Back to Journals » Clinical Ophthalmology » Volume 19
Capsule-Preserving Intraocular Lens Intrascleral Fixation for Zonular Weakness: Clinical Outcomes and Comparative Analysis
Authors Kato M , Kumashiro Y, Masatani I, Tanaka E, Ide N, Ariyasu K
Received 31 January 2025
Accepted for publication 5 May 2025
Published 26 May 2025 Volume 2025:19 Pages 1709—1720
DOI https://doi.org/10.2147/OPTH.S515743
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Scott Fraser
Haptics Fixation for Intraocular Lens Intrascleral Fixation – Video S1 [515743]
Views: 135
Mutsuko Kato, Yuri Kumashiro, Iori Masatani, Eizo Tanaka, Naohiro Ide, Kanazu Ariyasu
Department of Ophthalmology, Japan Red Cross Okayama Hospital, Okayama, Okayama-Prefecture, Japan
Correspondence: Mutsuko Kato, Department of Ophthalmology, Japan Red Cross Okayama Hospital, 2-1-1 Aoe, Kita-ward, Okayama, 700-8607, Japan, Tel +81 86 222 8811, Fax +81 86 222 8841, Email [email protected]
Purpose: To compare postoperative outcomes of haptic fixation sites, single versus dual haptics, in capsule-preserving intraocular lens (IOL) intrascleral fixation for zonular weakness.
Patients and Methods: This retrospective study analyzed 88 eyes (65 patients) with zonular weakness and at least two additional risk factors for IOL dislocation. Conducted at a single center from May 2019 to July 2023, the study followed patients for 6– 56 months (mean: 26.5± 16.0 months). Fixation methods included single or dual haptics, selected based on the operator’s subjective assessment of zonular weakness and patient age. Initially, scleral tunnel-style was used, transitioning to flange-style in October 2021. Outcomes assessed included IOL tilt, decentration, refractive error, and complications.
Results: Final assessments showed an average IOL tilt of 6.54± 3.14° and decentration of 0.60± 0.36mm. Refractive error at six months post-surgery averaged − 0.33± 0.99D. Dual fixation resulted in greater myopic shifts than single fixation (− 0.79± 0.93D vs − 0.16± 0.96D, p< 0.01), especially tunnel-dual fixation compared to tunnel-single fixation (− 1.31± 0.61D vs − 0.25± 0.89D, p< 0.001) and tunnel-dual fixation compared to flange-dual fixation (− 1.31± 0.61D vs − 0.17± 0.88D, p=0.001). Large IOL tilts (> 10°) occurred in six eyes (6.8%), all with tunnel style, with a refractive error of − 0.59± 0.78D; not statistically significant, but a correlation was observed between tilt and refractive error (R²=0.851, p=0.0176). Large IOL decentration (> 1mm) occurred in 12 eyes (13.6%), with a significant myopic shift of − 1.01± 0.93D. Capsule damage was noted in 15.9% of cases, vitreous prolapse was infrequent (4.5%), and no cases had iris capture or severe retinal complications.
Conclusion: Despite the risk of capsule damage, this method, which preserves the capsule and avoids posterior segment surgery, appears viable for cases with significant zonular weakness and anticipated progression, without iris capture or retinal complications. Improving the T-style or adopting F-style, particularly FD-fixation, may help prevent tilt and decentration, reduce refractive errors, and improve postoperative visual function.
Keywords: zonular weakness, capsule-preserving, single/dual-flange/tunnel IOL intrascleral fixation, optic capture, tilting, decentration
Introduction
The incidence of intraocular lens (IOL) dislocation is frequently reported to be around 1%.1–3 However, with advancements in surgical techniques and increased patient longevity due to earlier interventions, this rate is reportedly rising.4,5 In cases of zonular weakness, while standard cataract surgery with the use of a capsular tension ring (CTR) can often be performed, reports of CTR-IOL complex dislocation are also increasing.6,7 due to progressive zonular weakness, pseudoexfoliation syndrome, high myopia, trauma, or other long-term complications.
The capsule-preserving IOL fixation technique, which performs intrascleral fixation without removing the preserved capsule,8–10 is advantageous as it reduces surgical time and the risk of vitreoretinal complications and bullous keratopathy by eliminating the need for vitrectomy and peripheral iridectomy designed to prevent iris-capture of the IOL. Although this technique appears highly versatile for preventing future IOL dislocation compared to simple in-the-bag IOL fixation or IOL reverse optic capture (sulcus fixation) with or without CTR,11–13 reports remain limited. This study investigates the long-term postoperative outcomes of this method and whether postoperative outcomes differ based on the method of haptic fixation utilized.
Patients and Methods
We conducted a retrospective analysis of 65 patients (88 eyes) who underwent the aforementioned surgery and were observed for a minimum of six months between May 2019 and July 2023. Patients included in the study presented with zonular weakness such as phacodonesis or extensive zonular dialysis, and at least two of the following risk factors (resulting in a total of three or more factors when combined with the zonular issues): age under 80 years (indicating a longer postoperative lifespan, as fragility due to age is often accompanied by phacodonesis or progressive nuclear cataract covered by other factors), progressive nuclear cataract (Emery grade ≥ 4), pseudoexfoliation, a history of glaucoma attacks, narrow angles and/or poor mydriasis, diabetes, trauma or atopic dermatitis, ocular conditions such as high myopia or retinitis pigmentosa, and iatrogenic zonular damage (including vitrectomy). The exclusion criteria included cases with severe capsule damage where preservation was not possible, and cases where the CTR could not be inserted and fixed.
Surgical Technique
- Perform phacoemulsification and irrigation/aspiration (I/A) using a capsular tension ring (CTR, 130A0, HOYA, Tokyo, Japan). Fixate the haptics of the intraocular lens (IOL, NX-70S; Santen Pharmaceutical Co. Ltd., Osaka, Japan) from outside the anterior capsule to the sclera (Figure 1):
- Selection of Single or Dual Haptic Fixation: This decision is based on the degree of zonular weakness subjectively by one surgeon, dialysis extent, and patient age (life expectancy). In single fixation (S-fixation, S), Figure 1–1), the superior fixation is standard, but in cases of zonular dialysis, fixation is done at the dialysis site, and the unfixed haptic is secured outside the lens anterior capsule (“sulcus fixation”), Figure 1 Slice B. For dual fixation (D-fixation, D), Figure 1 and 2), stabilizers were used to secure the haptics at easily accessible positions (eg, 1:00–7:00, 4:00–10:00).
- Depress the anterior surface of the CTR-encased lens capsule posteriorly using an ophthalmic viscosurgical device (OVD). The externalization of the IOL haptics is performed by inserting them into 30-gauge ultra-thin wall needles (TSK; Tochigi Seiko, Tochigi, Japan) using IOL scleral fixation forceps (M.E. Technica, Tokyo, Japan). The insertion of the 30-gauge needles into the eye is assisted by the Yamane double-needle stabilizer (Geuder AG, Heidelberg, Germany). The stabilizer aids in positioning the 30-gauge needles 2mm from the corneal limbus, at an angle of 20° to the corneal limbus and 10° to the iris surface.14
- The fixation technique for the haptics involved inserting them 2mm into a scleral half-thickness tunnel and suturing with 8–0 Vicryl until October 2021 (tunnel-style, T-style, T), Figure 1–1.9 Post-October 2021, haptics fixation was achieved by creating flanges using the Accu-Temp ophthalmic cautery device (Beaver Visitec, Waltham, MA, USA) (flange-style, F-style, F), Figure 1–2.
- Achieve optic reverse capture of the IOL optics within the CTR-encased lens capsule with appropriately sized continuous curvilinear capsulorhexis.
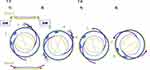 |
Figure 1 Style of the intrascleral haptics fixation 1–1 Tunnel style 1) Single (TS-fixation), 2) Dual (TD-fixation); 1–2 Flange style 1) Single (FS-fixation), 2) Dual (FD-fixation) Up to October 2021, scleral tunnel style suturing with 8–0 Vicryl of haptics was used (1–1), afterwards switching to flange style using the Accu-Temp ophthalmic cautery device (1–2). Depending on the severity of zonular weakness, dialysis extent, and patient age (life expectancy), either single (1-1-1, 1-2-1) or dual (1-1-2, 1-2-2) site fixation of haptics was chosen; in single cases, the opposite haptics were implanted out of the bag (sulcus fixation). Note: Figure 1-1 is adapted from Kato M, Namba M, Shimoyama S, Inoue M, Ouchi C, Shimizu T. Intrascleral intraocular lens fixation preserving the lens capsule in cases of cataract with insufficient zonular support. Clinical Ophthalmology. 2022;16:93–100.9 |
This method allows for scleral fixation of the IOL using only anterior segment surgery since the CTR-encased lens capsule is not removed. Vitrectomy and peripheral iridectomy were not performed. See Video S1 for the surgical technique.
Examination
The following outcomes and progress were measured and compared among the fixation site and style; visual acuity assessed at 5 meters preoperatively and at last visit postoperatively, refractive error at six months after surgery whose the deviation from the target refraction was calculated using the most appropriate formula based on axial length and corneal shape from amongst the Haigis, SRK/T, and Barrett Universal II formulas, corneal endothelial cell density measured preoperatively and at one month postoperatively using the CC-7000 (Konan Medical, Hyogo, Japan), IOL tilt and decentration which were evaluated via serial assessments using the CASIA2 anterior segment optical coherence tomography (OCT) system (Tomey Corporation, Nagoya, Japan). Intraoperative and postoperative complications were examined throughout the study period.
Statical Analysis
Visual acuity was expressed as the logarithm of the minimum angle of resolution (logMAR). Changes in postoperative visual acuity, endothelial cell density (ECD), and IOL tilt and decentration were assessed using the Wilcoxon signed-rank test for comparisons with preoperative values, following checks for normality. Mann–Whitney U-test, Student’s t-test and Fischer’s exam test were employed to compare parameters between groups. All values are reported as means ± standard deviations, and p-values of less than 0.05 were considered indicative of statistical significance. Statistical analyses were conducted using R (ver.4. 3. 1) (The R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient Characteristics
Table 1 shows the Patient Characteristics in overall and each group. There were 32 males and 33 females, with ages ranging from 47 to 94 years (mean: 75.3±10.6 years). The follow-up period ranged from 6 to 56 months (mean: 26.5±16.0 months). The risk score for progression of zonular weakness was 3.56±0.71. Among the site of the haptics fixation, S-fixation was performed in 63 eyes (tunnel-single fixation, TS-fixation, TS, in 41 eyes; flange-single fixation, FS-fixation, FS, in 22 eyes), D-fixation in 25 eyes (tunnel-dual fixation, TD-fixation, TD, in 13 eyes; flange-dual fixation, FD-fixation, FD, in 12 eyes). Regarding the patient backgrounds of each group, no significant differences in age, or risk scores were observed among these groups. Although the follow-up period was significantly shorter for TS-fixation (32.0±13.7 months) compared to TD-fixation (44.1±13.3 months) (p=0.007**), both groups had sufficiently long follow-up periods. The selection of S-fixation and D-fixation was subjectively determined by the surgeon based on the degree of zonular weakness, with D-fixation being performed in cases with severe weakness and longer life expectancy. Consequently, “iatrogenic zonular dialysis” was significantly more common in the D-fixation group (S vs D, 3.2% vs 20.0%, p=0.018*). Additionally, “narrow-angle and poor mydriasis” were significantly more common in the S-fixation group (S vs D, 61.9% vs 28.0%, p=0.005**).
 |
Table 1 Patient Characteristics |
Surgical Outcomes
Table 2 shows the postoperative data. Visual acuity improved significantly across all fixation methods and overall (p<0.001***, p<0.01**, p<0.05*), with no significant differences in postoperative visual acuity improvement. Corneal endothelial cell density (ECD) decreased significantly from 2423±484 preoperatively to 2207±497 cells/mm² postoperatively (p<0.01**), with an average reduction rate of 10.1±12.5%. There were no significant differences in endothelial cell loss among the fixation methods.
 |
Table 2 Summary of Surgical Outcomes |
The refractive error relative to the target refraction was −0.33±0.99D, with 52 eyes (59.1%) experiencing a myopic shift. When comparing fixation sites, D-fixation (−0.79±0.93D) resulted in a more significant myopic shift than S-fixation (−0.16±0.96D) (p=0.007**). This trend was more pronounced in the T-style (TS vs TD, −0.25±0.89D vs −1.31±0.61D; p<0.001***), while no significant difference was observed in the F-style (FS vs FD, 0.01±1.08D vs −0.17±0.88D, p=0.635, NS). In D-fixation, particularly with TD-fixation, there was a significantly greater myopic shift compared to FD-fixation (TD vs FD, −1.31±0.61D vs −0.17±0.88D, p=0.001). The Postoperative changes in the IOL tilt, as described in Table 2 were showed (Figure 2A). The tilt was 6.37±2.93° at 6 months (n=88), 6.39±3.03° at 1 year (n=71), and 7.20±3.37° at 2 years (n=47) postoperatively, with 6.58±3.14° at the final visit. Although sample sizes were smaller at 3 and 4 years, no significant increase in tilt was observed over time (7.18±3.25°, n=28; 7.15±3.11°, n=13). Similarly, changes in the IOL decentration were showed (Figure 2B). The decentration was 0.63±0.34mm at 6 months, 0.59±0.35mm at 1 year, and 0.56±0.37mm at 2 years, with 0.60±0.36mm at the final visit. No significant increase in decentration was observed over time, including at 3 years (0.54±0.36mm, n=28) and 4 years (0.56±0.42mm, n=13).
The changes in the IOL tilt based on the fixation site, as described in Table 2 were showed (Figure 3A). There was no significant increase at any visit. Postoperative changes of the IOL decentration by the fixation site showed no significant increase (Figure 3B). Although not statistically significant, a trend toward increased decentration was observed at 2 and 3 years in the S-fixation group (0.61±0.39mm, n=35; 0.64±0.40mm, n=17) compared to the D-fixation group (0.40±0.26mm, n=12; 0.39±0.26mm, n=11) (p=0.091; p=0.081, NS). Postoperative changes in IOL tilt and decentration were compared between S-fixation and D-fixation for both T-style and F-style (Figure 4). Due to the shorter follow-up period for the F-style (FS-fixation, FD-fixation, Figure 4C and D), tendencies towards increased tilt and decentration in single fixation were reflected by changes observed in the T-style (TS-fixation, TD-fixation, Figure 4A and B).
Changes After Capsulotomy
Laser capsulotomy was performed on six eyes, all of which had undergone TS-fixation. No significant differences in tilt or decentration were observed before and after capsulotomy (6.85±3.88° to 7.25±5.50° for tilt, p=0.456, NS and 0.43±0.16mm to 0.42±0.26mm for decentration, p=0.857, NS). However, one case showed an increase in tilt from 8.2° to 10.9° and in decentration from 0.94 to 1.24mm, involving a 51-year-old patient who underwent this procedure due to traumatic lens phacodonesis.
Complications
Complications, as described in Table 2, included lens capsule damage in 14 eyes (15.9%), vitreous herniation in 4 eyes (4.5%), transient intraocular pressure elevation in 2 eyes (2.3%), and spontaneously absorbed vitreous hemorrhage in 2 eyes (2.3%). Large tilts greater than 10° were observed in 6 eyes (6.8%), with a range of 10.5–14.6° (mean: 11.7±1.56°), and large decentration greater than 1mm was noted in 12 eyes (13.6%), with a range of 1.01–1.41mm (mean: 1.20±0.13mm). No cases of iris capture and bullous keratopathy. No macular edema or retinal trouble were observed in patients without concurrent retinal disease.
Analysis Between Methods
When stratified by fixation site and style, damage of lens capsule was more common with D-fixation (28.0% in 7 eyes) compared to S-fixation (11.1% in 7 eyes, p=0.102, NS), particularly among those with FD-fixation (n=12), where capsule damage occurred in 6 eyes (50%) and vitreous herniation in 2 eyes (16.7%). For FS-fixation (n=22), capsule damage was observed in 5 eyes (22.7%) and vitreous dislocation in 1 eye (4.5%).
Regarding large tilts (over 10°) in 6 eyes and decentration (over 1mm) in 12 eyes, there was no significant difference between S-fixation and D-fixation, although S-fixation exhibited a slightly higher incidence of large decentration (S vs D, 10 eyes 15.9% vs 2 eyes 8.0%, p=0.496). All six eyes with large tilts were in the T-style group (4 eyes in TS-fixation, 2 eyes in TD-fixation). In cases with large tilt (n=6), the refractive error was −0.59±0.78D, and there was no significant difference compared to cases without large tilt (−0.32±1.00D, p=0.511) (Figure 5A). In cases with large decentration (n=12; 3 eyes in TS-fixation, 1 eye in TD-fixation, 7 eyes in FS-fixation, 1 eye in FD-fixation), the refractive error was −1.01±0.93D, and there was a significant difference compared to cases without large decentration (−0.23±0.96D, p=0.00955*) (Figure 5B). Multiple regression analysis of the correlation between tilt/decentration and refractive error showed a significant correlation in cases with large tilt (R²=0.851, p=0.0176*) but not in cases with large decentration (R²=−0.1814, p=0.8581).
Discussion
The incidence of intraocular lens (IOL) dislocation, previously reported around 1%, is increasing due to advances in surgical techniques and longer patient lifespans.1–5 In cases of zonular weakness or dialysis, the use of FLACS (Femtosecond laser-assisted cataract surgery)15,16 combined with capsular tension rings (CTR) has increasingly facilitated conventional cataract surgery (phacoemulsification with in-the-bag IOL implantation).17
The insertion of a CTR in eyes with zonular weakness helps maintain the shape of the lens capsule postoperatively, reduces anterior capsule contraction, and stabilizes IOL tilt.18 However, the long-term effect of CTR on bag stability is uncertain, with reports of CTR-IOL complex dislocation occurring approximately 81.5±32.2 months (about 6.8 years) post-implantation at a rate of 0.76%.6 Although techniques such as the Cionni-modified CTR,19 capsular tension segment (CTS),20 and suturable CTR A2 (HOYA) have been developed, they are complex and not widely adopted. Thus, recently, in cases of zonular weakness or dialysis with capsule, three main options remain: fixing the IOL in the bag with or without a CTR, IOL reverse optic capture with or without a CTR,11–13 and removing the bag and using traditional scleral IOL fixation with vitrectomy especially in cases of significant zonular weakness or extensive dialysis.21–24 To address these challenges between IOL reverse optic capture and traditional scleral IOL fixation with vitrectomy, capsule-preserving IOL fixation techniques, which provide intrascleral fixation without removing the capsule and vitrectomy, have been reported.8–10
Sulcus IOL implantation with reverse optic capture has been reported to provide stability without scleral fixation, especially in cases where the capsule is partially compromised but still usable.11–13 Devranoğlu K. et al reported on 70 eyes with zonular weakness, using iris retractors combined with CCC and CTR for IOL optic capture, with an average follow-up of 13 months.12 They reported that IOL haptics provided additional support, particularly when IOL haptics are positioned against the subluxated area. They also said that even if the CTR-IOL complex dislocated scleral fixation was easier to perform. Bhaskaran J. et al studied 35 eyes with pseudoexfoliation (PE), with an average age of 75.21 years ± 5.74 (SD), and reported no late IOL subluxation, dislocation, or uveitis over a follow-up period of 4 months to 5 years (mean: 2 years 3 months).13 They concluded that this technique might prevent future dislocation in relatively younger patients with PE in their 50s and 60s. However, the longer-term frequency of IOL dislocation remains unknown in both reports. Additionally, the indications were limited to patients with clinical or intraoperative evidence of mild-to-moderate zonular weakness, excluding more severe cases. Their exclusion criteria included a history of trauma, corneal pathology, previous eye surgery, or decreased vision due to other reasons than cataracts (eg, exudative age-related macular degeneration, proliferative diabetic retinopathy, inflammatory eye diseases, etc.).12,13 In our study, 73.9% of cases had preoperative lens phacodonesis or extensive zonular dialysis, and 84.1% had lens phacodonesis either preoperatively or intraoperatively. Other conditions included traumatic eyes (9.1%), atopic dermatitis (5.7%), and iatrogenic zonular damage from previous ophthalmic surgery (8.0%). Objective quantification of zonular weakness is challenging,25 and no consensus evaluation method exists. Therefore, we applied a risk score concept, including cases with preoperative or intraoperative lens phacodonesis or extensive zonular dialysis and at least two additional risk factors for progressive zonular weakness (totaling three or more points). The risk scores of our cases ranged from 3 to 5 points, with an average of 3.56 points, indicating moderate-to-severe zonular weakness. Even with moderate-to-severe zonular weakness and extensive dialysis, this technique could be viable if enough capsule remains to secure the CTR. While reverse optic capture alone may risk early dislocation, this method avoids the need for vitrectomy and capsule removal as required in traditional scleral fixation.
The advantages of this technique include its minimally invasive nature as an anterior segment surgery,9 which eliminates the need for vitrectomy and consequently reduces the risk of vitro-retinal complications. Additionally, the IOL is securely supported by three components—scleral fixation via the haptics, reverse optic capture by the CTR-enclosed capsule, and the anterior vitreous9—preventing iris capture which may often annoy surgeons. Traditional scleral fixation methods involving vitrectomy have reported complications such as iris capture (1.59%,21 8%,23 2.9%26), macular edema (1.59%,21 1%,23 4.3%26), and retinal detachment (2%24). Moreover, peripheral iridectomy, often performed to prevent iris capture, has inherent risks of macular edema and long-term bullous keratopathy.27 In this study, there were no cases of iris capture, no macular edema or retinal trouble and no bullous keratopathy.
However, disadvantages include the possibility of capsule damage and vitreous dislocation caused by puncture of haptics guide needle. There may also be persistent vitreous hemorrhage without vitrectomy. In this study, capsule damage occurred in 15.9% of all cases, though vitreous dislocation was limited to 4.5%. Vitreous hemorrhage occurred in 2% of cases, all of which spontaneously resolved, and there were no retinal complications. There is also a risk of progressive IOL tilt and decentration due to contraction of remaining capsule, but no statistically significantly change in this study during follow-up periods.
Initially, tilt prevention strategies involved adjusting the extracted length of the haptics.28 However, Sotani et al demonstrated that IOL tilt is influenced by the vertical insertion angle of the haptic.29 With intraoperative anterior segment OCT guidance, excellent outcomes like 5.45±2.63°30 and 2.10±1.66°29 have been also reported. In our facility, the final tilt was 6.51±3.06° using the needle stabilizers14 without intraoperative anterior OCT, and the overall refractive error was −0.33±0.99D. Additionally, significantly greater myopic shifts were observed in D-fixation despite rather less tilt than in S-fixation (S vs D, −0.16±0.96D vs −0.79±0.93D, p<0.01; 6.61±2.91° vs 6.04±3.01°, p=0.508). This trend was stronger in the T-style (TS vs TD, −0.25±0.89D vs −1.31±0.61D, p<0.001***; 6.70±3.14° vs 7.08±3.55°, p=0.718), while no significant difference was observed in the F-style (FS vs FD, 0.01±1.08D vs −0.17±0.88D, p=0.635; 6.28±3.31° vs 5.88±2.50°, p=0.718). In D-fixation, particularly with TD-fixation, there was a significantly greater myopic shift compared to FD-fixation (TD vs FD, −1.31±0.61D vs −0.17±0.88D, p=0.001). These results may suggest that the myopic shift in D-fixation is not only due to the fixation site itself but rather to issues with T-style. In F-style, the flanged haptics are fixed along the direction of scleral insertion, resulting in less impact on the position of the optics. In contrast, in T-style, the haptic tips are inserted 2mm into the half-thickness scleral tunnel and sutured, which may cause the haptic tips to be restrained and the optics to move forward. Additionally, the surgeon’s increased proficiency with F-style post-October 2021 may have contributed to the improved outcomes. Sotani et al reported a tilt of 2.10±1.66° and a mean refractive error from the predicted value of −0.48±0.75 diopters.29 Postoperative visual function with scleral IOL fixation is heavily influenced by IOL tilt and decentration.31 Tokuhisa et al reported that tilts exceeding 10° exponentially affect refractive errors, leading to an exponential myopic shift as tilt increases.32 In this study, the myopic shift in 6 eyes with large tilt (>10°) was −0.59±0.78D, and not statistically significant but, consistent with previous reports, a correlation was observed between tilt/ decentration and refractive error (R²=0.851, p=0.0176*). On the other hand, significant myopic shift was observed in 12 eyes with large decentration (>1.0 mm), with a shift of −1.01±0.93D (p=0.00955**). The involvement of axial length,26 anterior chamber depth, IOL power, and higher-order wavefront aberration31 may need to be analyzed as potential causes. However, improving the T-style or adopting F-style, particularly FD-fixation, may help prevent tilt and decentration, reduce refractive errors, and improve postoperative visual function.
Except for prediction error, the evaluation of postoperative outcomes and complications by fixation methods revealed no significant differences. However, the choice between single and dual fixation was influenced by the degree of ciliary zonule fragility without objective evaluation and patient life expectancy, which involved the surgeon’s subjective judgment and may have impacted the results. Among the background factors, “narrow angles and poor mydriasis” were significantly more common in S-fixation, while “iatrogenic surgical zonular damage” was significantly more common in D-fixation. Although “narrow angles and poor dilation” might have increased complications in S-fixation, there was no significant difference in such as endothelial cell loss or the presence of all complications (p=0.12, p=0.102~1). Damage of lens capsule occurred during haptics scleral fixation, resulting in a higher incidence in D-fixation, though not statistically significant (p=0.102). “Iatrogenic zonular damage” might have negatively impacted postoperative outcomes, particularly decentration and tilt in D-fixation. However, in this study, S-fixation showed slightly larger decentration at 2 and 3 years postoperatively (p=0.091, 0.081), with no statistically significant difference in large tilt or large decentration (p=1, 0.496).
The limitations of this study include the non-randomized design, its retrospective nature, lack of a control group, small sample sizes, difficulty in objectively evaluating the selection of cases with zonular weakness, short follow-up periods, and significant differences in follow-up duration between fixation style. Additionally, subjective judgment by the surgeon in choosing between single and dual fixation, potential performance improvements due to surgical learning curves as fixation methods evolved over time are noted. We should also compare postoperative stability, tilt, and refractive outcomes between IOL optic capture and this capsule-preserving intrascleral fixation. Furthermore, long-term follow-up (over 10 years) is necessary to confirm whether this method, particularly S-fixation, does not lead to progressive tilt, decentration, or subluxation.
Conclusion
This method is suitable for cases with moderate-to-severe zonular weakness such as phacodonesis, where IOL reverse optic capture alone may not prevent future progression and dislocation. By preserving the capsule, it allows IOL implantation without the need for posterior segment surgery, making it a viable option for many anterior segment surgeons. Although capsule damage is more likely during scleral fixation of haptics, the frequency of vitreous prolapse is low, and the absence of iris capture or severe retinal vitreous complications makes this technique valuable. While there was no significant difference, large decentration was more common with S-fixation, and cases with large decentration had a significant myopic shift. D-fixation, particularly TD-fixation, also showed a significant myopic shift. Although the choice of cases and experience may influence the outcomes, FD-fixation might be a better option.
Ethics Approval and Informed Consent
This study protocol was approved by the Medical Ethics Committee of the Japanese Red Cross Okayama Hospital (approval number 2020-56). All clinical procedures adhered to the principles of the Declaration of Helsinki. Informed consent was obtained from all patients after providing a thorough explanation of the study protocol, surgical methods, ophthalmic examinations, and potential complications.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Mönestam EI. Incidence of dislocation of intraocular lenses and pseudophakodonesis 10 years after cataract surgery. Ophthalmology. 2009;116(12):2315–2320. doi:10.1016/j.ophtha.2009.05.015
2. Gimbel HV, Condon GP, Kohnen T, Olson RJ, Halkiadakis I. Late in-the-bag intraocular lens dislocation: incidence, prevention, and management. Journal of Cataract & Refractive Surgery. 2005;31(11):2193–2204. doi:10.1016/j.jcrs.2005.06.053
3. Mönestam E. Frequency of intraocular lens dislocation and pseudophacodonesis, 20 years after cataract surgery – a prospective study. American Journal of Ophthalmology. 2019;198:215–222. doi:10.1016/j.ajo.2018.10.020
4. Dabrowska-Kloda K, Kloda T, Boudiaf S, Jakobsson G, Stenevi U. Incidence and risk factors of late in-the-bag intraocular lens dislocation: evaluation of 140 eyes between 1992 and 2012. Journal of Cataract & Refractive Surgery. 2015;41(7):1376–1382. doi:10.1016/j.jcrs.2014.10.040
5. Ascaso FJ, Huerva V, Epidemiology GA. Etiology, and prevention of late IOL-capsular bag complex dislocation: review of the literature. Journal of Ophthalmology. Hindawi. 2015;e805706.
6. Werner L, Zaugg B, Neuhann T, Burrow M, Tetz M. In-the-bag capsular tension ring and intraocular lens subluxation or dislocation: a series of 23 cases. Ophthalmology. 2012;119(2):266–271. doi:10.1016/j.ophtha.2011.08.016
7. Gül Koçak Altıntaş AEO A. Spontaneous late intraocular lens and capsule tension ring dislocation [Internet]. Turkish Journal of Ophthalmology Available from: https://oftalmoloji.org/articles/doi/tjo.79836.
8. Taskin I, Altinbay D, Ozdemir VN. A different surgical approach to cases with zonular weakness or dialysis: sutureless transscleral fixated intraocular lens implantation and stabilization of lens capsule. Int Ophthalmol. 2020;40(9):2315–2323. doi:10.1007/s10792-020-01416-2
9. Kato M, Namba M, Shimoyama S, Inoue M, Ouchi C, Shimizu T. Intrascleral intraocular lens fixation preserving the lens capsule in cases of cataract with insufficient zonular support. Clinical Ophthalmology. 2022;16:93–100. doi:10.2147/OPTH.S344523
10. Ucar F. Intrascleral fixation of capsular bag and intraocular lens in cases with large zonular dialysis. Int Ophthalmol. 2022;43(1):131–140. doi:10.1007/s10792-022-02395-2
11. Gimbel HV, DeBroff BM. Intraocular lens optic capture. Journal of Cataract & Refractive Surgery. 2004;30(1):200–206. doi:10.1016/j.jcrs.2003.11.035
12. Devranoğlu K, Kılıç A, Özdamar A, Yurtsever AK. Intraocular lens optic capture in eyes with zonular weakness in cataract patients. J Cataract Refract Surg. 2013;39(5):669–672. doi:10.1016/j.jcrs.2013.02.035
13. Bhaskaran J, Narayanan S, Balamurali R. Three-piece intraocular lens in the sulcus with optic capture in patients with mild to moderate zonular weakness in exfoliation. Indian Journal of Ophthalmology. 2022;70(12):4312. doi:10.4103/ijo.IJO_1415_22
14. Yamane S, Maruyama-Inoue M, Kadonosono K. Needle stabilizer for flanged intraocular lens fixation. Retina. 2019;39(4):801. doi:10.1097/IAE.0000000000002455
15. Nagy Z, Takacs A, Filkorn T, Sarayba M. Initial clinical evaluation of an intraocular femtosecond laser in cataract surgery. Journal of Refractive Surgery. 2009;25(12):1053–1060. doi:10.3928/1081597X-20091117-04
16. Grewal DS, Basti S, Grewal SPS. Femtosecond laser–assisted cataract surgery in a subluxated traumatic cataract. Journal of Cataract & Refractive Surgery. 2014;40(7):1239. doi:10.1016/j.jcrs.2014.05.018
17. Gimbel HV, Sun R, Heston JP. Management of zonular dialysis in phacoemulsification and IOL implantation using the capsular tension ring. Ophthalmic Surg Lasers. 1997;28(4):273–281. doi:10.3928/1542-8877-19970401-03
18. Yang S, Jiang H, Nie K, Feng L, Fan W. Effect of capsular tension ring implantation on capsular stability after phacoemulsification in patients with weak zonules: a randomized controlled trial. CTR implantation in cataract patients with weak zonules. BMC Ophthalmol. 2021;21(1):19. doi:10.1186/s12886-020-01772-8
19. Cionni RJ, Osher RH, Marques DMV, Marques FF, Snyder ME, Shapiro S. Modified capsular tension ring for patients with congenital loss of zonular support. Journal of Cataract & Refractive Surgery. 2003;29(9):1668–1673.
20. Hasanee K, Butler M, Ahmed IIK. Capsular tension rings and related devices: current concepts. Asano. 2006;17(1):31–41.
21. Gabor SGB, Pavlidis MM. Sutureless intrascleral posterior chamber intraocular lens fixation. J Cataract Refract Surg. 2007;33(11):1851–1854. doi:10.1016/j.jcrs.2007.07.013
22. Yamane S, Inoue M, Arakawa A, Kadonosono K. Sutureless 27-gauge needle-guided intrascleral intraocular lens implantation with lamellar scleral dissection. Ophthalmology. 2014;121(1):61–66. doi:10.1016/j.ophtha.2013.08.043
23. Yamane S, Sato S, Maruyama-Inoue M, Kadonosono K. Flanged intrascleral intraocular lens fixation with double-needle technique. Ophthalmology. 2017;124(8):1136–1142. doi:10.1016/j.ophtha.2017.03.036
24. Ohta T, Toshida H, Murakami A. Simplified and safe method of sutureless intrascleral posterior chamber intraocular lens fixation: y-fixation technique. J Cataract Refract Surg. 2014;40(1):2–7. doi:10.1016/j.jcrs.2013.11.003
25. Yaguchi S, Yaguchi S, Yagi-Yaguchi Y, Kozawa T, Bissen-Miyajima H. Objective classification of zonular weakness based on lens movement at the start of capsulorhexis. PLoS One. 2017;12(4):e0176169. doi:10.1371/journal.pone.0176169
26. Kabata Y, Oki T, Nakano T. Comparison of refractive prediction error by axial length in flanged intrascleral intraocular lens fixation. Clinical Ophthalmology. 2024;18:895–900. doi:10.2147/OPTH.S455178
27. Ishii N, Yamaguchi T, Yazu H, Satake Y, Yoshida A, Shimazaki J. Factors associated with graft survival and endothelial cell density after descemet’s stripping automated endothelial keratoplasty. Sci Rep. 2016;6:25276. doi:10.1038/srep25276
28. Kurimori HY, Inoue M, Hirakata A. Adjustments of haptics length for tilted intraocular lens after intrascleral fixation. Tori. 10:180–184.
29. Sotani Y, Imai H, Iwane Y, et al. Usefulness of intraoperative optical coherence tomography to minimize the intraocular lens tilt during the intrascleral fixation: a clinical and experimental evaluation. Sci Rep. 2023;13(1):12065. doi:10.1038/s41598-023-39294-0
30. Fukumoto R, Inoue M, Ishida T, Koto T, Hirakata A. Adjustment of intraocular lens tilt during intrascleral fixation assisted by intraoperative OCT. J Cataract Refract Surg. 2021;47(10):1308–1313. doi:10.1097/j.jcrs.0000000000000615
31. Oshika T, Sugita G, Miyata K, et al. Influence of tilt and decentration of scleral-sutured intraocular lens on ocular higher-order wavefront aberration. British Journal of Ophthalmology. 2007;91(2):185–188. doi:10.1136/bjo.2006.099945
32. Tokuhisa T, Watanabe T, Watanabe A, Nakano T. Refractive error induced by intraocular lens tilt after intrascleral intraocular lens fixation. Int Ophthalmol. 2022;42(4):1213–1220. doi:10.1007/s10792-021-02106-3
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





