Back to Journals » Journal of Inflammation Research » Volume 17
Association of GWAS-Reported Variant of Matrix Metalloproteinase 12 Gene with Susceptibility to Ischemic Stroke in Southern Chinese Population
Authors Chen L, Liao K, Zhang Y, Zheng S, He J, Tang H, Wu H, Zhong W, Li S, Li Y
Received 16 July 2024
Accepted for publication 14 November 2024
Published 20 November 2024 Volume 2024:17 Pages 9231—9241
DOI https://doi.org/10.2147/JIR.S487321
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tara Strutt
Linfa Chen,1,2,* Keqi Liao,1,3,* Yutian Zhang,1,3,* Shutao Zheng,1,4 Jiawen He,1,3 Henglei Tang,1,4 Hailing Wu,1,3 Wangtao Zhong,4 Shengnan Li,1,3 You Li1,3
1Guangdong Key Laboratory of Age-Related Cardiac and Cerebral Diseases, Affiliated Hospital of Guangdong Medical University, Zhanjiang, 524001, People’s Republic of China; 2Department of Neurology, Huizhou Third People’s Hospital, Guangzhou Medical University, Huizhou, People’s Republic of China; 3Institute of Neurology, Affiliated Hospital of Guangdong Medical University, Zhanjiang, 524001, People’s Republic of China; 4Department of Neurology, Affiliated Hospital of Guangdong Medical University, Zhanjiang, 524001, People’s Republic of China
*These authors contributed equally to this work
Correspondence: You Li; Shengnan Li, Guangdong Key Laboratory of Age-Related Cardiac and Cerebral Diseases, Affiliated Hospital of Guangdong Medical University, Zhanjiang, 524001, People’s Republic of China, Email [email protected]; [email protected]
Background: Accumulating evidence suggests that matrix metalloproteinase (MMP) 12 plays a detrimental role in cerebro-cardiovascular diseases, including ischemic stroke (IS). Previous genome-wide association studies (GWAS) correlated the MMP12 rs660599 variant to IS risk in Europeans. However, this association is yet to be elucidated in the Chinese population. This study aims to assess the genetic predisposition of the MMP12 rs660599 G > A variant with regard to IS risk and short-term outcomes in individuals from Southern China.
Methods: The Multiplex SNaPshot assay was used to genotype rs660599 in 1035 IS patients and 1061 age-matched healthy controls. Multivariate logistic regression analyses evaluated the effect of the rs660599 G > A polymorphism on IS susceptibility and short-term outcomes.
Results: No significant association was found between the rs660599 G > A polymorphism and IS risk, even in dominant and recessive models. However, a relationship between rs660599 genotypes and diabetic status revealed that carriers of the A allele and the GA/AA genotype were more likely to develop IS. The presence of diabetes exacerbated the larger infarct volumes and elevated serum MMP12 levels seen in IS patients with the rs660599 A allele. The A allele of rs660599 and the GA/AA genotype were both correlated to moderate and severe stroke with poor short-term outcomes.
Conclusion: The MMP12 rs660599 polymorphism is associated with a higher incidence of IS in people with diabetes and can serve as a biomarker for assessing the severity of IS and its short-term consequences.
Keywords: Ischemic Stroke, Matrix Metalloproteinase 12, polymorphism, risk, short-term outcome
Introduction
Ischemic stroke (IS) affects countless individuals and their families globally and is one of the primary causes of mortality and disability.1 The pathogenesis of IS is influenced by both genetic variability and environmental risk factors, including age, hypertension, smoking, and diabetes. Numerous genome-wide association studies (GWAS) have shown several loci associated with IS.2 However, due to the disease’s heterogeneity, these genes account for only a small fraction of the genetic susceptibility to IS. Current GWAS have demonstrated that single nucleotide polymorphisms (SNPs) linked to IS are frequently connected to certain subtypes, including large artery atherosclerotic (LAA) stroke, cardioembolic stroke (CES), and small-vessel occlusion (SVO),3 suggesting distinct genetic patterns for different stroke subtypes.
Matrix Metalloproteinase (MMP) 12, part of the MMP family, degrades various extracellular matrix (ECM) components, including fibrin-1, laminin, vitreorenexin, type IV collagen, fibronectin, chondroitin sulfate, and heparin sulfate proteoglycans. It plays a crucial role in atherosclerosis.4 MMP12 is selectively confined to macrophages near the lipid core’s periphery in advanced-stage human plaques, and its expression is significantly increased in ruptured plaques.5 In models of IS (both in vitro and in vivo), MMP12 levels were significantly elevated, which compromises the blood-brain barrier (BBB), causing the cerebral microvascular endothelial cells’ tight junction proteins to break down.6 MMP12 also has a role in the expansion of the infarction volume and the apoptosis of ischemic brain cells.7 These developments have prompted us to hypothesize that the MMP12 gene may have a role in the pathogenesis of IS.
The rs660599 polymorphism at the MMP12 gene was found to be significantly associated with LAA stroke in a recent GWAS conducted in a European sample.3 Furthermore, Mahdessian (2017) revealed a link between the A allele of rs660599 and decreased plasma MMP12 levels and increased carotid intima-media thickness (cIMT).8 A recent study by Fan (2022) found no significant association between the MMP12 rs660599, rs2276109, and rs652438 polymorphisms and IS risk in the Chinese Hakka population.9 However, it remains unknown whether this GWAS-reported MMP12 polymorphism is linked to the risk and poor prognosis associated with IS. This study aimed to investigate the relationship between the MMP12 rs660599 polymorphism and the Southern Chinese Han population’s susceptibility, severity, and short-term outcome concerning IS.
Materials and Methods
Study Subjects
A total of 2096 participants—1061 healthy controls and 1035 IS patients—were progressively enrolled from the Affiliated Hospital of Guangdong Medical University, between 2015 and 2021. All of the participants were ethnically Han Chinese and had no genetic relationship. IS patients were admitted within 72 hours of neurological deficits onset, with symptoms lasting more than 24 hours. Magnetic resonance imaging (MRI) and computed tomography (CT) scans verified the diagnosis. The ORG 10172 Acute Stroke Treatment (TOAST) categorization method was used to divide the patients into SAO and LAA categories. The study did not select participants with a history of cerebral or transient ischemic attacks, autoimmune diseases, chronic infections, systemic inflammatory disorders, hematological maladies, coronary artery disease, or malignancies. Similar inclusion criteria were used to select healthy controls from the health examination center of the same hospital, who had no prior history of IS, chronic inflammation, autoimmune disorders, or cancer. The above criteria were utilized to characterize smoking, diabetes, and hypertension.10 The National Institute of Health Stroke Scale (NIHSS) score on admission assessed stroke severity, with scores < 6 indicating minor stroke and ≥ 6 indicating moderate to severe stroke. The modified Rankin Scale (mRS) score at 3 months’ post-onset was used to evaluate the functional outcomes, with mRS ≤ 2 denoted favorable outcomes and > 2 showing poor outcomes. Recurrent cases refer to those who experienced new neurological disorders or worsened pre-existing symptoms 21 days after the first occurrence. At the 3-month mark, a group of 975 patients was monitored either by telephone communication or in-person clinical visits. However, 60 patients (5.8% of the total) were not accounted for due to a loss of follow-up. The study was conducted in compliance with the Declaration of Helsinki and authorized by the Ethics Committee of the Affiliated Hospital of Guangdong Medical University, and all participants signed informed consent forms.
Genotyping
Using the TIANamp Blood DNA Kit (Tiangen Biotech, Beijing, China), genomic DNA was extracted from peripheral venous blood samples. The MMP12 gene polymorphism rs660599, reported in a GWAS study,3 was genotyped using the iMLDR-TM method with the following primers: forward primer:5’-ACACGACGCTCTTCCGATCTACTAGTTACTGCAATATGACCAGAAACAA-3’; reverse primer: 5’-TTCCTTGGCACCCGAGAATTCCAAAGTTAATGGGATATCTTAGGAGGACCAT-3’.
Detection of MMP12 Levels
Before analysis, serum samples were collected and kept at −80°C. ELISA (Tiangen Biotechnology, Beijing, China) was used to detect MMP12 levels following the manufacturer’s instructions.
Measurement of DWI Infarct Volume
Based on diffusion-weighted magnetic resonance imaging (DWI), infarct volumes were calculated using MIPAV software (Medical Image Processing, Analysis, and Visualization, version 3.0; NIH, Bethesda, MD).11 A semiautomated segmentation method based on apparent diffusion coefficient and fluid-attenuated inversion recovery imaging sequences was utilized to describe acute diffusion lesions on a slice-by-slice basis to distinguish acute from non-acute diffusion variation. Determining the DWI infarct volumes was achieved by multiplying the slice thickness by the total lesion area.
Statistical Analysis
For statistical analysis, GraphPad Prism 5.0 (GraphPad Software, Inc., San Diego, CA, USA) and SPSS version 19.0 (IBM, NY, USA) were used. The chi-square and t-tests were used to compute and compare the discrepancies between categorical and continuous data, respectively. A multivariate logistic regression model was used to examine the relationships between the MMP12 rs660599 variant and functional outcome following a stroke; 95% confidence interval (CI) and odds ratio (OR) were determined. The Cox proportional hazard regression model was used to determine the correlations between the rs660599 variation and the risk of stroke recurrence, as well as the hazard ratio and 95% confidence interval (CI). MMP12 levels with various genotypes in the patient and control groups were compared using the Student’s t-test for data with normal distribution or the Mann–Whitney U-test for nonparametric data. The associations between the DWI infarct volume and the genotypes of the rs660599 variation were investigated using a multiple linear regression model. Age, gender, smoking, hypertension, diabetes mellitus, and hyperlipidemia were all adjusted for linear regressions. The Bonferroni correction was employed for multiple comparisons and to reduce the occurrence of type 1 errors. A p-value less than 0.05 was deemed statistically significant.
Results
Demographic Characteristics
Table 1 lists the demographic details of the 1035 IS patients and 1061 healthy controls that were included in this investigation. Age (p = 0.65), serum uric acid (p = 0.72), total cholesterol (p = 0.94), and low-density lipoprotein (LDL) (p = 0.42) values did not significantly differ between the patient and control groups. However, there were significant differences between the control and IS groups (p < 0.001) in terms of sex (p < 0.001), smoking status (p < 0.001), hypertension (p < 0.001), diabetes (p < 0.001), triglycerides (p < 0.001), high-density lipoprotein (HDL) (p < 0.001), and homocysteine (p < 0.001).
 |
Table 1 Characteristics of IS Cases and Controls |
Relationship Between the MMP12 rs660599 Variant and the IS Risk
Table 2 summarizes the genotype and allele frequencies for the MMP12 rs660599 variation in the IS patients and controls. The Hardy-Weinberg equilibrium test showed no deviation in either group (p > 0.05). There was no significant correlation found by the χ²-test between the risk of IS and the MMP12 rs660599 variation. Between IS patients and controls, there was no significant difference in genotype or allele distributions (p > 0.05). Comparing IS patients and controls, there was no significant difference in the dominant (p = 0.066) or recessive (p = 0.79) models. In the examined genetic models, stratification based on TOAST categorization revealed no significant correlation (p > 0.05) between the rs660599 variant and the risk of LAA or SVO stroke.
 |
Table 2 Genotype and Allele Frequencies of MMP12 rs660599 Variants Between Controls and Overall IS Patients, and Patients with IS Subtype, and Corresponding Adjusted ORs |
Associations Between the MMP12 rs660599 Variant and Demographic Characteristics
The general characteristics of subjects according to MMP12 rs660599 genotypes and alleles are shown in Table 3. Upon adjusting for participant age, sex, hypertension, diabetes, and smoking status, it was observed that an increased risk of IS was associated with the MMP12 rs660599 GA/AA genotype and A allele in patients with diabetes (p = 0.0072) (Table 3).
 |
Table 3 A Comparison Between the Baseline Characteristics of the MMP12 rs660599 Genotypes and Alleles in the IS Patient and Control Groups |
MMP12 rs660599 Variant Serum Levels According to Various Genotypes
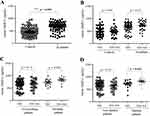
Serum MMP12 levels were assessed in 96 healthy controls and 98 IS patients. Patients with IS had significantly greater serum MMP12 levels than controls (p < 0.001; Figure 1A). Serum MMP12 levels did not significantly vary between patients with the rs660599 GA + AA genotype and those with the GG genotype in any group when stratified by MMP12 rs660599 genotypes (p > 0.05; Figure 1B). Serum MMP12 levels and the rs660599 variant did not significantly correlate in IS patients categorized on smoking status (p > 0.05; Figure 1C). However, diabetic individuals with the rs660599 A allele had significantly elevated blood MMP12 levels (p = 0.041; Figure 1D).
Correlation of the MMP12 rs660599 Variant with Infarct Volume
Figure 2 shows the diffusion-weighted magnetic resonance imaging of 78 IS patients, examined for the correlations between the MMP12 rs660599 variant and infarct volume. The mean infarct volumes of IS patients with the MMP12 rs660599 variant genotypes (GA + AA) were higher than those with the GG genotype (p = 0.042; Figure 2A). The rs660599 mutation and infarct volume were not significantly associated, according to stratification by smoking status (p > 0.05; Figure 2B). The infarct volumes of diabetic individuals with the rs660599 GA + AA genotypes were greater than those with the GG genotypes (p = 0.029; Figure 2C).
Association Between MMP12 rs660599 Variant and Stroke Severity
Table 4 displays the baseline characteristics of patients categorized according to their NIHSS scores. Patients with NIHSS ≥ 6 showed a greater proportion of diabetes (p = 0.0052) compared to those with NIHSS < 6. Multivariate logistic regression analysis, adjusted for diabetes, found a correlation between the rs660599 variant and a higher risk of severe stroke (p = 0.0031). In the recessive model, patients with GA + AA genotypes were linked with NIHSS ≥ 6 (p = 0.0031). Additionally, patients carrying the rs660599 variant A allele had a higher risk of NIHSS ≥ 6 (p = 0.0024).
 |
Table 4 Association of MMP12 rs660599 Polymorphism with Stroke Severity of IS |
MMP12 rs660599 Variant and Short-Term Outcome of IS
The short-term prognosis of IS was evaluated three months after the stroke using the mRS. The baseline characteristics of the two patient groups are shown in Table 5. Patients with mRS > 2 had a greater proportion of higher basal NIHSS scores (p < 0.001), intravenous thrombolysis (p = 0.017), and endovascular treatment (p = 0.018). The MMP12 rs660599 variant was significantly associated with short-term IS outcomes (p = 0.014). The rs660599 variant’s GA/AA genotype was statistically significantly (p = 0.0077) linked to a higher risk of a poor outcome compared to the GG genotype when confounding factors were adjusted. Patients with IS who carried the rs660599 A allele were more likely to experience unfavorable outcomes with greater disability (p = 0.0038).
 |
Table 5 Association of MMP12 rs660599 Polymorphism with Short-Term Outcome of IS |
MMP12 rs660599 Variant and IS Recurrence
For stroke recurrence analysis, 975 IS patients were included, with a median follow-up period of 12 months. Table 6 summarizes the basic features of individuals grouped according to stroke recurrence. Age was related to IS recurrence. Additional stratification based on rs660599 genotypes revealed no association between the rs660599 variation and the occurrence of stroke in any genetic models (Table 6).
 |
Table 6 Association Between MMP12 rs660599 Polymorphism and IS Recurrence |
Discussion
In this case-control study, which was hospital-based, no association between the MMP12 rs660599 variant and IS or its subtypes was found. However, stratification analysis revealed that individuals with diabetes or who had a smoking status carrying the MMP12 rs660599 A allele may have a higher susceptibility to developing IS. Additionally, it was found that, in comparison to individuals with the GG genotype, diabetic IS patients with the rs660599 A allele had elevated blood MMP12 levels with larger infarct volumes. Furthermore, a negative functional outcome for IS was significantly correlated with the rs660599 A allele, indicating that the rs660599 polymorphism may be a predictive biomarker for IS patients. Nevertheless, no association was found between this polymorphism and IS recurrence risk.
MMPs control a wide range of cellular processes, such as the breakdown of extracellular matrix (ECM), cell adhesion, proliferation, proteolysis, migration, loss of cell-surface proteins, and the release of cytokines that promote inflammation.12 MMP12, a member of the metalloelastase class, is mostly generated by macrophages and breaks down type IV collagen, laminin, and elastin, among other ECM components.13 Evidence indicates that following cerebral ischemia, MMP12 levels rise in the brain, promoting apoptosis, inflammation, blood-brain barrier breakdown, demyelination, and white matter damage.14 Additionally, pro-MMP1 and pro-MMP9 are activated by MMP12, and these MMPs, together with pro-MMP2 and pro-MMP3, function in the pathogenesis of IS.15 Inhibition of MMP12 may promote angiogenesis in the injured brain, aiding in the process of healing.16 Despite these advances, the role of MMP12 polymorphisms in IS susceptibility and prognosis has not been thoroughly addressed.
The MMP12 variant’s involvement in IS susceptibility has been the subject of several earlier investigations, with conflicting findings. According to Chehaibi (2014), among individuals with type 2 diabetes mellitus, the MMP12 −82A/G and MMP12 −1082A/G genotypes and haplotypes were found to be associated with IS risk.17 According to a meta-analysis, there may be a link between IS and the MMP12 −1082 A/G polymorphism.18 The MMP12 rs660599 variant has been correlated to an increased risk of major artery atherosclerotic stroke, according to prior GWAS.3 The study conducted by Fan concluded that the presence of the MMP12 rs660599 variant did not contribute to an increased risk of IS in the Han Hakka population. However, the study did find that there was a significant association between the interaction of MMP-9 rs17576 and MMP12 rs660599 and an increased risk of IS in the same group.9 In this study, the MMP12 rs660599 variant was not associated with the risk of IS or its LAA or SVO subtypes in a Southern Chinese Han population. However, after categorizing the data based on age, gender, smoking status, diabetes, and hypertension, it was shown that diabetic subgroups of IS patients had a higher risk of IS compared to the control group. This indicates that risk factors, such as diabetes, may combine with the genetic tendency of MMP12 to promote the development of IS.
Genetic polymorphisms influence the control of gene expression, which contributes to individual variations in the severity and susceptibility to disease. SNPs rs499459, rs613084, and rs1892971 at chr11q22.3 were independently related to plasma MMP12 in atherosclerotic plaque tissues, according to GWAS data of the plasma MMP12 concentrations.8 In reporter gene experiments, the A allele of the MMP12 −82A/G polymorphism (rs2276109) produces enhanced gene expression because of its affinity for the transcription factor activator protein-1 (AP-1).19 The MMP12 1082A/G polymorphism is located in the coding region of the hemopexin domain, which is important for regulating the activity of MMP12.20 In this study, MMP12 expression was significantly upregulated in IS patients compared to controls. However, MMP12 expression was similar between the A and G alleles of rs660599 in both the IS and control groups. Interestingly, a significant association was observed between the rs660599 GA + AA genotypes and higher levels of MMP12 in serum compared to the GG genotype in diabetic patients. This suggests that diabetic status and genetic variation may synergistically increase MMP12 levels, facilitating IS development.
Multiple pro-inflammatory and apoptotic pathways are activated by cerebral ischemia, which increases lymphocytes, infiltrates macrophages, and triggers microglia in the infarct region.21 MMP12 is involved in both increased infarct volume and ischemic brain cell death. MMP12 knockdown significantly lowered the amount of the post-ischemic infarct and enhanced motor and cognitive recovery, according to Arruri (2022).22 Silencing MMP12 has been shown to reduce tight junction protein degradation, BBB disruption, apoptosis, demyelination, MMP9 activation, TNF-α upregulation, and infarct volume development.4 In this investigation, it was revealed that patients with the major G alleles had smaller infarct sizes than those with the variation MMP12 rs660599 GA + AA genotype. Diabetic patients with the rs660599 GA + AA genotypes also had greater infarct volume than those with the GG genotype; however, no significance was observed in non-diabetic patients. This was also supported by the evidence that high glucose-induced oxidative stress and inflammation via MMP12 activation indicate that the MMP12 rs660599 variants and diabetes status may upregulate MMP12, contributing to infarct size development.23 These results indicate that environmental factors, particularly diabetes, and genetic factors (rs660599 A allele of MMP12) may synergistically influence infarct size expansion.
In vivo evidence also supports that MMP12 suppression significantly improves motor function and cognitive abilities in mouse stroke models.14,22,24 The mechanisms involved might include inflammation, apoptosis, attenuated BBB damage, demyelination, and decreased white matter damage. Thus, it was speculated that the prognosis for IS may be correlated with MMP12 polymorphisms. According to present research, the MMP12 rs660599 A allele was significantly linked to poor functional results in IS, and the rs660599 polymorphism may be able to predict an individual’s prognosis. This is the first study that has examined the impact of MMP12 polymorphisms on the severity of IS and its short-term outcomes in a Chinese population. These results show that patients carrying the rs660599 A allele have larger infarct volumes, supporting this conclusion. Elevated MMP12 levels were associated with stroke severity and poor neurological outcomes.4 The rs660599 A allele was linked to higher levels of MMP12 in the serum in this study. This means that the rs660599-MMP12 gene expression link is functionally linked to IS prognosis.
In this study, it was demonstrated that diabetic patients carrying the MMP12 rs660599 variants were linked to elevated MMP12 levels, increased IS risk, and poor outcomes of IS, consistent with Chehaibi’s (2014) report that in diabetic individuals, MMP12-82A/G may be a risk factor for IS in Tunisian population.17 This evidence suggests that diabetic status, in conjunction with genetic factors, may influence stroke onset and prognosis. Plasma levels of MMP12 are elevated in type 2 diabetes mellitus patients and show association with increased release of inflammatory cytokine, more severe atherosclerosis, and a higher incidence of coronary events.25,26 Upregulated MMP12 facilitates oxidative stress and inflammation via MAPK signaling, contributing to IS pathogenesis.23 According to a recent study, in vivo knockdown of MMP12 improves insulin sensitivity, systemic inflammation, and atherosclerotic characteristics in mice, hence reducing cardiometabolic disease.27 Based on this information, it is plausible to suggest that the presence of diabetes and the MMP12 polymorphism may contribute to the increased expression of MMP12. This, in turn, might lead to heightened inflammatory response, oxidative stress, and the advancement of atherosclerosis. These factors are closely linked to the incidence and prognosis of stroke.
There are several limitations to this study. Firstly, as this is a single-center research, larger sample numbers and multi-center, and multi-ethnic investigations are needed to confirm the results. Furthermore, because this is a hospital-based study, there may be an inherent selection bias. Second, this study focused mainly on the GWAS-reported variant rs660599 of MMP12. Other MMP12 genetic polymorphisms may also be important in the pathogenesis of IS. The potential interactions between MMP12 polymorphisms and other risky SNPs or other risk factors not investigated in this study were not excluded. Finally, the mechanism of rs660599 on the short-term prognosis after stroke and its synergy with diabetes in affecting stroke pathogenesis remains unclear and needs further clarification.
The present study demonstrated that the MMP12 rs660599 A allele confers an increased risk of IS and elevated MMP12 levels in diabetic patients. Moreover, in a Southern Chinese Han population, the MMP12 rs660599 A allele was associated with greater infarct volumes, moderate to severe stroke, and worse short-term outcomes of IS. Future studies exploring the exact biological mechanisms by which these genetic variants contribute to IS pathogenesis and its recovery need to be elucidated, which is essential for personalized treatment and prevention.
Acknowledgments
This work was supported by funding from the National Nature Science Foundation of China (grant numbers 81571157 and 81300929), the Natural Science Foundation of Guangdong Province (grant numbers 2023A1515012750), the Youth Cultivation Foundation of Guangdong Medical University (grant number GDMUQ2021006), the Medical Scientific Research Foundation of Guangdong Province, China (grant number A2022139), the Nonfunded Science and Technology Research Foundation of Zhanjiang City (grant number 2021B01370) and the discipline construction project of Guangdong Medical University (1015DFK20200003). We’d like to thank MJEditor (www.mjeditor.com) for its linguistic assistance during the prepare of this manuscript. We also thank the participants in this study.
Author Contributions
All authors have made significant contributions to the work reported, whether in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors have no actual or potential conflicts of interest related to this paper.
References
1. Dmytriw AA, Zhang Y, Mendes Pereira V. Mechanical thrombectomy and the future of acute stroke treatment. European j Radiology Mar. 2019;112:214–221. doi:10.1016/j.ejrad.2019.01.029
2. Lindgren A. Stroke genetics: a review and update. J Stro. 2014;16(3):114–123. doi:10.5853/jos.2014.16.3.114
3. Traylor M, Makela KM, Kilarski LL, et al. A novel MMP12 locus is associated with large artery atherosclerotic stroke using a genome-wide age-at-onset informed approach. PLoS Genetics. 2014;10(7):e1004469. doi:10.1371/journal.pgen.1004469
4. Chelluboina B, Nalamolu KR, Klopfenstein JD, et al. MMP-12, a Promising Therapeutic Target for Neurological Diseases. Molecular Neurob. 2018;55(2):1405–1409. doi:10.1007/s12035-017-0418-5
5. Morgan AR, Rerkasem K, Gallagher PJ, et al. Differences in matrix metalloproteinase-1 and matrix metalloproteinase-12 transcript levels among carotid atherosclerotic plaques with different histopathological characteristics. Stroke. 2004;35(6):1310–1315. doi:10.1161/01.STR.0000126822.01756.99
6. Chelluboina B, Klopfenstein JD, Pinson DM, Wang DZ, Vemuganti R, Veeravalli KK. Matrix Metalloproteinase-12 Induces Blood-Brain Barrier Damage After Focal Cerebral Ischemia. Stroke. 2015;46(12):3523–3531. doi:10.1161/STROKEAHA.115.011031
7. Chelluboina B, Warhekar A, Dillard M, et al. Post-transcriptional inactivation of matrix metalloproteinase-12 after focal cerebral ischemia attenuates brain damage. Sci Rep. 2015;5:9504. doi:10.1038/srep09504
8. Mahdessian H, Perisic Matic L, Lengquist M, et al. Integrative studies implicate matrix metalloproteinase-12 as a culprit gene for large-artery atherosclerotic stroke. J Internal Med. 2017;282(5):429–444. doi:10.1111/joim.12655
9. Fan D, Zheng C, Wu W, et al. MMP9 SNP and MMP SNP-SNP interactions increase the risk for ischemic stroke in the Han Hakka population. Brain and Behavior. 2022;12(2):e2473. doi:10.1002/brb3.2473
10. Li Y, Cui LL, Li QQ, et al. Association between ADAM17 promoter polymorphisms and ischemic stroke in a Chinese population. J Atheroscler Throm. 2014;21(8):878–893. doi:10.5551/jat.22400
11. Buck BH, Liebeskind DS, Saver JL, et al. Association of higher serum calcium levels with smaller infarct volumes in acute ischemic stroke. Arch. Neurol. 2007;64(9):1287–1291. doi:10.1001/archneur.64.9.1287
12. Cunningham LA, Wetzel M, Rosenberg GA. Multiple roles for MMPs and TIMPs in cerebral ischemia. Glia. 2005;50(4):329–339. doi:10.1002/glia.20169
13. Challa S, Fornal CA, Schaibley C, et al. Post-stroke suppression of matrix metalloproteinase-12 attenuates the expression of M1 and M2 markers and prevents the elevation of other matrix metalloproteinases. Paper presented at: stroke; 2022.
14. Veeravalli KK. Implications of MMP-12 in the pathophysiology of ischaemic stroke. Stroke Vasc Neurol. 2024;9(2):97–107. doi:10.1136/svn-2023-002363
15. Matsumoto S, Kobayashi T, Katoh M, et al. Expression and localization of matrix metalloproteinase-12 in the aorta of cholesterol-fed rabbits: relationship to lesion development. Am J Pathol. 1998;153(1):109–119. doi:10.1016/S0002-9440(10)65551-4
16. Dong Z, Kumar R, Yang X, Fidler IJ. Macrophage-derived metalloelastase is responsible for the generation of angiostatin in Lewis lung carcinoma. Cell. 1997;88(6):801–810. doi:10.1016/S0092-8674(00)81926-1
17. Chehaibi K, Hrira MY, Nouira S, Maatouk F, Ben Hamda K, Slimane MN. Matrix metalloproteinase-1 and matrix metalloproteinase-12 gene polymorphisms and the risk of ischemic stroke in a Tunisian population. J Neurol Sci. 2014;342(1–2):107–113. doi:10.1016/j.jns.2014.04.036
18. Misra S, Talwar P, Kumar A, et al. Association between matrix metalloproteinase family gene polymorphisms and risk of ischemic stroke: a systematic review and meta-analysis of 29 studies. Gene. 2018;672:180–194. doi:10.1016/j.gene.2018.06.027
19. Jormsjo S, Ye S, Moritz J, et al. Allele-specific regulation of matrix metalloproteinase-12 gene activity is associated with coronary artery luminal dimensions in diabetic patients with manifest coronary artery disease. Circulation Research. 2000;86(9):998–1003. doi:10.1161/01.RES.86.9.998
20. Joos L, He JQ, Shepherdson MB, et al. The role of matrix metalloproteinase polymorphisms in the rate of decline in lung function. Human Molecular Genetics. 2002;11(5):569–576. doi:10.1093/hmg/11.5.569
21. Jurcau A, Simion A. Neuroinflammation in cerebral ischemia and ischemia/reperfusion injuries: from pathophysiology to therapeutic strategies. Int J Mol Sci. 2021;23(1):14. doi:10.3390/ijms23010014
22. Arruri V, Chokkalla AK, Jeong S, et al. MMP-12 knockdown prevents secondary brain damage after ischemic stroke in mice. Neurochem Inter. 2022;161:105432. doi:10.1016/j.neuint.2022.105432
23. Quan X, Liu H, Ye D, Ding X, Su X. Forsythoside A Alleviates High Glucose-Induced Oxidative Stress and Inflammation in Podocytes by Inactivating MAPK Signaling via MMP12 Inhibition. Diabet metabolic syndrom obesi. 2021;14:1885–1895. doi:10.2147/DMSO.S305092
24. Challa SR, Nalamolu KR, Fornal CA, et al. Therapeutic efficacy of matrix metalloproteinase-12 suppression on neurological recovery after ischemic stroke: optimal treatment timing and duration. Front Neurosci. 2022;16:1012812. doi:10.3389/fnins.2022.1012812
25. Goncalves I, Bengtsson E, Colhoun HM, et al. Elevated Plasma Levels of MMP-12 Are Associated With Atherosclerotic Burden and Symptomatic Cardiovascular Disease in Subjects With Type 2 Diabetes. Arteriosclerosis Thrombosis Vasc Biol. 2015;35(7):1723–1731. doi:10.1161/ATVBAHA.115.305631
26. Kozakova M, Morizzo C, Goncalves I, Natali A, Nilsson J, Palombo C. Cardiovascular organ damage in type 2 diabetes mellitus: the role of lipids and inflammation. Cardiovascular Diabetology. 2019;18(1):61. doi:10.1186/s12933-019-0865-6
27. Amor M, Bianco V, Buerger M, et al. Genetic deletion of MMP12 ameliorates cardiometabolic disease by improving insulin sensitivity, systemic inflammation, and atherosclerotic features in mice. Cardiova Diab. 2023;22(1):327. doi:10.1186/s12933-023-02064-3
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.