Back to Journals » Research and Reports in Urology » Volume 16
Bladder Pain Syndrome (BPS): A Comprehensive Review of Treatment Strategies and Management Approaches
Authors Cacciatore L , Territo A, Minore A, Testa A , Mantica G, Esperto F
Received 27 August 2024
Accepted for publication 13 October 2024
Published 26 October 2024 Volume 2024:16 Pages 273—282
DOI https://doi.org/10.2147/RRU.S387749
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Panagiotis J Vlachostergios
Loris Cacciatore,1 Angelo Territo,2 Antonio Minore,1 Antonio Testa,1 Guglielmo Mantica,3 Francesco Esperto1
1Department of Urology, Fondazione Policlinico Universitario Campus Bio-Medico Di Roma, Rome, Italy; 2Department of Urology, Fundació Puigvert, Autonoma University of Barcelona, Barcelona, Spain; 3Department of Surgical and Diagnostic Integrated Sciences (DISC), University of Genova, Genoa, Italy
Correspondence: Loris Cacciatore, Department of Urology, Fondazione Policlinico Universitario Campus Bio-Medico di Roma, Rome, Italy, Email [email protected]
Abstract: Bladder pain Syndrome presents a multifaceted challenge in contemporary urological practice, marked by LUTS, negative behavioural, sexual, or emotional experiences, and the potential for sexual dysfunction. We meticulously explored the existing literature of conservative, non-invasive and invasive interventions, aiming to provide clinicians with a nuanced understanding of available options for comprehensive BPS management. We delve into the effectiveness and safety profiles from behavioural approaches through lifestyle changes and physical therapy, to oral or intravesical medications, until the definitive surgical treatment. The best option evaluated is the involvement of a multidisciplinary team, including urologists, urotherapists, gynaecologists, pain specialists, primary care physicians and psychologists, educating those patients regarding the condition and its chronic course and tailoring the perfect treatment for each person. Despite this, BPS remains a challenge for urologists. Indeed, our objective is to contribute to the evolving landscape of BPS management, fostering informed decision-making and personalized care for individuals grappling with this challenging condition.
Keywords: bladder pain syndrome, interstitial cystitis, lower urinary tract symptoms, females, continence, therapy, conservative treatment
Introduction
Bladder pain syndrome (BPS) is a chronic and debilitating condition with unknown aetiology that can be difficult to treat due to its frequent links to negative behavioural, sexual, or emotional experiences, as well as symptoms related to lower urinary tract (LUTS) and sexual dysfunction.1 Exact epidemiological data is challenging to define, considering that in population based studies, disease assessment is done by symptom-based questionnaires. However, estimates from the USA data suggest a prevalence of 100–300 per 100,000 women, with a male prevalence of 10–20% of the female estimate.2
Regarding the terminology, definition, and diagnostic criteria for BPS, there has been considerable debate in the urological landscape. The International Continence Society’s 2002 standardization report referred to it as “painful bladder syndrome/interstitial cystitis”, describing it as suprapubic pain associated with bladder filling, alongside increased day-time and night-time frequency, in the absence of identifiable urinary infection or other clear pathology.3 In contrast, the European Society for the Study of Interstitial Cystitis (ESSIC) refers to it as “bladder pain syndrome” characterized by chronic pelvic pain, pressure, or discomfort thought to be linked to the bladder, accompanied by at least one LUTS such as a persistent urge to void or urinary frequency.4
To date, there is no consensus on the best approach from conservative to surgical treatments or combination of more therapies, highlighting the need for further research to evaluate the effectiveness and safety of these procedures. This review aims to assess and explore the evidence of the existing literature on treatment options or recommendations and pragmatic frameworks for management of BPS in terms of efficacy and safety.
Diagnosis
The diagnosis of BPS is primarily one of exclusion, and various criteria have been proposed. Firstly, the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) introduced initial criteria in 1987 based on clinical symptoms and pathological findings from bladder biopsies,5 refined further in 1988. However, only about 40% of clinically diagnosed BPS patients meet these criteria, which are thus primarily used in research.6 The ESSIC criteria focus more on symptom-based diagnosis, where cystoscopic evaluation is not mandatory but can help with further classification. Indeed, the use of validated questionnaires, as Interstitial cystitis symptom index (ICSI), Bladder Pain/Interstitial Cystitis Symptom Score (BPIC-SS), Pain, Urgency, Frequency (PUF) score, International Consultation on Incontinence Questionnaires (ICIQs) such as Female Lower Urinary Tract Symptoms (ICIQ-FLUTS) and Lower Urinary Tract Symptoms Quality of Life Module (ICIQ-LUTSqol), are beneficial for the characterization of the disease.7 In clinical practice (Figure 1), most urologists diagnose the syndrome based on personal history, risk factors and symptoms after excluding other conditions and may use urine tests (urine culture and/or urinary cytology with 3 samples), cystoscopic assessment with bladder mapping and/or hydrodistension under light general anesthesia to evaluate physiological changes.8 Additionally, the diagnostic path should include radiological examinations such as ultrasound to exclude urinary dilation or pathological findings, CT scan abdomen and pelvic MRI as second level examinations.9 Consequently, the management of BPS has a multifaceted approach from conservative to pharmacological to minimally invasive treatments to definitive surgical treatment, ideally involving a multidisciplinary team, including urologists, specialist nurses (urotherapists), and gynaecologists with an extended network to involve pain specialists, primary care physicians (PCPs), and psychologists,10,11 educating those patients regarding the condition and its chronic course.
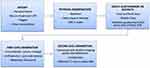 |
Figure 1 BPS diagnostic flow-chart. |
Conservative Treatments
Nearly half of all patients with Bladder Pain Syndrome (BPS) experience significant symptom improvement or resolution over time, even without consistent follow-up or new treatments. Figure 2 shows subsequent levels of treatment for BPS patients, starting from conservative and behavioral approaches, that should form the cornerstone of BPS management, given their risk-free nature and relatively low cost, making them accessible and sustainable options for long-term management.12 These strategies include lifestyle modifications such as stress reduction techniques, dietary changes (eg, reducing caffeine, spicy foods, and alcohol), smoking cessation, and physical therapies like timed voiding, bladder training, and pelvic floor.13 Studies have shown that these interventions can lead to significant improvements in urinary frequency, voiding intervals, and overall symptom relief, with some reports indicating that up to 88% of patients experience symptom improvement. Furthermore, a 12-week program focusing on controlled fluid intake and pelvic floor exercises significantly increased the time between voids and decreased urinary frequency.13
 |
Figure 2 Pathway of treatment from low/mild to severe/refractory BPS disease. |
Given the importance of personalized care, the early involvement of a urotherapist is crucial in BPS treatment. This approach allows for a thorough exploration of the patient’s history and the establishment of realistic expectations.14 Physiotherapy, particularly myofascial release therapy for patients with pelvic floor dysfunction or those exhibiting trigger points, has demonstrated up to 94% improvement in symptom scores.15 Additionally, a randomized trial showed that 59% of women with BPS who received myofascial physical therapy reported significant improvements compared to just 26% of those receiving general therapeutic massage.16
The role of education and support in managing BPS cannot be overstated. Involving the patient’s partner or family during consultations can provide emotional support and enhance the patient’s ability to cope with the disease. Given the prevalence of misleading information online, providing clear and accurate educational materials is essential. The EAU Patient Information (EAU PI) working group, supported by EAU guidelines,17 delivers high-quality video content about various clinical conditions and surgical procedures in patient-friendly language.18,19 Support groups also play a valuable role, offering community, understanding, and information on alternative therapies such as acupuncture,20 reflexology, hypnotherapy, or talking therapies like cognitive behavioral therapy (CBT), which can be facilitated by the primary care physician. This comprehensive, multidisciplinary approach to BPS management, grounded in conservative and behavioral strategies, offers a promising path to improving patients’ quality of life.
Pharmacological Treatments
When conservative management fails to alleviate symptoms in patients with BPS, the next step typically involves oral pharmacotherapy. Various oral agents have been utilized in BPS treatment, with guidelines offering differing levels of recommendation. While pharmacological therapies are commonly employed, they frequently have variable success rates and are associated with high discontinuation due to minimal long-term efficacy. The choice of treatment often depends on the individual patient’s response and tolerance to these medications.
Nonsteroidal Anti-Inflammatory Drugs (NSAIDs)
Nonsteroidal anti-inflammatory drugs (NSAIDs) are often the first-line analgesics, reducing pain and inflammation associated with BPS. They are usually combined with paracetamol and regular gastric protection.17 In clinical practice, combining conservative measures with pharmacotherapy may often serve as the most effective first-line therapy.
Tricyclic Antidepressants (TCAs)
Amitriptyline, a tricyclic antidepressant not officially approved for BPS, is commonly used to manage neuropathic pain, working by blocking the reuptake of serotonin and noradrenaline, which helps alleviate pain and symptoms related to storage LUTS. In randomized trials, it has shown significant symptom improvement in patients compared to placebo, particularly when patients could tolerate doses of 50 mg or higher.21 However, side effects like blurred vision, dry mouth, and constipation can limit its use (92% of patients reported at least one side effect), with fewer than half of patients able to tolerate higher doses.22
Pentosan Polysulphate Sodium (PPS)
PPS is a semi-synthetic drug used both orally and via bladder instillation for BPS. While licensed for BPS, evidence of its efficacy is mixed. Some studies show significant improvements in pain, urgency, and frequency compared to placebo,23 but recent trials have reported inconsistent results.24,25 Long-term use (median 186 months) has been associated with adverse events, particularly pigmentary maculopathy, in up to 16% of patients which may limit its future use.26
Antihistamines
These medications, such as cimetidine and hydroxyzine, are thought to mitigate bladder symptoms by preventing the effects of histamine release from mast cells. Cimetidine, with its action on a similar peptidergic pathway in the bladder, has shown some efficacy in reducing suprapubic pain and nocturia comparing to placebo,27 but evidence for antihistamines overall is limited. Hydroxyzine, especially when combined with PPS than PPS alone, may offer some benefit (40% vs 28%), but more large-scale studies are needed.28
Cyclosporin a (CyA)
An immunosuppressive drug traditionally used in transplant recipients, CyA is emerging as a treatment for refractory BPS. It has shown greater efficacy than PPS in improving symptoms like urinary frequency and pain scores, particularly in patients with Hunner lesions,29 although 94% of patients in the CyA group had high level of adverse events, including nephrotoxicity, hypertension, and immunosuppression.30 For these reasons, it requires administration by specialists with experience in its use and is reserved as a last-line treatment for severe cases.
Intravesical Treatment
A variety of intravesical therapies are employed globally for BPS, including chondroitin sulfate (CS), hyaluronic acid (HA), heparin, lidocaine, pentosan polysulfate sodium (PPS), and dimethyl sulfoxide (DMSO). These therapies generally aim to restore the glycosaminoglycan (GAG) layer in the bladder, which helps protect the bladder lining. The choice of therapy often depends on local regulations and practice guidelines. Overall, while these therapies provide options for managing BPS, the variability in response and side effects underscores the need for ongoing research to optimize treatment strategies and improve patient outcomes.
Chondroitin Sulfate (CS) and Hyaluronic Acid (HA)
CS and HA, Glycosaminoglycan (GAG) Layer Treatments are frequently used in Europe, typically administered once a week for six weeks, followed by monthly maintenance. These treatments can be used individually or combined (iAluRil®). Intravesical HA has demonstrated efficacy rates of 66–87% in observational studies.31,32 Similarly, intravesical CS has shown promising efficacy rates of around 60%.33 Common side effects include pain, irritation, and urinary tract infections (UTIs). Despite their frequent use, there is no definitive evidence favoring one over the other in terms of overall effectiveness.
Pentosan Polysulfate Sodium (PPS)
Pentosan polysulfate sodium (PPS) can be administered either orally or intravesically, showing variable effectiveness. Studies report a moderate efficacy rate of 40–62% for PPS compared to placebo, and combination therapies involving both intravesical and oral PPS appear to be more effective than oral PPS alone.34 Additionally, recent reports indicate that intravesical PPS is a safe and effective option, particularly when considering adverse events like pigmentary maculopathy and visual disturbances, which are more commonly associated with oral administration, which could impact its long-term utility.
Dimethyl Sulfoxide (DMSO)
DMSO is FDA-approved for interstitial cystitis and recommended by the European Association of Urology17 for BPS. Although its exact mechanism is not fully understood, it is believed to reduce inflammation and relax bladder muscles.35 DMSO is typically administered as a 50% solution once a week for six weeks via a temporary catheter, with additional courses and monthly maintenance if needed. Clinical studies show significant improvements in both subjective symptoms (53% vs 18% in placebo) and objective urodynamic measures (93% vs 35% in placebo).36 However, side effects such as headache and dizziness are common, with 21.8% of patients requiring additional oral medication for symptom management.37
Others Intravesical Treatments or Combinations
Intravesical heparin (40,000 IU) is often combined with anaesthetics like lidocaine (8 mL, 1% or 2%) or sodium bicarbonate (3 mL 8.4%). This combination has shown short-term success rates of 56–73% in cohort studies and provides relief for acute symptoms, though standalone studies are limited.38,39 Intravesical lidocaine, especially when combined with sodium bicarbonate, is recommended for short-term relief of acute symptoms.40
Intravesical “cocktails”, which combine various agents such as GAG-layer therapies, anaesthetics, and steroids, are used to manage severe symptoms, though evidence for their efficacy mainly comes from small cohort studies.41 Recent reports combined:
Minimally Invasive Treatments
Minimally invasive procedures like bladder hydrodistension, intravesical botulinum toxin A injections, platelet rich plasma, neuromodulations etc. are recommended by the EAU guidelines,17 although the supporting evidence is weak.
Hydrodistension
Hydrodistension is a long-standing technique used both for diagnosis and treatment. More in detail, irrigation was performed under gravity with a pressure of 100 cm until it stopped, and hydrodistension was maintained for 2 minutes. The urologist applied digital pressure against the urethra to prevent irrigant leakage around the cystoscope. After draining the bladder, the hydrodistension was repeated.44 A systematic review reported validated rates of 56% in 2–3 months with an acute exacerbation of symptoms in 9% of patients.45 Additionally, comparing patients undergoing versus not undergoing hydrodistension, the first group reported most sovrapubic pain/pressure, vaginal pain, dyspareunia and eyaculatory pain. Therefore, its efficacy remains under debate due to variability in techniques, complications and study outcomes. When combined with the transurethral destruction or fulguration of Hunner lesions, show high success rates in symptom relief with 40% reporting more than 3 years long-term efficacy.46 However, the majority required often multiple interventions for sustained efficacy.47
Botulinum Toxin Type a (BTT-A)
The function of BTT-A is related to reducing BPS symptoms through the inhibition of neurotransmitter release in the bladder’s sub-urothelial layer, thereby decreasing sensitivity and modulating detrusor contractility.48 It is particularly effective for patients unresponsive to standard intravesical treatments.49 Administered typically as a 100-unit dose via cystoscopy, injecting at approximately 10–20 sites, followed by careful monitoring and dose adjustments based on individual responses. Patients must be thoroughly informed about the risks, including the possibility of urinary retention and the potential need for self-catheterization. The effect of BTT-A was explored alone50 and against placebo.49 While compared with placebo (normal saline), the larger decreases in VAS scores were recorded in patients who received BTT-A injections in comparison to normal saline (2.6 vs 0.9 P=0.021). Moreover, in combination with hydrodistension showed a global response assessment (GRA) of 72% versus 48% in the cisto-hydrodistention alone group (P<0.05) at 3 months.48 Aforementioned comparative study indicate that BTT-A provides higher success rates and greater improvements in pain scores and bladder capacity compared to hydrodistention alone, with the average length of the effect of BTT-A of 5.4 months.
Platelet Rich Plasma
Transurethral suburothelial injections of PRP deliver growth factors and cytokines that support tissue healing, modulate inflammation, and reduce hypoxia-induced apoptosis in urothelial cells. Studies show multiple injections significantly improved symptoms in 70% of IC/BPS patients, though standardized PRP preparation protocols and a definitive understanding of its therapeutic mechanisms are still lacking. Further research is needed to determine optimal dosages, injection frequencies, and the long-term efficacy of PRP therapy for bladder disorders.51,52
Neuromodulation
Neuromodulation, including different options, such as transcutaneous electrical nerve stimulation (TENS), percutaneous tibial nerve stimulation (PTNS), and sacral nerve stimulation (SNS), offers a conservative yet potentially invasive approach to managing bladder conditions. TENS, a non-invasive method, can be self-administered at home, while more invasive techniques like PTNS, pudendal nerve stimulation (PNS), and SNS are typically considered for advanced treatment stages53 (PMID: 28458478). For patients with refractory bladder pain syndrome (BPS), particularly those with Hunner lesions, sacral neuromodulation (SNM) has shown promising outcomes. This procedure, which involves permanent implantation following a successful test phase, has demonstrated significant long-term efficacy in reducing pelvic pain, frequency, nocturia, and urgency, with success rates up to 80%.2,54 In some cases, pudendal nerve stimulation has proven even more effective than sacral nerve stimulation, especially in increasing voided volume and alleviating symptoms (59% vs 44%, P=0.05).55 Patients must be well-informed about potential adverse effects, such as infections. However, the need for device revision or permanent implants due to poor outcomes is a consideration, with limited evidence.
Surgical Treatments
Patients who have exhausted all other treatment options and continue to experience severe symptoms that significantly affect their quality of life, surgery may be considered as a last resort. However, this decision requires careful patient counselling due to the high risks associated with surgery, including complications such as infection, bowel obstruction, ureteric stricture, and stoma problems, as well as the potential impact on sexual function, body image, and lifestyle. Patients must understand that surgery does not guarantee pain relief and that they may be trading one set of problems for another.56 Surgical options include bladder augmentation cystoplasty or urinary diversion with or without cystectomy, and the choice between an ileal conduit or a continent urinary diversion depends on factors like patient preference, previous surgeries or radiotherapy.57 There is ongoing debate about whether the bladder should be left in place if a diversion is performed, as some evidence suggests that simply preventing the bladder from storing urine can lead to satisfactory symptom improvement. However, persistent issues like pyocystis (reported in 3.3% to 67% of cases) may still occur.58
Studies show mixed outcomes based on the presence of Hunner lesions. Indeed, research showed as patients with non-Hunner BPS were less likely to benefit from reconstructive procedures, then those with the Hunner subtype of BPS, that were pain-free following surgery in 82% of cases.59 A recent systematic review of 448 BPS patients undergoing radical surgery reported that 77% experienced symptomatic improvement, with the better clinical response observed in those who underwent total cystectomy with orthotopic neobladder formation. Despite these results, a significant proportion of patients (23%) did not have improvement, with considerable morbidity (26.5%) and mortality (1.3%). Given the variable outcomes and high risks, major surgical intervention should be reserved for the most severe cases, particularly in patients with Hunner lesions and small bladder capacity and should be approached with caution. There is a need for prospective randomised studies to answer questions regarding patient selection and optimal surgical approach.56
Emerging Treatments
BPS/IC is a complex condition with an unclear aetiology, driving ongoing research into new treatments. Current efforts focus on understanding the disease’s pathology, the microbiome, and identifying biomarkers to better classify and treat patients. These emerging therapies offer hope, but larger, long-term studies are required to validate their efficacy and safety in treating BPS/IC.
Several novel therapies are showing promise in early trials:
The Role of Urologists
Given the comprehensive approach advised for managing BPS, urologists are often in a prime position to spearhead the coordination of multidisciplinary efforts and build a local network of health professionals with specialized knowledge. This process is further streamlined when departments create a treatment protocol tailored to their available services. Appointing a lead urologist to drive and oversee these initiatives can be particularly effective. In cases where local treatment options, such as urinary diversion or nerve stimulation, are unavailable, establishing a referral pathway to a specialized centre is advisable. Maintaining strong communication with primary care providers is also crucial for updating referral guidelines, supporting patient care, and organizing educational programs.
Conclusions
BPS is a complex condition that poses significant challenges for clinicians. The absence of high-quality research, along with variability in study inclusion criteria and outcome measures, complicates the ability to draw definitive conclusions about treatment options. Effective management of BPS requires a multimodal approach tailored to each patient, emphasizing the symptoms that most impact their quality of life. Continued research into the underlying causes of BPS is essential for the development of more effective treatments, and several promising oral and intravesical therapies are currently emerging.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Disclosure
The authors declare no conflicts of interest.
References
1. Engeler D, Baranowski AP, Borovicka J, et al. Chronic pelvic pain. Uroweb. Available from: http://uroweb.org/guideline/chronic-pelvic-pain/.
2. Hanno P, Lin A, Nordling J, et al. Bladder pain syndrome committee of the international consultation on incontinence. Neurourol Urodyn. 2010;29(1):191–198. doi:10.1002/nau.20847
3. Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology. 2003;61(1):37–49. doi:10.1016/s0090-4295(02)02243-4
4. van de Merwe JP, Nordling J, Bouchelouche P, et al. Diagnostic criteria, classification, and nomenclature for painful bladder syndrome/interstitial cystitis: an ESSIC proposal. Eur Urol. 2008;53(1):60–67. doi:10.1016/j.eururo.2007.09.019
5. Gillenwater JY, Wein AJ Summary of the national institute of arthritis, diabetes, digestive and kidney diseases workshop on interstitial cystitis, national institutes of health, Bethesda, Maryland. J Urol. 1988;140(1):203–206. doi:10.1016/s0022-5347(17)41529-1
6. Hanno PM, Landis JR, Matthews-Cook Y, Kusek J, Nyberg L. The diagnosis of interstitial cystitis revisited: lessons learned from the national institutes of health interstitial cystitis database study. J Urol. 1999;161(2):553–557. doi:10.1016/s0022-5347(01)61948-7
7. Abrams P, Avery K, Gardener N, Donovan J, Advisory Board ICIQ. The international consultation on incontinence modular questionnaire. J Urol. 2006;175(3 Pt 1):1063–1066. doi:10.1016/S0022-5347(05)00348-4 discussion 1066.
8. Homma Y. Interstitial cystitis, bladder pain syndrome, hypersensitive bladder, and interstitial cystitis/bladder pain syndrome - clarification of definitions and relationships. Int J Urol. 2019;26(Suppl 1):20–24. doi:10.1111/iju.13970
9. Kivlin D, Lim C, Ross C, Whitmore K, Schellato T. The diagnostic and treatment patterns of urologists in the United States for interstitial cystitis/painful bladder syndrome. Urol Pract. 2016;3(4):309–314. doi:10.1016/j.urpr.2015.08.005
10. Colemeadow J, Sahai A, Malde S. Clinical management of bladder pain syndrome/interstitial cystitis: a review on current recommendations and emerging treatment options. Res Rep Urol. 2020;12:331–343. doi:10.2147/RRU.S238746
11. Juliebø-Jones P, Hjelle KM, Mohn J, et al. Management of Bladder Pain Syndrome (BPS): a practical guide. Adv Urol. 2022;2022:7149467. doi:10.1155/2022/7149467
12. Yeh HL, Jhang JF, Kuo YC, Kuo HC. Long-term outcome and symptom improvement in patients with interstitial cystitis/bladder pain syndrome with or without regular follow-up and treatment. Neurourol Urodyn. 2019;38(7):1985–1993. doi:10.1002/nau.24104
13. Chaiken DC, Blaivas JG, Blaivas ST. Behavioral therapy for the treatment of refractory interstitial cystitis. J Urol. 1993;149(6):1445–1448. doi:10.1016/s0022-5347(17)36411-x
14. Geissbuehler V, Forst S, Werner M, Schoenenberger CA, Berner R, Betschart C. Urotherapist activities in caring for patients with pelvic floor disorders: a prospective single-center observational study. Arch Gynecol Obstet. 2021;303(2):471–479. doi:10.1007/s00404-020-05810-0
15. Lukban J, Whitmore K, Kellogg-Spadt S, Bologna R, Lesher A, Fletcher E. The effect of manual physical therapy in patients diagnosed with interstitial cystitis, high-tone pelvic floor dysfunction, and sacroiliac dysfunction. Urology. 2001;57(6 Suppl 1):121–122. doi:10.1016/s0090-4295(01)01074-3
16. FitzGerald MP, Payne CK, Lukacz ES, et al. Randomized multicenter clinical trial of myofascial physical therapy in women with interstitial cystitis/painful bladder syndrome and pelvic floor tenderness. J Urol. 2012;187(6):2113–2118. doi:10.1016/j.juro.2012.01.123
17. European Association of Urology. (EAU) guidelines Available from: https://uroweb.org/guidelines.
18. Esperto F, Cacciatore L, Tedesco F, et al. Video consensus and radical prostatectomy: the way to chase the future? J Pers Med. 2023;13(6):1013. doi:10.3390/jpm13061013
19. Mantica G, Esperto F, Malinaric R, et al. Video-assisted informed consent in endoscopic urology: a randomized trial on ureteroscopy. J Endourol. 2024;38(9):916–920. doi:10.1089/end.2023.0674
20. Li SL, Lu GG, Zhao YB, et al. Research progress in treatment of chronic prostatitis/chronic pelvic pain syndrome with traditional Chinese medicine. Zhongguo Zhong Yao Za Zhi. 2024;49(10):2648–2653. doi:10.19540/j.cnki.cjcmm.20240211.501
21. Foster HE, Hanno PM, Nickel JC, et al. Effect of amitriptyline on symptoms in treatment naïve patients with interstitial cystitis/painful bladder syndrome. J Urol. 2010;183(5):1853–1858. doi:10.1016/j.juro.2009.12.106
22. van Ophoven A, Pokupic S, Heinecke A, Hertle L. A prospective, randomized, placebo controlled, double-blind study of amitriptyline for the treatment of interstitial cystitis. J Urol. 2004;172(2):533–536. doi:10.1097/01.ju.0000132388.54703.4d
23. Hwang P, Auclair B, Beechinor D, Diment M, Einarson TR. Efficacy of pentosan polysulfate in the treatment of interstitial cystitis: a meta-analysis. Urology. 1997;50(1):39–43. doi:10.1016/S0090-4295(97)00110-6
24. Nickel JC, Herschorn S, Whitmore KE, et al. Pentosan polysulfate sodium for treatment of interstitial cystitis/bladder pain syndrome: insights from a randomized, double-blind, placebo controlled study. J Urol. 2015;193(3):857–862. doi:10.1016/j.juro.2014.09.036
25. Al-Zahrani AA, Gajewski JB. Long-term efficacy and tolerability of pentosan polysulphate sodium in the treatment of bladder pain syndrome. Can Urol Assoc J. 2011;5(2):113–118. doi:10.5489/cuaj.10095
26. Pearce WA, Chen R, Jain N. Pigmentary maculopathy associated with chronic exposure to pentosan polysulfate sodium. Ophthalmology. 2018;125(11):1793–1802. doi:10.1016/j.ophtha.2018.04.026
27. Thilagarajah R, Witherow RO, Walker MM. Oral cimetidine gives effective symptom relief in painful bladder disease: a prospective, randomized, double-blind placebo-controlled trial. BJU Int. 2001;87(3):207–212. doi:10.1046/j.1464-410x.2001.02031.x
28. Sant GR, Propert KJ, Hanno PM, et al. A pilot clinical trial of oral pentosan polysulfate and oral hydroxyzine in patients with interstitial cystitis. J Urol. 2003;170(3):810–815. doi:10.1097/01.ju.0000083020.06212.3d
29. Forrest JB, Payne CK, Erickson DR. Cyclosporine A for refractory interstitial cystitis/bladder pain syndrome: experience of 3 tertiary centers. J Urol. 2012;188(4):1186–1191. doi:10.1016/j.juro.2012.06.023
30. Sairanen J, Tammela TLJ, Leppilahti M, et al. Cyclosporine A and pentosan polysulfate sodium for the treatment of interstitial cystitis: a randomized comparative study. J Urol. 2005;174(6):2235–2238. doi:10.1097/01.ju.0000181808.45786.84
31. Riedl CR, Engelhardt PF, Daha KL, Morakis N, Pflüger H. Hyaluronan treatment of interstitial cystitis/painful bladder syndrome. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(5):717–721. doi:10.1007/s00192-007-0515-5
32. Daha LK, Riedl CR, Lazar D, Hohlbrugger G, Pflüger H. Do cystometric findings predict the results of intravesical hyaluronic acid in women with interstitial cystitis? Eur Urol. 2005;47(3):393–397. doi:10.1016/j.eururo.2004.10.022 discussion 397.
33. Nickel JC, Egerdie B, Downey J, et al. A real-life multicentre clinical practice study to evaluate the efficacy and safety of intravesical chondroitin sulphate for the treatment of interstitial cystitis. BJU Int. 2009;103(1):56–60. doi:10.1111/j.1464-410X.2008.08028.x
34. Davis EL, El Khoudary SR, Talbott EO, Davis J, Regan LJ. Safety and efficacy of the use of intravesical and oral pentosan polysulfate sodium for interstitial cystitis: a randomized double-blind clinical trial. J Urol. 2008;179(1):177–185. doi:10.1016/j.juro.2007.08.170
35. Rawls WF, Cox L, Rovner ES. Dimethyl sulfoxide (DMSO) as intravesical therapy for interstitial cystitis/bladder pain syndrome: a review. Neurourol Urodyn. 2017;36(7):1677–1684. doi:10.1002/nau.23204
36. Perez-Marrero R, Emerson LE, Feltis JT. A controlled study of dimethyl sulfoxide in interstitial cystitis. J Urol. 1988;140(1):36–39. doi:10.1016/s0022-5347(17)41478-9
37. Lim YN, Dwyer P, Murray C, Karmakar D, Rosamilia A, Thomas E. Long-term outcomes of intravesical dimethyl sulfoxide/heparin/hydrocortisone therapy for interstitial cystitis/bladder pain syndrome. Int Urogynecol J. 2017;28(7):1085–1089. doi:10.1007/s00192-016-3232-0
38. Parsons CL. Successful downregulation of bladder sensory nerves with combination of heparin and alkalinized lidocaine in patients with interstitial cystitis. Urology. 2005;65(1):45–48. doi:10.1016/j.urology.2004.08.056
39. Kuo HC. Urodynamic results of intravesical heparin therapy for women with frequency urgency syndrome and interstitial cystitis. J Formos Med Assoc. 2001;100(5):309–314.
40. Nickel JC, Moldwin R, Lee S, Davis EL, Henry RA, Wyllie MG. Intravesical alkalinized lidocaine (PSD597) offers sustained relief from symptoms of interstitial cystitis and painful bladder syndrome. BJU Int. 2009;103(7):910–918. doi:10.1111/j.1464-410X.2008.08162.x
41. Cox A, Golda N, Nadeau G, et al. CUA guideline: diagnosis and treatment of interstitial cystitis/bladder pain syndrome. Can Urol Assoc J. 2016;10(5–6):E136–E155. doi:10.5489/cuaj.3786
42. Welk BK, Teichman JMH. Dyspareunia response in patients with interstitial cystitis treated with intravesical lidocaine, bicarbonate, and heparin. Urology. 2008;71(1):67–70. doi:10.1016/j.urology.2007.09.067
43. Lukban JC, Whitmore KE, Sant GR. Current management of interstitial cystitis. Urol Clin North Am. 2002;29(3):649–660. doi:10.1016/s0094-0143(02)00055-1
44. Olson LE, Dyer JE, Haq A, Ockrim J, Greenwell TJ. A systematic review of the literature on cystodistension in bladder pain syndrome. Int Urogynecol J. 2018;29(2):251–257. doi:10.1007/s00192-017-3355-y
45. Ottem DP, Teichman JMH. What is the value of cystoscopy with hydrodistension for interstitial cystitis? Urology. 2005;66(3):494–499. doi:10.1016/j.urology.2005.04.011
46. Ryu J, Pak S, Song M, Chun JY, Hong S, Choo MS. Elimination of hunner’s ulcers by fulguration in patients with interstitial cystitis: is it effective and long lasting? Korean J Urol. 2013;54(11):767–771. doi:10.4111/kju.2013.54.11.767
47. Ko KJ, Cho WJ, Lee YS, Choi J, Byun HJ, Lee KS. Comparison of the efficacy between transurethral coagulation and transurethral resection of hunner lesion in interstitial cystitis/bladder pain syndrome patients: a prospective randomized controlled trial. Eur Urol. 2020;77(5):644–651. doi:10.1016/j.eururo.2020.01.002
48. Kuo HC, Chancellor MB. Comparison of intravesical botulinum toxin type A injections plus hydrodistention with hydrodistention alone for the treatment of refractory interstitial cystitis/painful bladder syndrome. BJU Int. 2009;104(5):657–661. doi:10.1111/j.1464-410X.2009.08495.x
49. Kuo HC, Jiang YH, Tsai YC, Kuo YC. Intravesical botulinum toxin-A injections reduce bladder pain of interstitial cystitis/bladder pain syndrome refractory to conventional treatment - A prospective, multicenter, randomized, double-blind, placebo-controlled clinical trial. Neurourol Urodyn. 2016;35(5):609–614. doi:10.1002/nau.22760
50. Akiyama Y, Nomiya A, Niimi A, et al. Botulinum toxin type A injection for refractory interstitial cystitis: a randomized comparative study and predictors of treatment response. Int J Urol. 2015;22(9):835–841. doi:10.1111/iju.12833
51. Kuo H. Intravesical injections of autologous platelet‐rich plasma for the treatment of refractory interstitial cystitis. LUTS. 2023;15(6):210–215. doi:10.1111/luts.12504
52. Wu ZS, Luo HL, Chuang YC, Lee WC, Wang HJ, Chancellor MB. Platelet lysate therapy attenuates hypoxia induced apoptosis in human uroepithelial SV-HUC-1 cells through regulating the oxidative stress and mitochondrial-mediated intrinsic apoptotic pathway. Biomedicines. 2023;11(3):935. doi:10.3390/biomedicines11030935
53. Sharma N, Rekha K, Srinivasan JK. Efficacy of transcutaneous electrical nerve stimulation in the treatment of chronic pelvic pain. J Midlife Health. 2017;8(1):36–39. doi:10.4103/jmh.JMH_60_16
54. Wang J, Chen Y, Chen J, Zhang G, Wu P. Sacral neuromodulation for refractory bladder pain syndrome/interstitial cystitis: a global systematic review and meta-analysis. Sci Rep. 2017;7(1):11031. doi:10.1038/s41598-017-11062-x
55. Peters KM, Feber KM, Bennett RC. A prospective, single-blind, randomized crossover trial of sacral vs pudendal nerve stimulation for interstitial cystitis. BJU Int. 2007;100(4):835–839. doi:10.1111/j.1464-410X.2007.07082.x
56. Osman NI, Bratt DG, Downey AP, Esperto F, Inman RD, Chapple CR. A systematic review of surgical interventions for the treatment of bladder pain syndrome/interstitial cystitis. Eur Urol Focus. 2021;7(4):877–885. doi:10.1016/j.euf.2020.02.014
57. Moon A, Vasdev N, Thorpe AC. Continent urinary diversion. Indian J Urol. 2013;29(4):303–309. doi:10.4103/0970-1591.120111
58. Kamel MH, Gardner R, Tourchi A, et al. Pyocystis: a systematic review. Int Urol Nephrol. 2017;49(6):917–926. doi:10.1007/s11255-017-1562-6
59. Rössberger J, Fall M, Jonsson O, Peeker R. Long-term results of reconstructive surgery in patients with bladder pain syndrome/interstitial cystitis: subtyping is imperative. Urology. 2007;70(4):638–642. doi:10.1016/j.urology.2007.05.028
60. Chen H, Wang F, Chen W, et al. Efficacy of daily low-dose sildenafil for treating interstitial cystitis: results of a randomized, double-blind, placebo-controlled trial--treatment of interstitial cystitis/painful bladder syndrome with low-dose sildenafil. Urology. 2014;84(1):51–56. doi:10.1016/j.urology.2014.02.050
61. Bosch PC. A randomized, double-blind, placebo controlled trial of Adalimumab for interstitial cystitis/bladder pain syndrome. J Urol. 2014;191(1):77–82. doi:10.1016/j.juro.2013.06.038
62. Qu HC, Zhang W, Yan S, Liu YL, Wang P. Urinary nerve growth factor could be a biomarker for interstitial cystitis/painful bladder syndrome: a meta-analysis. PLoS One. 2014;9(9):e106321. doi:10.1371/journal.pone.0106321
63. Krenn H, Daha LK, Oczenski W, Fitzgerald RD. A case of cannabinoid rotation in a young woman with chronic cystitis. J Pain Symptom Manage. 2003;25(1):3–4. doi:10.1016/s0885-3924(02)00601-2
64. Peters KM, Hasenau D, Killinger KA, Chancellor MB, Anthony M, Kaufman J. Liposomal bladder instillations for IC/BPS: an open-label clinical evaluation. Int Urol Nephrol. 2014;46(12):2291–2295. doi:10.1007/s11255-014-0828-5
65. Rappaport YH, Zisman A, Jeshurun-Gutshtat M, et al. Safety and feasibility of intravesical instillation of botulinum toxin-a in hydrogel-based slow-release delivery system in patients with interstitial cystitis-bladder pain syndrome: a pilot study. Urology. 2018;114:60–65. doi:10.1016/j.urology.2017.12.028
66. Chuang YC, Kuo HC. A prospective, multicenter, double-blind, randomized trial of bladder instillation of liposome formulation onabotulinumtoxinA for interstitial cystitis/bladder pain syndrome. J Urol. 2017;198(2):376–382. doi:10.1016/j.juro.2017.02.021
67. Gallego-Vilar D, García-Fadrique G, Povo-Martin I, Salvador-Marin M, Gallego-Gomez J. Maintenance of the response to dimethyl sulfoxide treatment using hyperbaric oxygen in interstitial cystitis/painful bladder syndrome: a prospective, randomized, comparative study. Urol Int. 2013;90(4):411–416. doi:10.1159/000343697
68. Chuang YC, Meng E, Chancellor M, Kuo HC. Pain reduction realized with extracorporeal shock wave therapy for the treatment of symptoms associated with interstitial cystitis/bladder pain syndrome-A prospective, multicenter, randomized, double-blind, placebo-controlled study. Neurourol Urodyn. 2020;39(5):1505–1514. doi:10.1002/nau.24382
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

