
23. Wei C, Kim IK, Li L, Wu L, Gupta S. Thymosin Beta 4 protects mice from monocrotaline-induced pulmonary hypertension and right ventricular hypertrophy. PLoS One. 2014;9(11):e110598. doi:10.1371/journal.pone.0110598
24. Choi HM, Lee YA, Yang HI, Yoo MC, Kim KS. Increased levels of thymosin β4 in synovial fluid of patients with rheumatoid arthritis: association of thymosin β4 with other factors that are involved in inflammation and bone erosion in joints. Int J Rheum Dis. 2011;14(4):320–324. doi:10.1111/j.1756-185X.2011.01652.x
25. Liao Y, Xiao N, Wang X, Dai S, Wang G. Promoting effect of Tmsb4x on the differentiation of peripheral blood mononuclear cells to dendritic cells during septicemia. Int Immunopharmacol. 2022;111:109002. doi:10.1016/j.intimp.2022.109002
26. Huang WQ, Wang QR. Bone marrow endothelial cells secrete thymosin beta4 and AcSDKP. Exp Hematol. 2001;29(1):12–18. doi:10.1016/s0301-472x(00)00634-2
27. Xu GJ, Hannappel E, Morgan J, Hempstead J, Horecker BL. Synthesis of thymosin beta 4 by peritoneal macrophages and adherent spleen cells. Proc Natl Acad Sci U S A. 1982;79(13):4006–4009. doi:10.1073/pnas.79.13.4006
28. Xing Y, Ye Y, Zuo H, Li Y. Progress on the function and application of thymosin β4. Front Endocrinol. 2021;12:767785. doi:10.3389/fendo.2021.767785
29. Gupta M, Noel GJ, Schaefer M, Friedman D, Bussel J, Johann-Liang R. Cytokine modulation with immune gamma-globulin in peripheral blood of normal children and its implications in Kawasaki disease treatment. J Clin Immunol. 2001;21(3):193–199. doi:10.1023/a:1011039216251
30. Tian F, Ma L, Zhao R, et al. Correlation between matrix metalloproteinases with coronary artery lesion caused by Kawasaki disease. Front Pediatr. 2022;10:802217. doi:10.3389/fped.2022.802217
31. Charley KR, Ramstead AG, Matous JG, et al. Effector-phase IL-2 signals drive Th1 effector and memory responses dependently and independently of TCF-1. J Immunol Baltim Md 1950. 2024;212(4):586–595. doi:10.4049/jimmunol.2300570
32. Landoni E, Woodcock MG, Barragan G, et al. IL-12 reprograms CAR-expressing natural killer T cells to long-lived Th1-polarized cells with potent antitumor activity. Nat Commun. 2024;15(1):89. doi:10.1038/s41467-023-44310-y
33. Zheng XY, Lv YF, Li S, et al. Recombinant adeno-associated virus carrying thymosin β4 suppresses experimental colitis in mice. World J Gastroenterol. 2017;23(2):242–255. doi:10.3748/wjg.v23.i2.242
34. Hirao J, Hibi S, Andoh T, Ichimura T. High levels of circulating interleukin-4 and interleukin-10 in Kawasaki disease. Int Arch Allergy Immunol. 1997;112(2):152–156. doi:10.1159/000237447
35. Kubo M. The role of IL-4 derived from follicular helper T (TFH) cells and type 2 helper T (TH2) cells. Int Immunol. 2021;33(12):717–722. doi:10.1093/intimm/dxab080
36. Lee YW, Lee WH, Kim PH. Role of NADPH oxidase in interleukin-4-induced monocyte chemoattractant protein-1 expression in vascular endothelium. Inflamm Res off J Eur Histamine Res Soc Al. 2010;59(9):755–765. doi:10.1007/s00011-010-0187-3
37. Wang Y, Wang W, Gong F, et al. Evaluation of intravenous immunoglobulin resistance and coronary artery lesions in relation to Th1/Th2 cytokine profiles in patients with Kawasaki disease. Arthritis Rheum. 2013;65(3):805–814. doi:10.1002/art.37815
38. Wang C, Liu S, Yang Y, et al. Interleukin-4 and Interleukin-17 are associated with coronary artery disease. Clin Cardiol. 2024;47(2):e24188. doi:10.1002/clc.24188
39. Yamaji N, Da silva Lopes K, Shoda T, et al. TNF-α blockers for the treatment of Kawasaki disease in children. Cochrane Database Syst Rev. 2019;8(8):CD012448. doi:10.1002/14651858.CD012448.pub2
40. Inoue T, Miyashita M, Murakami S, et al. IL-1β and IL-17A are involved in IVIG resistance through activation of C/EBPβ and δ in a coronary artery model of Kawasaki disease. Allergy. 2020;75(8):2102–2105. doi:10.1111/all.14281
41. Gonda L, Torner B, Ghansah H, et al. Monoclonal whole IgG impairs both fibrin and thrombin formation: hemostasis and surface plasmon resonance studies. Clin Chem Lab Med. 2024;62(9):1863–1869. doi:10.1515/cclm-2024-0252
42. Huff T, Otto AM, Müller CSG, Meier M, Hannappel E. Thymosin beta4 is released from human blood platelets and attached by factor XIIIa (transglutaminase) to fibrin and collagen. FASEB J off Publ Fed Am Soc Exp Biol. 2002;16(7):691–696. doi:10.1096/fj.01-0713com
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Carrier-Free Binary Self-Assembled Nanomedicines Originated from Traditional Herb Medicine with Multifunction to Accelerate MRSA-Infected Wound Healing by Antibacterial, Anti-Inflammation and Promoting Angiogenesis

Lu J, Wang Z, Cai D, Lin X, Huang X, Yuan Z, Zhang Y, Lei H, Wang P
International Journal of Nanomedicine 2023, 18:4885-4906
Published Date: 30 August 2023
Exploring Interleukin-10 Levels in Diabetes Patients with and without Oral Diseases: A Systematic Review
Novianti Y, Nur’aeny N
Journal of Inflammation Research 2024, 17:541-552
Published Date: 31 January 2024

