Back to Journals » Journal of Inflammation Research » Volume 17
Calycosin Ameliorates Neuroinflammation via TLR4-Mediated Signal Following Cerebral Ischemia/Reperfusion Injury in vivo and in vitro
Authors Yang X, Pan Y, Cai L, Wang W, Zhai X, Zhang Y, Wu Q, Chen J, Zhang C, Wang Y
Received 29 May 2024
Accepted for publication 29 November 2024
Published 10 December 2024 Volume 2024:17 Pages 10711—10727
DOI https://doi.org/10.2147/JIR.S480262
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Adam Bachstetter
Xin Yang,1,* Yanjin Pan,1,* Le Cai,1,* Wenbo Wang,2 Xiaoya Zhai,1 Yuhui Zhang,1 Qiguang Wu,1 Jian Chen,1 Chong Zhang,3,4 Yong Wang1,4,5
1Key Laboratory of Tumor Immunology and Microenvironmental Regulation, Guilin Medical University, Guilin, 541199, People’s Republic of China; 2Department of Neurosurgery, Nanxishan Hospital of Guangxi Zhuang Autonomous Region, Guilin, 541002, People’s Republic of China; 3Department of Neurology, The Second Affiliated Hospital of Guilin Medical University, Guilin, 541199, People’s Republic of China; 4Guangxi Medical and Health Key Cultivation Discipline Construction Project, Guilin, 541199, People’s Republic of China; 5Department of Physiology, Guilin Medical University, Guilin, 541199, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yong Wang, Key Laboratory of Tumor Immunology and Microenvironmental Regulation, Guilin Medical University, No. 1 Zhiyuan Road, Guilin, 541199, People’s Republic of China, Email [email protected] Chong Zhang, Department of Neurology, The Second Affiliated Hospital of Guilin Medical University, No. 212 Renmin Road, Guilin, 541199, People’s Republic of China, Email [email protected]
Background: Cerebral ischemia-reperfusion injury (CIRI) is a key pathophysiological process that leads to stroke mortality, with TLR4-mediated inflammation playing a crucial role. Our previous research highlighted the neuroprotective effects of the phytoestrogen calycosin on CIRI, although the precise mechanism remains unclear. This study aimed to explore the effects of calycosin on the HMGB1/TLR4/NF-κB signaling pathway in rat models of CIRI, both in vivo and in vitro.
Methods: In vivo, a rat CIRI model was established using middle cerebral artery occlusion (MCAO), inducing ischemia for 1.5 h followed by 24 h of reperfusion. Calycosin was administered intraperitoneally 1 h after ischemia. Neurological deficits and brain infarct volumes were evaluated. Histological changes and key protein expressions around the ischemic penumbra were assessed by H&E staining and immunofluorescence. In vitro, primary neurons and PC12 cells were subjected to oxygen-glucose deprivation/reoxygenation (OGD/R) to mimic CIRI. Cell viability was measured using a CCK8 assay, and alterations in HMGB1/TLR4/NF-κB pathway components were analyzed using qRT-PCR, Western blotting, and ELISA.
Results: In the MCAO rat model, calycosin significantly reduced neurological deficits and infarct sizes, and improved brain tissue damage following reperfusion. Similarly, in the OGD/R model, calycosin attenuated neuronal injury in PC12 cells and in primary neurons. Additionally, calycosin inhibited LPS-induced activation of the HMGB1/TLR4/NF-κB signaling pathway in PC12 cells. Both in vitro and in vivo studies have shown that calycosin effectively downregulates HMGB1 and TLR4 expression, decreases NF-κB and IκB phosphorylation, and reduces the secretion of inflammatory cytokines such as IL-6 and IL-18.
Conclusion: These findings suggest that calycosin mitigates cerebral ischemia-reperfusion injury and neuroinflammation by inhibiting the HMGB1/TLR4/NF-κB signaling pathway, thereby providing neuroprotection.
Keywords: calycosin, cerebral ischemia/reperfusion injury, TLR4-mediated signaling, neuroinflammation, middle cerebral artery occlusion, oxygen-glucose deprivation/reoxygenation
Graphical Abstract:

Introduction
Stroke is the leading cause of disability and mortality worldwide. The incidence of stroke is rising in developing countries, constituting approximately two-thirds of all cases, highlighting the huge burden on families and societies in these regions.1 Stroke is primarily categorized into ischemic and hemorrhagic types. Globally, ischemic stroke constitutes approximately 70% of all stroke cases, whereas hemorrhagic stroke accounts for the remaining 30%.2–4 In terms of sex differences, women generally have a lower risk of stroke than men, although this risk varies with age and stroke type.5 Ischemic stroke, the most common form of stroke, is mainly caused by blockage of blood vessels in the brain. The principal approach for treating ischemic stroke is timely thrombolysis to restore the blood and oxygen supply to the occluded vessels. However, delayed vascular recanalization may induce detrimental effects on the brain tissue due to calcium overload, generation of free radicals, and infiltration of inflammatory cells.6,7 This phenomenon is referred to as cerebral ischemia/reperfusion (I/R) injury. Consequently, prompt intervention is imperative for patients with ischemic stroke, with a limited time window of approximately 4 hours for thrombolytic treatment, as failure to do so may lead to secondary injury from cerebral ischemia/reperfusion.8
High Mobility Group Box 1 (HMGB1) is a nuclear DNA-binding protein involved in regulating gene expression, DNA repair, and chromatin structure. Additionally, HMGB1 can be released into the extracellular space as a damage-associated molecular pattern (DAMP) molecule, triggering inflammation and immune responses.9,10 TLR4, a member of the Toll-like receptor (TLR) family of protein receptors, can recognize specific molecular patterns associated with a variety of endogenous and exogenous ligands, including HMGB1, to control the immune system and defense mechanisms.11,12 Binding of HMGB1 to TLR4 promotes the transcriptional activation of NF-κB, which subsequently induces the expression of inflammatory factors.13 The pivotal role of the HMGB1/TLR4/NF-κB signaling pathway in inflammatory and immune responses following cerebral ischemia/reperfusion injury has become increasingly well-understood in recent years.14–16 Therefore, HMGB1/TLR4/NF-κB pathway may be a therapeutic target for ischemic stroke.
There has been an increasing interest in natural products with anti-inflammatory and neuroprotective properties to alleviate cerebral I/R injury.17–19 Phytoestrogens are nonsteroidal polyphenolic natural products found in certain medicinal plants that structurally resemble 17-β-estradiol, a primary member of the estrogen family of female sex hormones. These compounds can bind to estrogen receptors, exerting biological effects via genomic as well as non-genomic mechanisms.20 Research has indicated that both estrogens and phytoestrogens may positively influence brain function.21 The administration of phytoestrogens or estrogen replacement therapy has been shown to enhance cognitive function, delay the progression of neurodegenerative diseases, such as Alzheimer’s disease and Parkinson’s disease, and provide neuroprotection.22–24 Calycosin is a bioactive isoflavone phytoestrogen extracted from the traditional Chinese medicine Astragalus membranaceus.25 Owing to its molecular structure similar to estrogen, calycosin has estrogenic and anti-estrogenic effects, and recent studies have shown that it also has anti-inflammatory, antioxidant, and anticancer properties.26,27 In our previous studies, calycosin prevented ischemic brain injury in rats by inhibiting neuronal autophagy, apoptosis, and inflammatory processes.28,29 However, the therapeutic potential of calycosin and its ability to regulate HMGB1/TLR4/NF-κB signaling pathway activity in cerebral ischemia/reperfusion injury remain unclear.
In this study, we examined the effects of therapeutic administration of calycosin against cerebral ischemia/reperfusion injury in rats, and further investigated its neuroprotective mechanisms based on the HMGB1/TLR4/NF-κB signaling pathway.
Materials and Methods
Animals, Cells, and Reagents
Adult Sprague-Dawley (SD) rats (male rats weighing 250–280 g, female rats weighing 230–260 g; license number SCXK, Hunan, 2019–004) were purchased from Hunan SJA Laboratory Animal Co., Ltd. (Changsha, China). At the SPF-level experimental area of the Experimental Animal Center of Guilin Medical University, rats were fed a standard chow diet and sterile water every day at room temperature (22±2 °C) under a 12h light / 12h dark cycle. Follow-up experiments were carried out after one week of adaptive feeding. The experimental protocol was reviewed and approved by the Laboratory Animal Welfare and Ethics Committee of Guilin Medical University (approval number: GLMC-IACUC-202203293) and conducted in accordance with the Institutional Animal Care and Use Committee (IACUC) Handbook of Guilin Medical University.
The PC12 cells (catalog number: SCSP-517) were purchased from the Cell Bank of the Typical Culture Preservation Committee of the Chinese Academy of Sciences (Shanghai, China).
For cell experiments, calycosin (Figure 1A, C16H12O5, purity>98.0%, Sigma-Aldrich Company, MO, USA) was dissolved in dimethyl sulfoxide (DMSO) and freshly diluted to the target concentration in the culture medium. For animal experiments, calycosin (purity>90.0%, Biopurify Phytochemicals Ltd., Chengdu, China) was dissolved in DMSO and diluted with saline to the target concentration. The final DMSO concentration in the culture medium was less than 0.5%. Lipopolysaccharide (LPS, product number: L4391, Escherichia coli 0111: B4) was obtained from Sigma-Aldrich. The Enzyme-linked immunosorbent assay (ELISA) kits were purchased from Fankew (Shanghai, China). Specific primers were provided by Gene Create (Wuhan, China).
 |
Figure 1 The chemical structure of calycosin (A) and the schematic diagram of the in vivo experimental process (B). |
Ischemic Brain Injury Model
The MCAO (middle cerebral artery occlusion) model in rats was established using the modified Longa’s method.30 Briefly, male SD rats were anesthetized with an intraperitoneal injection of 10% chloral hydrate (350 mg/kg). After anesthesia, the rats were fixed in the supine position, and their hair was removed from the ventral neck using a hair shaver. Subsequently, a median cervical incision is made above the scapular line. The right common, external, and internal carotid arteries were then exposed and isolated. The distal end of the external carotid artery was ligated and the internal carotid artery was clamped. A small incision was made at the common carotid artery, and a thread plug (RWD Life Science Co., Ltd., Shenzhen, China) with a diameter of 0.36 mm at the top was inserted into the internal carotid artery. Thread insertion was stopped if the sensation of obstruction was felt 1.8–2.0 cm away from the common carotid artery bifurcation, and the common carotid artery was ligated to fix the thread plug. After 1.5 h of ischemia, the thread plug was removed, and reperfusion was achieved. No plug operation was performed in the sham operation group and the other operation procedures were the same as those in the MCAO group (Figure 1B). After the operation, the rats were kept warm in the light until they were fully awake.
Animal Experimental Protocols
SD rats were randomly assigned to the sham operation, MCAO, or calycosin administration groups. The calycosin administration groups were further divided into low (5 mg/kg), medium (10 mg/kg), and high (20 mg/kg) dosage groups. After 1 h of local cerebral ischemia, each administration group was intraperitoneally injected with the corresponding dose of calycosin, whereas the sham operation and MCAO groups were administered saline according to their body weight.
Neurobehavioral Evaluation and Infarct Measurement (TTC Staining)
After 1.5 h of ischemia and 24 h of reperfusion, the neurological behavior of the rats was evaluated according to Longa’s method.30 Scores were based on the following criteria: 0-point, no neurological deficit or normal; 1-point, when the tail was suspended, the rat could not fully extend its left forepaw; 2-point, when crawling, the rat turned to the left side; 3-point, when crawling, the body of the rat involuntarily fell to the left side; 4-point, the rat was unable to walk spontaneously or was accompanied by impaired consciousness.
After 24 h of cerebral reperfusion, the whole brains of the rats in each group were quickly isolated and frozen at −20 °C for 15 min. Subsequently, the brain was sliced into 2-mm thick sections on a coronal brain mold for a total of six pieces. The slices were stained with a 1% TTC (2,3,5-triphenyltetrazolium chloride) solution (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) at 37 °C for 15 min and then fixed with 4% paraformaldehyde solution overnight. The stained slices were photographed, and the infarct volumes were analyzed using ImageJ software 6.0. There was no infarction in the red area, but there was an infarction in the white area. The infarction size was calculated using the following formula: percentage of cerebral infarct volume = (total infarction area of brain section/total area of brain section) ×100%.
H&E Staining
Rats were anesthetized after 24 h of cerebral reperfusion. Subsequently, their brains were rapidly isolated, fixed in a 4% paraformaldehyde solution for 48 h, and embedded in paraffin. Each brain sample was coronally sectioned at a thickness of 4µm, using a rotary microtome. Paraffin sections were dewaxed with xylene, dehydrated in gradient concentrations of ethanol (75%, 85%, 95%, and 100%), and stained with hematoxylin and eosin (H&E) solution (Zhongshan Golden Bridge Biotechnology, Beijing, China).31 The sections were photographed under a light microscope to observe brain tissue damage.
Immunofluorescence Staining
The paraffin-embedded brain samples were dewaxed and rehydrated. After rinsing with PBS, the slices were blocked with 5% BSA for 15 min and then incubated with primary rabbit antibodies against rat overnight at 4°C. The primary antibodies used were anti-HMGB1 (1:100; Abcam, Cambridge, UK), anti-IκBα (1:100; Abcam), and anti-p-IκBα (1:100; Abcam). Subsequently, the primary antibody was washed with PBST and incubated with the corresponding Alexa Fluor 488-labeled goat anti-rabbit IgG (H+L) secondary antibody (1:200) for 1 h at 37°C in the dark. After rinsing with PBS for 30 min, the slides were sealed with DAPI (4’,6-diamidino-2-phenylindole)-containing mounting medium to visualize the nuclei. Finally, the slices were imaged using a fluorescence microscope (Leica, Germany), and the fluorescence intensity was analyzed.32
Cell Culture and Oxygen-Glucose Deprivation/Reoxygenation (OGD/R) Treatment
Primary cortical neuronal cultures were prepared as previously described in our article.33 Briefly, timed pregnant SD rats were sacrificed on day 17–19 of gestation. Subsequently, fetal rats were removed from the pregnant rats under sterile conditions, and the embryonic brains were isolated and placed in Petri dishes containing DMEM/F12 complete medium. After stripping the meninges and blood vessels of the brain, the cerebral cortex was isolated, and a suitable quantity of papain digestion solution (2 mg/mL) and DNA ligase solution (1 mg/mL) were introduced and allowed to digest for 20 min. The digestion process was terminated using DMEM/F12 medium containing 10% fetal bovine serum at 37 °C. Afterwards, the cells were seeded onto dishes or 96-well plates that had been pre-coated with poly-L-lysine (PLL), and cultured in a 5% CO2 incubator. Following a 24-hour period, the medium was replaced with maintenance medium, specifically Neurobasal, supplemented with 1% B27 and 1% glutamine. Subsequent experiments were conducted once the neuronal cells reached maturation.
PC12 cells were seeded in 1640 complete medium supplemented with 10% fetal bovine serum, and cultured routinely at 37 °C in a 5% CO2 incubator.34 Cells in the logarithmic growth phase were harvested for subsequent experiments.
According to the experimental design, primary neurons or PC12 cells were divided into control group, OGD/R group, and OGD/R combined with calycosin pretreatment groups. Except for the control group, all groups were treated with OGD/R for an appropriate time to simulate the cerebral ischemia/reperfusion model in vitro. After substituting glucose-containing EBSS with a glucose-free EBSS, the cells were placed in a portable hypoxia chamber (Billups-Rothenberg, CA, USA) with 95% N2 and 5% CO2 at 37 °C for an appropriate time. Following OGD injury, the corresponding complete medium was substituted, and the cells were again cultured for 24 h at 37°C in a 5% CO2 incubator, thereby achieving full recovery of oxygen and glucose.33 The OGD/R combined with calycosin groups were pretreated with the corresponding doses of calycosin for 24 h before OGD/R.
Cell Viability Assay
Calycosin was dissolved DMSO to prepare a stock solution at a concentration of 0.1M. Similarly, LPS was dissolved in sterile saline to prepare a stock solution at a concentration of 0.1 mg/mL. The stock solutions were maintained at 4 °C and diluted to working concentrations using the corresponding medium as required in the experiment. PC12 cells or primary neurons in the logarithmic growth phase were seeded into 96-well plates and subjected to OGD treatment for the indicated times. To induce inflammation, PC12 cells were starved overnight (in 1% FBS) and treated with LPS at different concentrations for 12 h. Cell viability was assessed using the cell counting kit 8 (CCK8; CK04, Dojindo, Kumamoto, Japan) in accordance with the manufacturer’s guidelines, thereby establishing the duration of OGD for the cells.33 The OGD durations of PC12 cells and primary nerve cells were determined to be 4 and 2 h, respectively, followed by pretreatment with varying doses of calycosin or LPS for 24 h. After 24 h of glucose and reoxygenation incubation of both cell types, the wells were added to CCK8 solution and then were placed in a 5% CO2 cell incubator at 37°C for 4 h. A microplate reader (BioTek Instruments, Winooski, USA) was used to measure optical density (OD) at 450 nm.
Enzyme-Linked Immunosorbent Assay (ELISA)
IL-18 and HMGB1 levels in the culture supernatants were determined by ELISA.35 Following the collection of the supernatants, the assays were performed strictly according to the instructions of the ELISA kit (Fankew). First, 100 μL of the enzyme-labeling reagent was added to each well of the microplate. Next, the microplates were incubated at a temperature of 37°C. After incubation, washing solution, chromogen agent, and termination solution were sequentially added. Finally, the OD density of the samples was measured at 450 nm using a microplate reader (BioTek Instruments, Winooski, USA).
Quantitative Real-Time PCR (qRT-PCR)
Total RNA was extracted from the rat brain tissue and cells according to the instructions of the Total RNA Kit (Tiangen Biotech, Beijing, China).33 Following the assessment of RNA concentration, RNA was reverse-transcribed into cDNA using a reverse transcription kit (MonScriptTM RTIII All-in-One Mix with dsDNase, MR05101S; Monad Biotech Co., Ltd., Shanghai, China), and then PCR amplification reactions were performed using an ABI PRISM 7500 Sequence Detector System (Applied Biosystems, Foster City, USA). The qRT-PCR reaction system consisted of a total of 20 μL, which contained 10 μL SYBR Green qPCR mixture, 0.4 μL upstream primer, 0.4 μL downstream primer, 8.2 μL enzyme-free water and 1 μL template cDNA.
The reaction conditions were as follows: one cycle of pre-denaturation at 95 °C for 30s, 40 cycles of denaturation at 95 °C for 10s, annealing at 55 ° C for 10s, and extension at 72 ° C for 30s. Each tested gene was confirmed to show a single product based on melting curve analysis using the instrument default acquisition procedure. The relative expression of the target genes was calculated using the 2−∆∆CT method and normalized to that of a housekeeping gene (β-actin or GAPDH). The primer sequences used are listed in Table 1.
 |
Table 1 The Specific Primer Sequences Used for qRT-PCR |
Western Blot Analysis
Following a 24-hour period of reperfusion, brain tissue samples were obtained from the ischemic penumbra of the rats and homogenized using a precooled RIPA lysate to prepare tissue protein samples.33 After 24 h of reoxygenation, the cells were collected, washed with PBS, and treated with precooled RIPA lysate to prepare cell protein samples. The BCA method was used to quantify the total protein concentrations. Tissue proteins were separated on 10% polyacrylamide gels and cellular proteins were separated on 12% polyacrylamide gels at 80 V. The separated proteins were then transferred onto PVDF membranes for 90 min at 260 mA. Following this, the membranes were blocked with 5% skim milk at room temperature for 2 h. Subsequently, the membranes were incubated with different primary antibodies at 4°C overnight. The following primary antibodies were used: mouse anti-rat TLR4 polyclonal antibody (1:1000), rabbit anti-rat NF-κB monoclonal antibody (1:1000), rabbit anti-rat p-NF-κB monoclonal antibody (1:1000), and rabbit anti-rat IL-6 polyclonal antibody (1:500). Subsequently, horseradish peroxidase (HRP)-labeled secondary antibodies were added and incubated at room temperature for 1.5 h. The ECL reagent was used to detect the protein bands bound by the antibody, which were visualized using a gel imager. Finally, the gray values of the protein bands were analyzed using the ImageJ software. Protein levels were expressed as the ratio of the band gray value of the target protein to that of the internal reference.
Statistical Analysis
Data were analyzed using SPSS 27 statistics, and the measurement data were expressed as the mean ± SD. Student’s t-test was used to compare two independent samples. One-way analysis of variance (ANOVA) followed by the least significant difference (LSD) post-test were used for comparisons between multiple groups. Non-normally distributed data were analyzed using non-parametric tests. P < 0.05 was considered statistically significant.
Results
Calycosin Improved the Neurological Damage in the MCAO Model Rats
To ascertain the potential of calycosin to facilitate the restoration of neurological function in cerebral ischemia/reperfusion rats, it was administered following the MCAO procedure, and neurological function scores were subsequently assessed at 1.5 h of ischemia and 24 h of reperfusion.
The results showed that the Longa neurological deficit scores in the model group were significantly higher than those in the sham-operated group after 1.5 h of regional cerebral ischemia in rats (P<0.001). Treatment with calycosin resulted in a gradual decrease in neurological scores as the calycosin concentration increased, and the high-dose group (20 mg/kg) scored significantly lower than the model group (P<0.05) (Figure 2A).
The neurological deficit score at 24 h of reperfusion in the model group was significantly higher than those in the sham-operated group (P<0.001). However, the scores in the calycosin treatment groups decreased in a dose-dependent manner, and the scores in the middle (10 mg/kg) and high (20 mg/kg) dose groups were significantly lower than those in the model group (P<0.05, P<0.01, respectively) (Figure 2B).
Calycosin Reduced Cerebral Infarction Volumes in the MCAO Model Rats
TTC staining was used to determine the degree of brain damage and investigate the neuroprotective effects of calycosin in MCAO model rats. Figure 3A illustrates that the sham-operated group exhibited no signs of cerebral infarction, whereas the MCAO model group displayed distinct white infarct areas. Notably, treatment with calycosin resulted in a dose-dependent reduction of these infarct areas. As depicted in Figure 3B, quantitative analysis revealed that the infarct volume in the MCAO model group was 29.4%. Calycosin treatment significantly decreased the infarct volume, with reductions to 14%, 10%, and 4% in the low-dose (5 mg/kg), medium-dose (10 mg/kg), and high-dose (20 mg/kg) groups, respectively, indicating a pronounced neuroprotective effect.
Calycosin Alleviated Brain Histopathological Damage in the MCAO Model Rats
Hematoxylin and eosin (H&E) staining was used to examine the pathological changes in the rat brain tissue in each group. Figure 4A presents the H&E staining results for the cortex. In the sham-operated group, the neuronal cell structure and morphology remained intact and regularly arranged. Conversely, the model group displayed cortical cells that underwent liquefaction necrosis due to ischemia and hypoxia. These cells exhibited a disordered arrangement, loss of cell microstructure, light coloration, and shrinkage of the nucleus upon dark staining. In the calycosin treatment groups, a dose-dependent improvement was observed in the reduction of nerve cells, degree of swelling, and disintegration of nerve fibers compared to the model group. Figure 4B indicates that the H&E staining results of the striatum were similar to those observed in the cortex. In the model group, the striatum areas exhibited a scarcity of neural cells, along with vacuolar changes and evident disintegration of the nerve fibers. Treatment with calycosin effectively mitigated structural damage in the striatal region caused by ischemia and hypoxia. Importantly, the high-dose calycosin treatment group showed the most notable improvements in the pathological alterations of neurons in both the cortex and the striatum. This was evidenced by an increased number of surviving cells surrounding the neurons, relatively intact cell structures, and a substantial reduction in vacuolar changes.
Calycosin Inhibited Inflammatory Response Induced by HMGB1/TLR4/NF-κB Signaling Pathway in the MCAO Model Rats
Prior studies have demonstrated the significance of the inflammatory response triggered by the HMGB1/TLR4/NF-κB signaling pathway in pathological damage caused by cerebral ischemia. To ascertain whether calycosin can ameliorate inflammatory damage from cerebral ischemia/reperfusion by modulating the HMGB1/TLR4/NF-κB signaling pathway in MCAO model rats, we examined the expression of molecules involved in this signaling pathway.
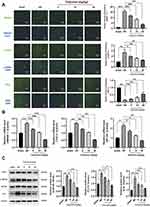
Immunofluorescence staining demonstrated a significant increase in HMGB1 fluorescence intensity in the MCAO model group compared to that in the sham-operated group, whereas a dose-dependent decrease in HMGB1 fluorescence intensity was observed in the calycosin treatment groups. Immunofluorescence staining of IκBα and IκBα revealed that in contrast to the sham group, the model group exhibited a significant increase in the fluorescence intensity of p-IκBα, accompanied by a significant decrease in the fluorescence intensity of IκBα. Conversely, the calycosin treatment groups displayed a decline in p-IκBα fluorescence intensity, whereas the fluorescence intensity of IκBα significantly increased in a dose-dependent manner (Figure 5A).
The effects of calycosin on the expression levels of HMGB1, TLR4, NF-κB, and IL-6 were evaluated using qRT-PCR and Western blotting. As shown in Figure 5B, the mRNA levels of HMGB1, TLR4, and NF-κB were significantly higher in the MCAO model group than those in the sham-operated group. However, the administration of calycosin resulted in dose-dependent suppression of these mRNA expressions. From the results of the Western blot analysis (Figure 5C), the protein expression of TLR4 and IL-6, as well as the phosphorylation level of NF-κB in the MCAO model group, were significantly higher than those in the sham-operated group. Treatment with calycosin significantly reduced the protein expression of TLR4 and IL-6, as well as the phosphorylation level of NF-κB. These results suggest that calycosin may alleviate the inflammatory response by inhibiting activation of the HMGB1/TLR4/NF-κB signaling pathway in cerebral ischemia/reperfusion injury.
Calycosin Attenuates OGD/R-Induced Neuronal Injury in PC12 Cells and Primary Neurons
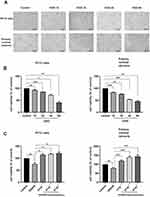
To further assess the neuroprotective properties of calycosin against cerebral ischemia/reperfusion injury, a series of in vitro experiments was conducted using PC12 cells and primary cortical neurons. The morphology and proliferation of both cell types were examined after OGD for varying durations of 1h, 2h, 4h, and 8h. As the duration of OGD increased, there was a significant decrease in the cell count, accompanied by a gradual rounding of cell bodies and a substantial reduction or complete disappearance of cellular protrusions (Figure 6A).
The CCK8 assay revealed that, compared to the control group, the viability of PC12 cells decreased by 7.1%, 14.8%, 27.9%, and 58.8% after 1, 2, 4, and 8 h of OGD, respectively. Similarly, the viability of the primary neurons decreased by 16.1%, 23.7%, 46.3%, and 54.0% under the same OGD conditions (Figure 6B). These results demonstrated a clear time-dependent relationship. Consequently, to avoid irreversible severe cellular damage caused by excessive hypoxia, in the subsequent experiments in this study, the OGD duration was determined to be 4 h for PC12 cells and 2 h for primary neurons, followed by a 24 h treatment of oxygen-glucose restoration treatment to establish the OGD/R model.
Using the CCK8 assay, we determined that calycosin significantly enhanced the viability of both PC12 cells and primary cortical neurons within a concentration range of 10-7-10−6 mol/L. Notably, the highest level of cell viability was observed at a concentration of approximately 10−6 mol/L (data not shown). For subsequent experiments, calycosin was administered at concentrations of 2×10−7, 4×10−7, and 8×10−7 mol/L to PC12 cells, representing low, medium, and high doses, respectively. Similarly, calycosin was administered at low, medium, and high doses of 1×10−7, 2×10−7, and 4×10−7 mol/L, respectively, in primary cortical neurons.
According to the results of the CCK8 assay shown in Figure 6C, exposure to OGD/R led to a notable decline in the viability of both PC12 cells and primary cortical neurons. However, the administration of calycosin resulted in a dose-dependent enhancement in the viability of these two cell types compared to the OGD/R model group. These results indicated that calycosin possesses neuroprotective properties in PC12 cells and primary cortical neurons following OGD/R treatment.
Calycosin Alleviates the Inflammatory Injury After OGD/R in PC12 Cells and Primary Neurons by Inhibiting the HMGB1/TLR4/NF-κB Pathway
To investigate the impact of calycosin on the inflammatory damage induced by the HMGB1/TLR4/NF-κB signaling pathway following OGD/R, the levels of HMGB1 and IL-18 in the cellular supernatant were assessed using ELISA. Additionally, the expression levels of molecules associated with the HMGB1/TLR4/NF-κB signaling pathway were determined using qRT-PCR and Western blot analysis.
The ELISA results (Figure 7A) demonstrated a significant increase in the levels of HMGB1 and the inflammatory factor IL-18 following OGD/R, and the administration of calycosin reduced their secretion. The qRT-PCR results, as depicted in Figure 7B, indicated a significantly higher mRNA expression of HMGB1 and TLR4 in the OGD/R group than in the control group, with a subsequent decrease in expression levels observed after calycosin administration compared to the OGD/R group. Furthermore, Western blotting results (Figure 7C) revealed an increase in both the expression level of TLR4 protein and the phosphorylation level of NF-κB in the OGD/R group when compared to the control group. However, the groups treated with calycosin exhibited a dose-dependent decrease in TLR4 protein expression and NF-κB phosphorylation. These results suggest that calycosin may inhibit the activation of the HMGB1/TLR4/NF-κB signaling pathway to ameliorate the inflammatory damage to nerve cells caused by OGD/R.
Calycosin Prevents LPS-Induced Activation of HMGB1/TLR4/NF-κB Signaling Pathway in PC12 Cells
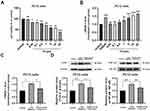
To confirm the LPS-induced excretion of HMGB1 from PC12 cells in vitro, ELISA was used to measure the release of HMGB1 from the supernatant of PC12 cells treated with various concentrations of LPS (0.05, 0.1, 0.5, 1, 5, 10, and 20 μg/mL) for 24 h. The results depicted in Figure 8A and B demonstrate a gradual decline in cell activity and a gradual increase in the release of HMGB1 from PC12 cells with increasing LPS concentration. Notably, when the LPS concentration was 5 μg/mL, the extracellular secretion of HMGB1 closely resembled that observed in the OGD/R group, with no significant differences between the two groups. Consequently, 5 μg/mL LPS was selected to induce the release of HMGB1 in PC12 cells for 24 h.
To further investigate the neuroprotective mechanisms of calycosin, we used ELISA and Western blotting to assess the expression of HMGB1, TLR4, and NF-κB in PC12 cells following LPS stimulation and calycosin pretreatment. As shown in Figure 8C and D, calycosin effectively mitigated the LPS-induced release of HMGB1. Additionally, calycosin pretreatment significantly attenuated the LPS-induced upregulation of TLR4 protein and NF-κB phosphorylation. Taken together, these findings indicate that calycosin inhibits LPS-induced activation of the HMGB1/TLR4/NF-κB signaling pathway in PC12 cells.
Discussion
Neuroinflammation is a critical pathophysiological process in ischemic stroke. Following cerebral ischemia, glial cells become activated, leading to the infiltration of peripheral immune cells.36 This cascade results in extensive neuronal cell death and the release of numerous inflammatory mediators. The neuroinflammatory response not only exacerbates ischemia-reperfusion injury in the brain but also affects neural tissue regeneration.37 Understanding the key molecular mechanisms involved in the inflammatory response to brain ischemia and rescuing neurons at risk of death are essential for the development of novel therapeutics for the prevention and treatment of ischemic stroke.38,39 In the present study, we investigated the therapeutic potential of calycosin for ischemic stroke. Calycosin decreased neurological deficit scores and infarct size, and significantly improved brain tissue damage caused by ischemia/reperfusion in the MCAO rat model. In vitro and in vivo experiments showed that calycosin downregulated the expression of HMGB1 and TLR4; reduced the phosphorylation of NF-κB and IκB; and decreased the secretion of IL-6, IL-18, and other inflammatory factors. These results suggest that calycosin may play a neuroprotective role in cerebral ischemia/reperfusion injury by inhibiting the activation of the HMGB1/TLR4/NF-κB signaling pathway and alleviating the inflammatory response.
Cerebral ischemia/reperfusion injury frequently coincides with neuroinflammation, resulting in the disturbance of brain homeostasis.40,41 The onset of acute cerebral ischemia not only induces the activation of inflammatory and immune cells within the central nervous system (CNS), but also facilitates the infiltration and accumulation of inflammatory and immune cells from the peripheral circulation system, thereby releasing a significant quantity of proinflammatory cytokines.37,42,43 Consequently, the regulation of the neuroinflammatory response is a critical factor in mitigating the effects of cerebral ischemia/reperfusion injury.
Numerous studies have demonstrated that HMGB1, functioning as a DAMP, can be passively released by necrotic neurons, or actively secreted by immunocompetent cells such as microglia and macrophages.44–47 The active involvement of HMGB1 in the inflammatory injury process of cerebral ischemia/reperfusion has been well documented.48,49 Furthermore, TLR4, a crucial pattern recognition receptor (PRR) for HMGB1, plays a pivotal role in neuroinflammation.50 Following ischemia/reperfusion injury, TLR4 expression increases in cerebral cortical neurons, and TLR4 inhibition or functional deficits have been shown to effectively mitigate the inflammatory injury induced by cerebral ischemia.50–54 NF-κB is an important nuclear transcription factor that regulates the expression of genes encoding proinflammatory cytokines. The interaction between HMGB1 and TLR4 promotes dissociation of the NF-κB/IκB heterodimer in the cytoplasm, leading to nuclear translocation of NF-κB, thus enhancing the expression of downstream proinflammatory cytokines.55 These cytokines possess the ability to not only attract additional immune cells from the peripheral circulation system to the CNS, thereby exacerbating the progression of neuroinflammation but also prompt microglia and macrophages to actively secrete HMGB1.56 It has been reported that NF-κB plays an essential role in the acute phase of cerebral ischemia, as evidenced by its rapid increase in transcriptional activity following cerebral ischemia.57 Notably, studies involving NF-κB (p50) knockout mice or treatment with an NF-κB inhibitor have shown a reduction in ischemic damage after a permanent focal insult.58 In our study, the expression of HMGB1 and TLR4 was significantly increased in the MCAO rat brain tissue and OGD/R primary neurons. Additionally, there was an increase in the phosphorylation activity of NF-κB and I-κB, as well as the release of the inflammatory cytokines IL-6 and IL-18. These findings suggest that the HMGB1/TLR4/NF-κB signaling pathway is activated in cerebral ischemia/reperfusion injury.
Calycosin is a natural compound with various pharmacological activities, which have been extensively investigated. Numerous reports have confirmed its potential in the prevention and treatment of tumors, cardiovascular and cerebrovascular diseases, diabetes, and other diseases.26,59 Notably, the protective effect of calycosin against ischemic stroke has received much attention, with its neuroprotective mechanisms being multifaceted. It has been reported that calycosin can alleviate neurological deficits and brain infarction in rats following cerebral ischemia/reperfusion, through its antioxidant properties.60 Guo et al demonstrated that calycosin exhibits neuroprotective effects in MCAO rats and OGD/R primary neurons by upregulating TRPC6 expression and CREB phosphorylation as well as by inhibiting calpain activation.61 Additionally, a recent study by Xu et al revealed that calycosin inhibits autophagy by regulating the STAT3/FOXO3a signaling pathway, thereby ameliorating cerebral ischemia/reperfusion injury.62 Furthermore, Liu et al reported that calycosin exhibited a protective effect against cerebral ischemic damage by suppressing ACSL4-dependent ferroptosis.63 Our recent study also indicated that calycosin inhibits activation of the HMGB1/TLR4/NF-κB signaling pathway and proinflammatory depolarization in microglia subjected to OGD/R.35 In this study, we further demonstrated that the neuroprotective effect of calycosin on cerebral ischemia/reperfusion injury is associated with the suppression of the inflammatory response mediated by the HMGB1/TLR4/NF-κB signaling pathway, both in vivo and at the neuronal level.
The present study explored the effects and potential mechanisms of calycosin on cerebral ischemia-reperfusion injury and acknowledged several limitations. First, although we propose that calycosin may exert neuroprotective effects by inhibiting the HMGB1/TLR4/NF-κB signaling pathway, evidence supporting this mechanism remains inconclusive. Further studies employing specific inhibitors, gene knockout techniques, or siRNA interference are necessary to comprehensively elucidate the underlying molecular mechanisms. Second, future research should incorporate a positive control group using a known effective drug for ischemic stroke to allow for a comparative assessment of the efficacy and advantages of calycosin. Furthermore, the current study primarily addressed the short-term effects of calycosin. The long-term efficacy, toxicity, and safety of calycosin require further validation to fully assess its potential as a therapeutic agent for the prevention and treatment of ischemic stroke.
Conclusion
In conclusion, our findings suggest that calycosin post-conditioning can alleviate cerebral ischemia/reperfusion injury and that its neuroprotective effects are exerted through the inhibition of HMGB1/TLR4/NF-κB pathway signaling. This study contributes to a better understanding of the molecular mechanisms underlying ischemic stroke and presents a potential new drug target for ischemic stroke therapy.
Funding
This work was supported by the National Natural Science Foundation of China (nos. 82260711 and 81860231), Independent Research Project of Guangxi Key Laboratory of Tumor Immunology and Microenvironmental Regulation (no. 203030302313 and 203030302413), Guilin Technology Application and Extension Plan (no. 20220139-13-3), and Guangxi Medical and Health Key Cultivation Discipline Construction Project.
Disclosure
The authors declare no conflicts of interest.
References
1. Fan J, Li X, Yu X, et al. Global burden, risk factor analysis, and prediction study of ischemic stroke, 1990-2030. Neurology. 2023;101(2):e137–e150. doi:10.1212/WNL.0000000000207387
2. Collaborators GBDSRF. Global, regional, and national burden of stroke and its risk factors, 1990-2021: a systematic analysis for the Global Burden of Disease Study 2021. Lancet Neurol. 2024;23(10):973–1003. doi:10.1016/S1474-4422(24)00369-7.
3. Tu WJ, Wang LD, Special Writing Group of China Stroke Surveillance R. China stroke surveillance report 2021. Mil Med Res. 2023;10(1):33. doi:10.1186/s40779-023-00463-x
4. Tsao CW, Aday AW, Almarzooq ZI, et al. Heart disease and stroke statistics-2023 update: a report from the American heart association. Circulation. 2023;147(8):e93–e621. doi:10.1161/CIR.0000000000001123
5. Vyas MV, Silver FL, Austin PC, et al. Stroke Incidence by Sex Across the Lifespan. Stroke. 2021;52(2):447–451. doi:10.1161/STROKEAHA.120.032898
6. Kalogeris T, Baines CP, Krenz M, Korthuis RJ. Ischemia/Reperfusion. Compr Physiol. 2016;7(1):113–170. doi:10.1002/cphy.c160006
7. Campbell BCV, De Silva DA, Macleod MR, et al. Ischaemic stroke. Nat Rev Dis Primers. 2019;5(1):70. doi:10.1038/s41572-019-0118-8
8. Simon E, Forghani M, Abramyuk A, et al. Intravenous Thrombolysis by Telestroke in the 3- to 4.5-h Time Window. Front Neurol. 2021;12:756062. doi:10.3389/fneur.2021.756062
9. Chen R, Kang R, Tang D. The mechanism of HMGB1 secretion and release. Exp Mol Med. 2022;54(2):91–102. doi:10.1038/s12276-022-00736-w
10. Ge Y, Huang M, Yao YM. The effect and regulatory mechanism of high mobility group box-1 protein on immune cells in inflammatory diseases. Cells. 2021;10(5):1044. doi:10.3390/cells10051044
11. Gong T, Liu L, Jiang W, Zhou R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat Rev Immunol. 2020;20(2):95–112. doi:10.1038/s41577-019-0215-7
12. Kim HJ, Kim H, Lee JH, Hwangbo C. Toll-like receptor 4 (TLR4): new insight immune and aging. Immun Ageing. 2023;20(1):67. doi:10.1186/s12979-023-00383-3
13. Park JS, Svetkauskaite D, He Q, et al. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem. 2004;279(9):7370–7377. doi:10.1074/jbc.M306793200
14. Xu Y, Zhang J, Gao F, et al. Engeletin alleviates cerebral ischemia reperfusion-induced neuroinflammation via the HMGB1/TLR4/NF-kappaB network. J Cell Mol Med. 2023;27(12):1653–1663. doi:10.1111/jcmm.17758
15. Duran-Laforet V, Pena-Martinez C, Garcia-Culebras A, et al. Role of TLR4 in neutrophil dynamics and functions: contribution to stroke pathophysiology. Front Immunol. 2021;12:757872. doi:10.3389/fimmu.2021.757872
16. Xu X, Piao HN, Aosai F, et al. Arctigenin protects against depression by inhibiting microglial activation and neuroinflammation via HMGB1/TLR4/NF-kappaB and TNF-alpha/TNFR1/NF-kappaB pathways. Br J Pharmacol. 2020;177(22):5224–5245. doi:10.1111/bph.15261
17. Ri MH, Xing Y, Zuo HX, et al. Regulatory mechanisms of natural compounds from traditional Chinese herbal medicines on the microglial response in ischemic stroke. Phytomedicine. 2023;116:154889. doi:10.1016/j.phymed.2023.154889
18. Li XH, Yin FT, Zhou XH, et al. The Signaling pathways and targets of natural compounds from traditional Chinese medicine in treating ischemic stroke. Molecules. 2022;27(10). doi:10.3390/molecules27103099
19. Tao T, Liu M, Chen M, et al. Natural medicine in neuroprotection for ischemic stroke: challenges and prospective. Pharmacol Ther. 2020;216:107695. doi:10.1016/j.pharmthera.2020.107695
20. Petrine JCP, Del Bianco-Borges B. The influence of phytoestrogens on different physiological and pathological processes: an overview. Phytother Res. 2021;35(1):180–197. doi:10.1002/ptr.6816
21. Gorzkiewicz J, Bartosz G, Sadowska-Bartosz I. The potential effects of phytoestrogens: the role in neuroprotection. Molecules. 2021;26(10):2954. doi:10.3390/molecules26102954
22. Al-Shami AS, Essawy AE, Elkader H. Molecular mechanisms underlying the potential neuroprotective effects of Trifolium pratense and its phytoestrogen-isoflavones in neurodegenerative disorders. Phytother Res. 2023;37(6):2693–2737. doi:10.1002/ptr.7870
23. Ren Y, Qu S. Constituent isoflavones of Puerariae radix as a potential neuroprotector in cognitive impairment: evidence from preclinical studies. Ageing Res Rev. 2023;90:102040. doi:10.1016/j.arr.2023.102040
24. Song YJ, Li SR, Li XW, et al. The effect of estrogen replacement therapy on Alzheimer’s disease and parkinson’s disease in postmenopausal women: a meta-analysis. Front Neurosci. 2020;14:157. doi:10.3389/fnins.2020.00157
25. Li M, Han B, Zhao H, et al. Biological active ingredients of Astragali Radix and its mechanisms in treating cardiovascular and cerebrovascular diseases. Phytomedicine. 2022;98:153918. doi:10.1016/j.phymed.2021.153918
26. Deng M, Chen H, Long J, Song J, Xie L, Li X. Calycosin: a review of its pharmacological effects and application prospects. Expert Rev Anti Infect Ther. 2021;19(7):911–925. doi:10.1080/14787210.2021.1863145
27. Sohel M, Zahra Shova FT, Shuvo S, et al. Unveiling the potential anti-cancer activity of calycosin against multivarious cancers with molecular insights: a promising frontier in cancer research. Cancer Med. 2024;13(3):e6924. doi:10.1002/cam4.6924
28. Wang Y, Ren Q, Zhang X, Lu H, Chen J. Neuroprotective mechanisms of calycosin against focal cerebral ischemia and reperfusion injury in rats. Cell Physiol Biochem. 2018;45(2):537–546. doi:10.1159/000487031
29. Wang Y, Dong X, Li Z, Wang W, Tian J, Chen J. Downregulated RASD1 and upregulated miR-375 are involved in protective effects of calycosin on cerebral ischemia/reperfusion rats. J Neurol Sci. 2014;339(1–2):144–148. doi:10.1016/j.jns.2014.02.002
30. Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20(1):84–91. doi:10.1161/01.STR.20.1.84
31. Zhang QQ, Luo L, Liu MX, Wang CJ, Wu Y, Yu KW. Enriched environment-induced neuroprotection against cerebral ischemia-reperfusion injury might be mediated via enhancing autophagy flux and mitophagy flux. Mediators Inflamm. 2022;2022:2396487. doi:10.1155/2022/2396487
32. Zhang J, Zhou R, Xiang C, et al. Enhanced thioredoxin, glutathione and Nrf2 antioxidant systems by safflower extract and aceglutamide attenuate cerebral ischaemia/reperfusion injury. J Cell Mol Med. 2020;24(9):4967–4980. doi:10.1111/jcmm.15099
33. Guo MM, Qu SB, Lu HL, et al. Biochanin A alleviates cerebral ischemia/reperfusion injury by suppressing endoplasmic reticulum stress-induced apoptosis and p38MAPK signaling pathway in vivo and in vitro. Front Endocrinol (Lausanne). 2021;12:646720. doi:10.3389/fendo.2021.646720
34. Wu Y, Fan X, Chen S, et al. Geraniol-mediated suppression of endoplasmic reticulum stress protects against cerebral ischemia-reperfusion injury via the PERK-ATF4-CHOP Pathway. Int J Mol Sci. 2022;24(1):544. doi:10.3390/ijms24010544
35. Li X, Yang X, Lu H, et al. Calycosin attenuates the inflammatory damage of microglia induced by oxygen and glucose deprivation through the HMGB1/TLR4/NF-kappaB signaling pathway. Acta Biochim Biophys Sin (Shanghai). 2023;55(8):1–10. doi:10.3724/abbs.2023125
36. Wang H, Zhang S, Xie L, Zhong Z, Yan F. Neuroinflammation and peripheral immunity: focus on ischemic stroke. Int Immunopharmacol. 2023;120:110332. doi:10.1016/j.intimp.2023.110332
37. Alsbrook DL, Di Napoli M, Bhatia K, et al. Neuroinflammation in acute ischemic and hemorrhagic stroke. Curr Neurol Neurosci Rep. 2023;23(8):407–431. doi:10.1007/s11910-023-01282-2
38. Qin C, Yang S, Chu YH, et al. Signaling pathways involved in ischemic stroke: molecular mechanisms and therapeutic interventions. Signal Transduct Target Ther. 2022;7(1):215. doi:10.1038/s41392-022-01064-1
39. Paul S, Candelario-Jalil E. Emerging neuroprotective strategies for the treatment of ischemic stroke: an overview of clinical and preclinical studies. Exp Neurol. 2021;335:113518. doi:10.1016/j.expneurol.2020.113518
40. Shichita T, Ooboshi H, Yoshimura A. Neuroimmune mechanisms and therapies mediating post-ischaemic brain injury and repair. Nat Rev Neurosci. 2023;24(5):299–312. doi:10.1038/s41583-023-00690-0
41. Candelario-Jalil E, Dijkhuizen RM, Magnus T. Neuroinflammation, stroke, blood-brain barrier dysfunction, and imaging modalities. Stroke. 2022;53(5):1473–1486. doi:10.1161/STROKEAHA.122.036946
42. Endres M, Moro MA, Nolte CH, Dames C, Buckwalter MS, Meisel A. Immune pathways in etiology, acute phase, and chronic sequelae of ischemic stroke. Circ Res. 2022;130(8):1167–1186. doi:10.1161/CIRCRESAHA.121.319994
43. Qin X, Akter F, Qin L, et al. Adaptive immunity regulation and cerebral ischemia. Front Immunol. 2020;11:689. doi:10.3389/fimmu.2020.00689
44. Qiu J, Nishimura M, Wang Y, et al. Early release of HMGB-1 from neurons after the onset of brain ischemia. J Cereb Blood Flow Metab. 2008;28(5):927–938. doi:10.1038/sj.jcbfm.9600582
45. Kim JB, Lim CM, Yu YM, Lee JK. Induction and subcellular localization of high-mobility group box-1 (HMGB1) in the postischemic rat brain. J Neurosci Res. 2008;86(5):1125–1131. doi:10.1002/jnr.21555
46. Andersson U, Yang H, Harris H. High-mobility group box 1 protein (HMGB1) operates as an alarmin outside as well as inside cells. Semin Immunol. 2018;38:40–48. doi:10.1016/j.smim.2018.02.011
47. Wang S, Zhang Y. HMGB1 in inflammation and cancer. J Hematol Oncol. 2020;13(1):116. doi:10.1186/s13045-020-00950-x
48. Gao B, Wang S, Li J, et al. HMGB1, angel or devil, in ischemic stroke. Brain Behav. 2023;13(5):e2987. doi:10.1002/brb3.2987
49. Gou X, Ying J, Yue Y, et al. The roles of high mobility group box 1 in cerebral ischemic injury. Front Cell Neurosci. 2020;14:600280. doi:10.3389/fncel.2020.600280
50. Tang SC, Arumugam TV, Xu X, et al. Pivotal role for neuronal Toll-like receptors in ischemic brain injury and functional deficits. Proc Natl Acad Sci U S A. 2007;104(34):13798–13803. doi:10.1073/pnas.0702553104
51. Suzuki Y, Hattori K, Hamanaka J, et al. Pharmacological inhibition of TLR4-NOX4 signal protects against neuronal death in transient focal ischemia. Sci Rep. 2012;2(1):896. doi:10.1038/srep00896
52. Garcia-Culebras A, Duran-Laforet V, Pena-Martinez C, et al. Role of TLR4 (toll-like receptor 4) in N1/N2 neutrophil programming after stroke. Stroke. 2019;50(10):2922–2932. doi:10.1161/STROKEAHA.119.025085
53. Caso JR, Pradillo JM, Hurtado O, Lorenzo P, Moro MA, Lizasoain I. Toll-like receptor 4 is involved in brain damage and inflammation after experimental stroke. Circulation. 2007;115(12):1599–1608. doi:10.1161/CIRCULATIONAHA.106.603431
54. Gong P, Jia HY, Li R, et al. Downregulation of Nogo-B ameliorates cerebral ischemia/reperfusion injury in mice through regulating microglia polarization via TLR4/NF-kappaB pathway. Neurochem Int. 2023;167:105553. doi:10.1016/j.neuint.2023.105553
55. Napetschnig J, Wu H. Molecular basis of NF-kappaB signaling. Annu Rev Biophys. 2013;42(1):443–468. doi:10.1146/annurev-biophys-083012-130338
56. Yu M, Huang H, Dong S, Sha H, Wei W, Liu C. High mobility group box-1 mediates hippocampal inflammation and contributes to cognitive deficits in high-fat high-fructose diet-induced obese rats. Brain Behav Immun. 2019;82:167–177. doi:10.1016/j.bbi.2019.08.007
57. Liu Q, Zhang Y. PRDX1 enhances cerebral ischemia-reperfusion injury through activation of TLR4-regulated inflammation and apoptosis. Biochem Biophys Res Commun. 2019;519(3):453–461. doi:10.1016/j.bbrc.2019.08.077
58. Nurmi A, Lindsberg PJ, Koistinaho M, et al. Nuclear factor-kappaB contributes to infarction after permanent focal ischemia. Stroke. 2004;35(4):987–991. doi:10.1161/01.STR.0000120732.45951.26
59. Pan L, Zhang XF, Wei WS, Zhang J, Li ZZ. The cardiovascular protective effect and mechanism of calycosin and its derivatives. Chin J Nat Med. 2020;18(12):907–915. doi:10.1016/S1875-5364(20)60034-6
60. Guo C, Tong L, Xi M, Yang H, Dong H, Wen A. Neuroprotective effect of calycosin on cerebral ischemia and reperfusion injury in rats. J Ethnopharmacol. 2012;144(3):768–774. doi:10.1016/j.jep.2012.09.056
61. Guo C, Ma Y, Ma S, et al. The role of TRPC6 in the neuroprotection of calycosin against cerebral ischemic injury. Sci Rep. 2017;7(1):3039. doi:10.1038/s41598-017-03404-6
62. Xu S, Huang P, Yang J, Du H, Wan H, He Y. Calycosin alleviates cerebral ischemia/reperfusion injury by repressing autophagy via STAT3/FOXO3a signaling pathway. Phytomedicine. 2023;115:154845. doi:10.1016/j.phymed.2023.154845
63. Liu H, Zhao Z, Yan M, Zhang Q, Jiang T, Xue J. Calycosin decreases cerebral ischemia/reperfusion injury by suppressing ACSL4-dependent ferroptosis. Arch Biochem Biophys. 2023;734:109488. doi:10.1016/j.abb.2022.109488
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.