Back to Journals » Journal of Inflammation Research » Volume 17
Case Report and Literature Review of “Treatment Failure” of Aspergillus Infection Secondary to Influenza a Virus
Authors Teng P, Liu Y, Zhang X , Luan N, Han X , Liu X
Received 15 September 2024
Accepted for publication 28 November 2024
Published 4 December 2024 Volume 2024:17 Pages 10305—10311
DOI https://doi.org/10.2147/JIR.S496441
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tara Strutt
Peikun Teng,1 Yuzhan Liu,1 Xingyu Zhang,2 Nianxu Luan,1 Xiudi Han,1 Xuedong Liu1
1Department of Respiratory and Critical Care Medicine, Qingdao Hospital, University of Health and Rehabilitation Sciences (Qingdao Municipal Hospital), Qingdao, 266000, People’s Republic of China; 2Human Resources Department, Qingdao Hospital, University of Health and Rehabilitation Sciences (Qingdao Municipal Hospital), Qingdao, 266000, People’s Republic of China
Correspondence: Xuedong Liu; Xiudi Han, Department of Respiratory and Critical Care Medicine, Qingdao Hospital, University of Health and Rehabilitation Sciences (Qingdao Municipal Hospital), Qingdao, 266000, People’s Republic of China, Tel +86-18661678256 ; +86-15315002781, Email [email protected]; [email protected]
Purpose: Increased clinical manifestations combined with increased lung imaging findings during antifungal therapy are often misjudged as failure of antifungal therapy, and should be vigilant against immune reconstitution inflammatory syndrome.
Case: We describes a case of invasive pulmonary Aspergillus infection after Influenza A Virus (IAV). After active antifungal therapy, the patient’s clinical symptoms continued to worsen, imaging lesions continued to progress, laboratory indicators improved, and immune reconstitution inflammatory syndrome was considered.
Conclusion: The clinical characteristics and treatment process of this case were summarized, and related literature search was carried out, in order to provide a new perspective for the treatment failure of fungal infection in the future, and to avoid the random change of antifungal drugs, which may lead to the increase of drug resistance.
Keywords: Aspergillus infection, influenza A virus, immune reconstitution inflammatory syndrome
Introduction
Fungal diseases claim the lives of over 1.5 million individuals annually and cumulatively impact more than 1 billion people.1 It is estimated that approximately 250,000 people worldwide develop invasive aspergillosis each year, mainly in people with chronic obstructive pulmonary disease, admission to intensive care units, lung cancer or hematologic malignancies with relatively low immune function.1,2 The growth of Aspergillus in the lungs results in tissue destruction, invasion of blood vessels, and sepsis accompanied by hemoptysis. At present, the case fatality rate of invasive pulmonary aspergillosis is as high as 30% to 80%.3 Although early and accurate diagnosis can advance antifungal therapy, the therapeutic effect is not always satisfactory, so that clinical diagnosis and treatment enter a bottleneck. This article describes a case of invasive pulmonary aspergillus infection after influenza A virus (IAV) infection. After adequate antifungal therapy, the patient’s clinical symptoms continued to worsen, imaging lesions continued to progress, and laboratory indicators improved. Clinical diagnosis and treatment were often misjudged as the failure of antifungal treatment, which led to the random replacement of antifungal drugs, and should be vigilant against immune reconstitution inflammatory syndrome (IRIS). IRIS refers to the excessive and intense immune response against various antigens in the process of recovery from immunosuppression, and the deterioration of clinical symptoms and imaging changes, which usually occur in Human immunodeficiency virus (HIV), tuberculosis.4–6 This case provides a new perspective for the diagnosis and treatment of clinical fungal infection.
Case Presentation
Patients were male, 68 years, due to the “continuous cough, fever” 10 days on April 2, 2023 to the hospital. On March 24, 2023, the patient developed cough and yellow phlegm due to cold, accompanied by fever and chills, which were not continuous and irregular, and the temperature was up to 38.9°C. The anti-infection treatment in the hospital was not effective, but he still had cough and fever. He had smoked 20 cigarettes per day for more than 40 years. He had no previous underlying diseases such as diabetes and had not used immunosuppressants.
On admission, Physical examination on admission revealed the patient had clear consciousness, thick breathing sound in both lungs, wet rales could be heard, slight wheezing in the right lung, no abnormal heart, and no edema in both lower limbs. A complete chest CT scan showed double pneumonia (Figure 1).
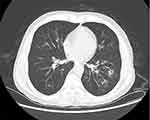 |
Figure 1 Chest CT showed that the double lung lesions were found distributed along the air bundle, with some of them forming spots on April 2, 2023. |
The laboratory tests after admission showed the Leukocytes were 21.66×109/L, Neutrophils was 18.57×109/L, C-reactive protein was 104.84mg/l, Interleukin-6 was 171.09pg/mL, IgG antibody detection value of Aspergillus was 65.055 AU/mL (< 80AU/mL was negative). The anti-infective treatment of cefoperazone sulbactam (3.0g intravenous q8h) + minocyclic (100mg oral q12h, double the first dose) was given first. Improved Influenza A Virus (IAV) nucleic acid test positive (Ct value 27.24), blood GM test: Aspergillus galactomannan determination 2.317↑ (cut off: ≤0.499). Bronchoscopy + alveolar lavage (Figure 2): viscous secretions and mucosal congestion are visible on both bronchus. Bronchoscopic alveolar lavage fluid (BALF) -Metagenomics next generation sequencing (mNGS) results return: Aspergillus fumigatus (sequence number 303, relative abundance 53%). BALF-GM test: Determination of Aspergillus galactomannan 4.539↑ (cut off: ≤0.499). BALF- Culture of bacteria and fungi: Aspergillus fumigatus and Aspergillus flavus.
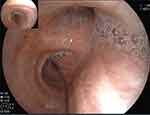 |
Figure 2 Bronchoscopy showed that more white sputum retention and mucous hyperemia in the main and left and right bronchus on April 4, 2023. |
On April 4, 2023, the treatment regimen was adjusted to voriconazole (0.2g q12h, double the dose on the first day) + Peramivir. The patient still had persistent fever with no decrease in peak value. On April 6, 2023, chest CT showed that the lesion was worse than before (Figure 3). On April 11, 2023, the reexamination of blood GM test 0.395 (negative) showed that leukocyte, neutrophil, CRP and procalcitonin all decreased, and chest CT showed that the inflammation continued to progress more than before (Figure 4). Considering the poor efficacy of voriconazole and drug resistance, the antifungal treatment was replaced with amphotericin B cholesterol sulfate complex (200mg/ day pump).
 |
Figure 3 Chest CT showed that both lung lesions were more severe than before, and some lesions were fused into slices on April 6, 2023. |
 |
Figure 4 Chest CT showed that the lesions of double lung disease were more advanced on April 11, 2023. |
After active treatment, the patient still had fever, cough and asthma, which continued to worsen, and the inflammation index (leukocyte, neutrophil, CRP) decreased in reexamination. Blood gas analysis showed PO2 61mmHg (oxygen concentration 37%), and lymphocyte subgroup test showed T lymphocytes 71.72% (reference range 61–85%). B lymphocyte 20.67% (reference range 8.01–15.47%), improved chest CT showed continued progression of double pneumonia with ground glass exudation on April 17, 2023 (Figure 5). Considering the presence of immune reconstructive inflammatory syndrome during the treatment of fungal infection, methylprednisolone 30mg bid (0.5mg/kg) was added on April 17, 2023, and the patient’s symptoms of dyspnea and cough were alleviated. The chest CT examination on April 26, 2023 showed (Figure 6): The absorption of the ground glass shadow in both lungs improved, and she was discharged on April 27, 2023. After discharge, she continued to take oral hormones and gradually reduced the amount.
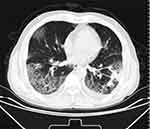 |
Figure 5 Chest CT showed that the lesion continued to progress, and the original solid lesion was slightly reduced, accompanied by ground glass shadow exudation on April 17, 2023. |
 |
Figure 6 Chest CT showed, that both lung glass shadow absorption improved on April 26, 2023. |
Discussion
Influenza is closely related to Aspergillus infection, the most common fungus being Aspergillus fumigatus (17.8%).7 In recent years, the incidence of influenza-associated pneumomycosis (IAPA) has been as high as 20% in influenza patients. The incidence of invasive pulmonary aspergillus (IPA) in patients admitted to intensive care units (ICU) with severe influenza was 19.2% in a retrospective cohort study. Influenza (aOR 5.19; 95% CI 2.63–10.26; p<0.0001) is a risk factor for IPA and is associated with a high mortality rate (51% 90-day mortality, p=0.0001).8 The 2018 European Guidelines for the Diagnosis and Management of Aspergillus disease clearly listed influenza patients as a high-risk group for Aspergillus infection.9 It has also been reported that HIV patients can develop invasive aspergillosis and chronic pulmonary aspergillosis secondary, especially in those with severely compromised immune function and who do not respond to conventional treatment should still consider invasive aspergillosis infection. In HIV patients with tuberculosis, attention should be paid to the occurrence of chronic pulmonary aspergillosis.10 Clinical attention should be paid to immunocompromised people, and early fungal screening should be called for, and early diagnosis and treatment should be suggested to improve the prognosis of patients.
In this case, it was clear that the patient was infected with IAV at the early stage when he was admitted to the hospital. In combination with imaging, Aspergillus infection was highly suspected, and etiological detection was actively improved. Finally, Aspergillus infection was clinically diagnosed by mNGS and sputum culture. Only by providing sufficient etiological basis, strengthening the communication and cooperation between clinical and microbiological room, and improving the diagnostic level of microbiological room, can we provide guidance and support for subsequent treatment.
Although the patient was clearly diagnosed as a secondary aspergillus infection of IAV, after adequate antifungal treatment, the patient still had persistent fever accompanied by worsening clinical symptoms, and chest CT indicated that the inflammation continued to progress. Clinical diagnosis and treatment were often misjudged as the failure of antifungal treatment, which led to the random replacement of antifungal drugs, and then the occurrence of antifungal drug resistance. In the treatment of this patient, despite adjustment of antifungal drugs, the effect was still not well, but the inflammatory indicators decreased, and no obvious immune function depletion was observed in the lymphocyte subgroup. Considering the presence of immune reestablishment inflammatory syndrome (IRIS), the patient’s condition gradually improved after subsequent hormone therapy, further confirming the diagnosis.
IRIS refers to the clinical phenomenon of excessive and intense immune response in response to various antigens, including disease-causing microorganisms, drugs and unknown autoantigens, in the process of recovery from immunosuppression, which destroys normal tissues and leads to deterioration of clinical symptoms and aggravation of imaging changes.11 It is commonly seen in the inflammatory reaction of AIDS patients after receiving antiretroviral therapy (ART) or in the process of immune function recovery in immunocompromised patients, and it can also be seen in tuberculosis patients.4–6 However, fungal infections are rarely reported, mainly in cryptococcus.12
Delliere et al diagnosed IRIS as a new or worsening clinical or radiographic manifestation consistent with the inflammatory process; Symptoms that cannot be explained by emerging infection and negative culture results and/or decreased levels of fungal antigens (G test, GM test, etc).13 Miceli et al defined IPA-IRIS as “an infectious/inflammatory lung disease with worsening clinical and lung imaging findings, temporary association with neutrophil recovery, and microbiological evidence. (50% decrease in serum GM titer measured twice over 4 days)”14 In this case, the etiological diagnosis was clear, and the blood GM titer decreased by more than 50% twice 4 days apart. On the basis of the original antifungal treatment, combined with hormones, the clinical symptoms and imaging absorption of the patient were improved.
At present, there is still a lack of treatment guidelines for non-HIV-IRIS. The pathology of IRIS is related to a variety of innate and adaptive immune factors, including CD4+T cells, CD8+T cells, macrophages, pro-inflammatory cytokines and chemokines, and the most commonly used drugs are corticosteroids for anti-inflammatory activity.5,15 It can reduce the clinical symptoms, shorten the length of hospital stay, and reduce the need for surgery in TB related IRIS patients.16 The specific dosage and course of treatment still need to be further discussed. In addition, the process of Aspergillus infection is involved by a variety of cytokines and chemokines, including IFN-γ, CCR4, CCR17 and so on. Invasive aspergillosis involves Th1 and Th17 cells activating various immune cells through IFN-γ, IL-1, IL-6, and IL-17, and stimulating the production of other immune molecules that help clear fungal pathogens, such as antimicrobial peptides and reactive oxygen species.17 TNF-α inhibitors, anti-IL-6 antibodies, and statins can also be used as anti-inflammatory treatment options.18–20 Aerosol Pam2ODN immunomodulatory therapy significantly improved disease severity and mortality in IAPA mice and alleviated suppressed immune responses due to fungal infection, the study reported.21 It has been reported in the literature that corticosteroid treatment can significantly reduce the anti-Aspergillus IgG antibodies in patients and increase the susceptibility to aspergillus.22 Receiving glucocorticoids increases the risk of aspergillus infection in COVID-19 patients.23 Our case suggested that although the patient had secondary aspergillus after IAV and complicated with IRIS after treatment failure, the treatment with methylprednisolone (0.5mg/kg) was sufficient to improve the prognosis of the patient.
Conclusion
Early molecular identification of the microbial pathogen including Aspergillus and drug sensitivity and resistance pattern could assist in selecting the drug of choice for better treatment. In addition, increased clinical manifestations combined with increased lung imaging findings during antifungal therapy are often misjudged as failure of antifungal therapy, and should be vigilant against IRIS combined with the patient’s condition.
Abbreviations
IAV, Influenza A Virus; BALF, Bronchoscopic Alveolar Lavage Fluid; IRIS, Immune Reestablishment Inflammatory Syndrome; CRP, C-Reactive Protein; MNGS, Metagenomics Next Generation Sequencing; IAPA, Influenza-Associated Pneumomycosis; IPA, Invasive Pulmonary Aspergillus.
Ethics Statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. Ethical review and approval were not required to publish the case details in accordance with the institutional requirements.
Acknowledgments
The authors thank the patient who participated in this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
National Clinical Key Specialty Construction Project (Department of Respiratory Medicine) (GJZDZK-002).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bongomin F, Gago S, Oladele RO, et al. Global and multi-national prevalence of fungal diseases-estimate precision. J Fungi. 2017;3(4):57. doi:10.3390/jof3040057
2. Denning DW. Global incidence and mortality of severe fungal disease. Lancet Infect Dis. 2024;24(7):e428–e438. doi:10.1016/S1473-3099(23)00692-8
3. Latgé J-P, Chamilos G. Aspergillus fumigatus and Aspergillosis in 2019. Clin Microbiol Rev. 2019;33:e00140–18. doi:10.1128/CMR.00140-18
4. Vinhaes CL, Araujo-Pereira M, Tibúrcio R, et al. Systemic inflammation associated with immune reconstitution inflammatory syndrome in persons living with HIV. Life. 2021;11(1):65. doi:10.3390/life11010065
5. Gopal R, Rapaka RR, Kolls JK. Immune reconstitution inflammatory syndrome associated with pulmonary pathogens. Eur Respir Rev. 2017;26(143):160042. doi:10.1183/16000617.0042-2016
6. Nayak B, Panda RK, Mohanty T, et al. Incidence and pattern of tuberculosis immune reconstitution inflammatory syndrome (TB-IRIS) in patients on anti-retroviral therapy: an observational study. Indian J Tuberc. 2024;71(3):291–296. doi:10.1016/j.ijtb.2023.05.015
7. Beumer MC, Koch RM, van Beuningen D, et al. Influenza virus and factors that are associated with ICU admission, pulmonary co-infections and ICU mortality. J Crit Care. 2019;50:59–65. doi:10.1016/j.jcrc.2018.11.013
8. Schauwvlieghe AFAD, Rijnders BJA, Philips N, et al. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: a retrospective cohort study. Lancet Respir Med. 2018;6(10):782–792. doi:10.1016/S2213-2600(18)30274-1
9. Ullmann AJ, Aguado JM, Arikan‐Akdagli S, et al. Diagnosis and management of Aspergillus diseases: executive summary of the 2017 ESCMID‐ECMMERS guideline. Clin Microbiol Infect. 2018;24(Suppl 1):
10. Truda VSS, Falci DR, Porfírio FMV, et al. A contemporary investigation of burden and natural history of aspergillosis in people living with HIV/AIDS. Mycoses. 2023;66(7):632–638. doi:10.1111/myc.13589
11. Sueki H, Mizukawa Y, Aoyama Y. Immune reconstitution inflammatory syndrome in non-HIV immunosuppressed patients. J Dermatol. 2018;45(1):3–9. doi:10.1111/1346-8138.14074
12. Vlasova-St Louis I, Mohei H. Molecular diagnostics of Cryptococcus spp. and immunomics of cryptococcosis-associated immune reconstitution inflammatory syndrome. Diseases. 2024;12(5):101. doi:10.3390/diseases12050101
13. Dellière S, Guery R, Candon S, et al. Understanding pathogenesis and care challenges of immune reconstitution inflammatory syndrome in fungal infections. J Fungi. 2018;4(4):139. doi:10.3390/jof4040139
14. Miceli MH, Maertens J, Buvé K, et al. Immune reconstitution inflammatory syndrome in cancer patients with pulmonary aspergillosis recovering from neutropenia: proof of principle, description, and clinical and research implications. Cancer. 2007;110(1):112–120. doi:10.1002/cncr.22738
15. Marais S, Wilkinson RJ, Pepper DJ, et al. Management of patients with the immune reconstitution inflammatory syndrome. Curr HIV/AIDS Rep. 2009;6(3):162–171. doi:10.1007/s11904-009-0022-z
16. Meintjes G, Wilkinson RJ, Morroni C, et al. Randomized placebo-controlled trial of prednisone for paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome. AIDS. 2010;24:2381–2390. doi:10.1097/QAD.0b013e32833dfc68
17. Shankar J, Thakur R, Clemons KV, et al. Interplay of cytokines and chemokines in Aspergillosis. J Fungi. 2024;10(4):251. doi:10.3390/jof10040251
18. Armange L, Lacroix A, Petitgas P, et al. The use of TNF-α antagonists in tuberculosis to control severe paradoxical reaction or immune reconstitution inflammatory syndrome: a case series and literature review. Eur J Clin Microbiol Infect Dis. 2023;42(4):413–422. doi:10.1007/s10096-023-04564-2
19. Watanabe S, Kaneko Y, Kawamoto H, et al. Paradoxical response with increased tumor necrosis factor-α levels to anti-tuberculosis treatment in a patient with disseminated tuberculosis. Respir Med Case Rep. 2017;20:201–204. doi:10.1016/j.rmcr.2017.02.011
20. Falagas ME, Makris GC, Matthaiou DK, et al. Statins for infection and sepsis: a systematic review of the clinical evidence. J Antimicrob Chemother. 2008;61(4):774–785. doi:10.1093/jac/dkn019
21. García JP, Wurster S, Albert ND, et al. Immunotherapy with nebulized pattern recognition receptor agonists restores severe immune paralysis and improves outcomes in mice with influenza-associated pulmonary aspergillosis. bioRxiv. 2024. doi:10.1101/2024.10.02.616293
22. Sarden N, Sinha S, Potts KG, et al. A B1a-natural IgG-neutrophil axis is impaired in viral- and steroid-associated aspergillosis. Sci Transl Med. 2022;14(674):eabq6682. doi:10.1126/scitranslmed.abq6682
23. Feys S, Almyroudi MP, Braspenning R, et al. A visual and comprehensive review on COVID-19-associated pulmonary Aspergillosis (CAPA). J Fungi. 2021;7(12):1067. doi:10.3390/jof7121067
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

