Back to Journals » Journal of Inflammation Research » Volume 17
Circulating Protectin D1 and Neutrophils Extracellular Traps in the Diagnosis and Progression of Acute Pancreatitis
Authors Wu Z, Lu W, Zhang X, Xia Q, Zuo H, Guo X, Liu Y, Zhang F, Zhang X, Zhang L
Received 17 September 2024
Accepted for publication 29 October 2024
Published 5 November 2024 Volume 2024:17 Pages 8215—8225
DOI https://doi.org/10.2147/JIR.S494649
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tara Strutt
Zhiyang Wu,1,* Wenjun Lu,2,* Xin Zhang,1 Qiaoying Xia,2 Han Zuo,2 Xi Guo,1 Yu Liu,1 Fan Zhang,3 Xin Zhang,4 Luyao Zhang2
1Department of Critical Care Medicine, Qilu Hospital (Qingdao), Cheeloo College of Medicine, Shandong University, Qingdao, Shandong, People’s Republic of China; 2Department of Pathology and Pathophysiology, School of Medicine, Nanjing University of Chinese Medicine, Nanjing, Jiangsu, People’s Republic of China; 3Department of Critical Care Medicine, Qilu Hospital, Cheeloo College of Medicine, Shandong University, Qingdao, Shandong, People’s Republic of China; 4Department of General Surgery, Qilu Hospital (Qingdao), Cheeloo College of Medicine, Shandong University, Qingdao, Shandong, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Luyao Zhang, Department of Pathology and Pathophysiology, School of Medicine, Nanjing University of Chinese Medicine, Nanjing, Jiangsu, People’s Republic of China, Email [email protected] Xin Zhang, Department of General Surgery, Qilu Hospital (Qingdao), Cheeloo College of Medicine, Shandong University, Qingdao, Shandong, People’s Republic of China, Email [email protected]
Purpose: Protectin D1 (PD1), a biologically active molecule derived from docosahexaenoic acid (DHA), plays a major role in the body’s endogenous lipid-mediated inflammatory response. The study aims to explore the relationship between PD1, inflammatory response and the severity of acute pancreatitis (AP).
Patients and Methods: Sixty consecutive AP patients within 72h of disease onset were enrolled as the study group, a further thirty healthy people were enrolled as the control group. General demographics collected at the time of enrollment. Serum PD1, Citrullinated Histone 3 (CitH3), myeloperoxidase-Deoxyribonucleic acid (MPO-DNA), tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6) level were measured in AP patients on enrollment day 0, day 1, day 3 and day 7. Meanwhile, the Acute Physiology and Chronic Health evaluation II (APACHE II) scores, Sequential Organ Failure Assessment (SOFA) scores were also evaluated on day 0, day 1, day 3 and day 7.
Results: AP was severe in 29 patients (48.3%), moderately severe acute pancreatitis (MSAP) was found in 9 patients (15%), and mild acute pancreatitis (MAP) was found in 22 patients (36.7%). The level of PD1, CitH3 and MPO-DNA were statistically significantly higher in AP patients than in the healthy population. Serum PD1, CitH3 and MPO-DNA concentration increased with AP severity. In AP patients, PD1 has a strong linear association with TNF-α, CitH3 and MPO-DNA. The AUC for SAP predicted by PD1 was 0.938. The calculated cut-off point for prognosis SAP is 7.94 pg/mL. The AUC for infected pancreatic necrosis (IPN) predicted by PD1 was 0.836 and the cut-off point was 8.65 pg/mL. The AUC for organ failure (OF) predicted by PD1 was 0.719 and the cut-off point was 7.94 pg/mL.
Conclusion: PD1 is associated with both the presence of AP and the severity of pancreatitis.
Keywords: PD1, acute pancreatitis, CitH3, MPO-DNA, infected pancreatic necrosis, organ failure
Introduction
Acute pancreatitis (AP) is an inflammatory disease of the pancreas, the leading cause of hospitalization for gastrointestinal disorders.1 It is characterized by the activation of digestive enzymes within the acinar cells, leading to a subsequent inflammatory response. The course of AP is variable and complex, and is often unpredictable in the early stages of the disease. Approximately 80% of patients develop mild to moderately severe disease (organ failure not exceeding 48 hours) which runs a self-limiting course.2 Nevertheless, one in five patients are severely ill, with a mortality rate of approximately 20%.2 Despite improvements in access to care, imaging and interventional techniques, AP continues to be associated with significant morbidity and mortality.3 The search for early prediction and diagnosis of AP severity, infected pancreatic necrosis (IPN) and organ failure (OF) through the exploration of bioindicators is of paramount importance in determining further treatment and prognosis.4
Protective protectin D1 (PD1) is a biologically active product derived from docosahexaenoic acid,5 which has been demonstrated to exert anti-inflammatory effects in a range of diseases, including acute kidney injury and neurodegenerative disorders.6,7 It modulates the innate immune response and stimulates resolution. Our previous study on animals also demonstrated that exogenous supplementation PD1 ameliorated AP by reducing the early infiltration of neutrophils into the pancreas and the formation of neutrophil extracellular traps (NETs).8
The aim of this study was to examine the potential correlation between PD1, circulating indicators of NETs formation and disease severity in the acute phase of AP. Furthermore, we also attempted to see whether PD1 can predict the severity of AP and the occurrence of IPN and OF.
Materials and Methods
Study Population
A total of 60 adult patients diagnosed with SAP were admitted to Qilu Hospital, Qingdao, China. The study population consisted of individuals who experienced abdominal pain between November 2023 and March 2024 and were enrolled within 72 hours of the onset of symptoms. The study was approved by the Ethics Committee of the Qilu Hospital and registered with Chictr.cn (identifier ChiCTR2400085321) and adhered to ethical principles outlined in the Helsinki Declaration. Informed consent was obtained from the patients or their next of kin before enrollment. The diagnostic criteria for AP were defined in accordance with the Atlantic criteria.9 Patients with one or more of the following conditions were excluded from the study: (1) chronic pancreatitis, (2) pre-existing OF, (3) prior surgical intervention, (4) malignant tumor. All patients received standard treatment for AP.10
Definitions
The diagnosis of AP9 requires the presence of two or more of the following three features: (1) abdominal pain consistent with AP (acute onset of a persistent, severe, epigastric pain often radiating to the back); (2) serum lipase activity (or amylase activity) at least three times greater than the upper limit of normal; and (3) characteristic findings of AP on contrast-enhanced computer tomography and in less common cases, magnetic resonance imaging or transabdominal ultrasonography may be employed.11 Infection may be suspected if percutaneous image-guided fine-needle aspiration, gram stains and cultures are positive for bacteria and/or fungi. IPN may be associated with variable amounts of septicemia (pus), which increases with liquefaction over time.11 IPN includes acute necrotic collection and walled-off necrosis. Acute necrotic collection occurs early in the course of the disease and is characterized by an accumulation of mixed fluid and necrotic tissue. Walled-off necrosis is a solid cystic structure containing pancreatic and/or peripancreatic necrotic tissue with a well-defined inflammatory envelope.3 OF was defined using the modified Marshall scoring system, and OF was defined as a score of 2 or more for any of these three organ systems.12
Mild acute pancreatitis (MAP) is defined by the absence of OF and the absence of local or systemic complications.13 Moderately severe acute pancreatitis (MSAP) is defined by the presence of transient OF (within 48h) or local or systemic complications.14 Severe acute pancreatitis (SAP) is defined by the persistence of OF for a period exceeding 48h.15
Laboratory Assessments
Blood samples were obtained on admission, day 1, day 3 and day 7. Levels of amylase were uniformly measured by department of clinical laboratory in the hospital. Blood samples were immediately centrifuged and stored at −80° Celsius. Serum PD1, citrullinated Histone 3 (CitH3), myeloperoxidase-Deoxyribonucleic acid (MPO-DNA), tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) levels were measured by using commercially available Human ELISA Kits. The sensitivity by the kit is 0.1ng/mL and variation range less than 15%. All ELISA kits were purchased from FANKEW (Shanghai, China).
Clinical Data Collection
Demographic information and clinical relevant data of the patients were duly recorded. The Acute Physiology and Chronic Health evaluation II (APACHE II) scores and Sequential Organ Failure Assessment (SOFA) scores were collected at admission, day 1, day 3 and day 7. Systemic inflammatory response syndrome (SIRS) scores and computed tomography (CT) scores were assessed only at admission.
Statistical Analysis
Data were presented with a description of the median and interquartile range (25th to 75th percentiles). Descriptive data was presented as an absolute number or a percentage number. Continuous variables were tested for normality or lognormality, and the S-W test results were viewed and the normal QQ plot was drawn to determine whether the distribution was normal. Differences between groups were analyzed by one-way ANOVA for normally distributed, variance-aligned, independent data. The Kruskal–Wallis rank sum test was used for non-normally distributed data. Serum parameters over time in different subgroups were compared using repeated measures ANOVA. Correlation analyses between PD1 and other indicators employed either Pearson or Spearman tests, depending on the characteristics of the data. The comparison of the efficacy of the prediction of AP severity, IPN and OF was calculated by the area under the receiver operating characteristic curve (ROC). A p value beneath 0.05 was deemed to be statistically significant. All data were analyzed with SPSS version 27.0 and GraphPad Prism version 8.0.
Results
Characteristics of Patients
A total of 60 patients with AP were included in the study. AP was mild in 22 patients (36.7%); moderate in 9 patients (15%) and severe in 29 patients (48.3%). The etiology of pancreatitis was biliary in 24 patients (40%), hyperlipidemic in 22 patients (36.7%), alcoholic in 2 patients (3.3%) and due to other causes in 12 patients (20%). Of 60 enrolled patients, 5 patients (8.3%) developed IPN, 4 patients (6.7%) developed OF (Table 1).
 |
Table 1 Characteristics of Patients with AP Included in the Study |
PD1, CitH3 and MPO-DNA Level in Prediction of AP Incidence
On admission, the levels of PD1, CitH3 and MPO-DNA in the AP group were significantly higher than non-AP group (p<0.0001). The median PD1, CitH3, MPO-DNA level was 8.38 pg/mL, 12.73 pg/mL, 65.31 pg/mL in AP patients and 0.99 pg/mL, 0.77 pg/mL, 6.01 pg/mL in non-AP patients (Figure 1).
 |
Figure 1 Comparison of serum PD1, CitH3, MPO-DNA levels in non-AP and AP patients. (a) Serum PD1 levels. (b) Serum CitH3 levels. (c) Serum MPO-DNA levels. AP, Acute pancreatitis. ****p<0.0001. |
PD1, CitH3 and MPO-DNA Level in Prediction of AP Severity
Serum PD1 concentration values on admission were compared between MAP, MSAP and SAP, with concentrations of PD1 4.01 ± 0.9 pg/mL, 7.83 ± 2.25 pg/mL, and 11.83 ± 3.74 pg/mL. These differences were statistically significant (p<0.01). Concentrations of CitH3 on admission of MAP, MSAP and SAP were 6.81 ± 1.38 pg/mL, 11.53 ± 1.88 pg/mL and 17.59 ± 7.95 pg/mL (p<0.01). MPO-DNA values of these three subgroups on admission were 27.26 ± 8.14 pg/mL, 74.43 ± 25.95 pg/mL and 91.35 ± 29.34 pg/mL (p<0.01) (Figure 2). Overall, serum PD1, CitH3 and MPO-DNA values increased with AP severity.
Predictive Value of PD1 in SAP, IPN and OF
The optimal PD1 concentration threshold to distinguish SAP from MAP and MSAP was determined by creating a ROC curve. PD1 had a higher value in the prediction of SAP compared to APACHE II score and SOFA score. The cut-off value, sensitivity and specificity were shown in Table 2.
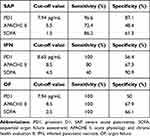 |
Table 2 Values of PD1, APACHE II Score and SOFA Score in the Prediction of SAP, IPN and OF |
We further evaluated the ability of serum PD1 levels to predict IPN. Serum PD1 had the higher AUC of 0.836 compared with APACHE II score and SOFA score in the setting of IPN and non-IPN. For prediction of OF, the AUC was 0.719 for serum PD1, 0.824 for APACHE II score and 0.868 for SOFA score as shown in Figure 3c. PD1 is less valuable than APAPCHE II score in predicting OF.
Change of PD1, CitH3 and MPO-DNA Within the First Week of Hospital Admission
Serum concentrations of PD1, CitH3 and MPO-DNA changes over time (Table 3). All these indexes were markedly elevated in SAP patients relative to non-SAP patients at various time points. There was a decline in serum PD1 levels in both non-SAP and SAP patients when compared to those at the time of admission. A statistically significant difference was observed in serum PD1 levels between day 7 and day 0 (p<0.01). Serum levels of PD-1, CitH3 and MPO-DNA remained consistently high in patients with OF.
 |
Table 3 Serum Concentration of PD1 of Patients in Different Subgroups |
Correlation Analysis Between PD1 and Inflammation/Disease Severity-Related Parameters
The correlation results showed that on the first after admission, there were positive relativity the levels of PD1 and CitH3 (r = 0.7818, p<0.0001), MPO-DNA (r = 0.7655, p<0.0001), TNF-α (r = 0.8222, p<0.0001) and IL-6 (r = 0.7851, p<0.0001). Therefore, there is a strong correlation between PD1 and the indexes of NETS formation and the level of inflammatory factors.
The correlation results also showed positive relativity the levels of PD1 and APACHE II scores (r = 0.3518, p<0.01), SOFA scores (r = 0.3520, p<0.01), CT scores (r = 0.4029, p<0.01) and SIRS scores (r = 0.3280, p<0.05) (Figure 4).
Discussion
The main findings of the present study were as follows. (1) Serum PD1, CitH3 and MPO-DNA were significantly higher in AP patients than in non-AP patients. (2) Serum PD1, CitH3 and MPO-DNA demonstrated a statistically significant increase with increasing AP severity. (3) There is a high correlation between serum PD1 and TNF-α; a significant correlation between PD1 and CitH3, and MPO-DNA; and significant correlation between APACHE II scores, SOFA scores, CT scores and SIRS scores. (4) Serum PD1 has high value in predicting AP severity and IPN.
AP is defined as an inflammatory disease of self-digestion of pancreatic tissue. After the initial injury, infiltration of neutrophils in pancreas is observed.16 On the one hand, activated neutrophils have the capability to capture microbes and to release bactericidal enzymes and radicals, one the other hand, these enzymes and radicals can also be harmful to pancreatic tissue. Upon arrival at the site of injury, neutrophils are activated by various cytokines and bacterial products.17 Neutrophils release DNA from their nuclei and bind it to intracellular proteins to form a network of NETs. In addition to neutralizing pathogens, NETs are involved in sterile inflammation, promote thrombosis and mediate tissue damage.18 They also contribute to tissue remodeling. Merza et al19 first found large amounts of NETs formation in pancreatic tissue in a mouse model of SAP, and NETs exacerbated pancreatitis. Our previous study also identified that NETs played a crucial role in the anti-inflammatory effects of PD1.8 Consistent with prior findings, our current study demonstrated that circulating CitH3 and MPO-DNA—both indicators of NETs—were significantly elevated in patients with AP compared to healthy controls. Moreover, these markers exhibited an increase correlating with the severity of the disease.
PD1 is a potent lipid anti-inflammatory mediator that is produced on demand in the brain and other peripheral immune cells. Schwab et al20 reported that PD1 promotes phagocytic clearance during acute inflammation by modulating leukocyte infiltration, increasing the uptake of apoptotic polymorphonuclear neutrophils by macrophages in vivo and in vitro, and enhancing the appearance of phagocytic enzyme-bearing phagocytes in lymph nodes and spleen. For the first time, PD1 has been demonstrated to exert a protective effect during the acute inflammatory regression phase in an animal model of self-limiting inflammation.21 PD1 has a pro-inflammatory protective effect in cardiovascular disease, COVID19 and neurological disease.22 Gobbetti et al23 provides evidence that systemic treatment with PD1n-3 DPA had beneficial effects on leukocyte reactivity and cytokine production, modulating the outcome of intestinal inflammation. To date, there have been few studies investigating changes in serum PD1 levels in patients experiencing acute inflammation. Our research indicates that serum PD1 was elevated in patients during the acute phase of AP when compared to healthy individuals. Moreover, the degree of elevation correlated with disease severity. It can be hypothesized that this endogenous increase in PD1 serves a negative feedback mechanism within the body, aiming at limiting the progression of inflammation.
Over the past 40 years, various scoring systems have been proposed to predict the severity of AP.24 However, there is no definitive scoring system with high sensitivity and specificity. The interest in identifying new biomarkers and predictive models for SAP demonstrates the clinical importance of early severity prediction. Several clinical scoring systems have been developed and employed and their effectiveness and accuracy compared.25 The APACHE II and SOFA scores are currently commonly used in patients with AP. Our study compared the predictive value of serum PD1 levels with APACHE II and SOFA scores. We found a higher sensitivity and specificity of PD1 as a metric in predicting pancreatitis severity, IPN and OF incidence.
There are some limitations in our study, first, our study was a single-center observation and included a relatively small number of patients; it may be possible to include a larger sample size to obtain more accurate results later. Second, there were only 5 patients developed IPN and 4 patients developed OF, which made it challenging to assess the efficacy of PD1 in these patient populations. PD1 did not show a significant advantage in predicting OF, possibly related to the severity of pancreatitis in the patients included.
In conclusion, our results suggest that PD1 levels correlates with AP severity and predicts the occurrence of SAP, OF and IPN, which are a promising new prognostic marker of SAP.
Conclusion
In the early stage of AP, serum PD1 concentration risen much higher than milder patients and also developed a poorer prognosis. Our study found may be a new prognostic marker for AP.
Data Sharing Statement
The data that support the findings of this study are available on request from the corresponding author (Please contact Luyao Zhang, [email protected]). The data are not publicly available due to privacy or ethical restrictions.
Acknowledgments
This study was supported by Qilu Hospital and Nanjing University of Chinese Medicine. The study was supported by investigators at Qilu Hospital and Nanjing University of Chinese Medicine. We thank all the investigators at these institutions.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was funded by Shandong Provincial Natural Science Foundation (ZR2022QH043), Jiangsu Province Traditional Chinese Medicine Technology Development Plan Project (QN202305) and National Natural Science Foundation of China (82474636).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Lankisch PG, Apte M, Banks PA. Acute pancreatitis [published correction appears in Lancet. 2015 Nov 21;386(10008):2058]. Lancet. 2015;386(9988):85–96. doi:10.1016/S0140-6736(14)60649-8
2. Forsmark CE, Vege SS, Wilcox CM. Acute pancreatitis. N Engl J Med. 2016;375(20):1972–1981. doi:10.1056/NEJMra1505202
3. Greenberg JA, Hsu J, Bawazeer M, et al. Clinical practice guideline: management of acute pancreatitis. Can J Surg J Canadien de Chirurgie. 2016;59(2):128–140. doi:10.1503/cjs.015015
4. Wu BU. Prognosis in acute pancreatitis. Canadian Med Assoc J. 2011;183(6):673–677. doi:10.1503/cmaj.101433
5. Calandria JM, Marcheselli VL, Mukherjee PK, et al. Selective survival rescue in 15-lipoxygenase-1-deficient retinal pigment epithelial cells by the novel docosahexaenoic acid-derived mediator, neuroprotectin D1. J Biol Chem. 2009;284(26):17877–17882. doi:10.1074/jbc.M109.003988
6. Xu ZZ, Liu XJ, Berta T, et al. Neuroprotectin/protectin D1 protects against neuropathic pain in mice after nerve trauma. Ann. Neurol. 2013;74(3):490–495. doi:10.1002/ana.23928
7. Duffield JS, Hong S, Vaidya VS, et al. (2006). Resolvin D series and protectin D1 mitigate acute kidney injury. J Immunol. 1950;177(9):5902–5911. doi:10.4049/jimmunol.177.9.5902
8. Wu Z, Lu G, Zhang L, et al. Protectin D1 decreases pancreatitis severity in mice by inhibiting neutrophil extracellular trap formation. Int Immunopharmacol. 2021;94:107486. doi:10.1016/j.intimp.2021.107486
9. Banks PA, Bollen TL, Dervenis C, et al. Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62(1):102–111. doi:10.1136/gutjnl-2012-302779
10. Heinrich S, Schäfer M, Rousson V, Clavien PA. Evidence-based treatment of acute pancreatitis: a look at established paradigms. Ann Surg. 2006;243(2):154–168. doi:10.1097/01.sla.0000197334.58374.70
11. Banks PA, Freeman ML, Practice Parameters Committee of the American College of Gastroenterology. Practice guidelines in acute pancreatitis. Am J Gastroenterol. 2006;101(10):2379–2400. doi:10.1111/j.1572-0241.2006.00856.x
12. Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med. 1995;23(10):1638–1652. doi:10.1097/00003246-199510000-00007
13. Singh VK, Bollen TL, Wu BU, et al. An assessment of the severity of interstitial pancreatitis. Clin Gastroenterol Hepatol. 2011;9(12):1098–1103. doi:10.1016/j.cgh.2011.08.026
14. Vege SS, Gardner TB, Chari ST, et al. Low mortality and high morbidity in severe acute pancreatitis without organ failure: a case for revising the Atlanta classification to include ”moderately severe acute pancreatitis”. Am J Gastroenterol. 2009;104(3):710–715. doi:10.1038/ajg.2008.77
15. Johnson CD, Abu-Hilal M. Persistent organ failure during the first week as a marker of fatal outcome in acute pancreatitis. Gut. 2004;53(9):1340–1344. doi:10.1136/gut.2004.039883
16. Ishqi HM, Ali M, Dawra R. Recent advances in the role of neutrophils and neutrophil extracellular traps in acute pancreatitis. Clin Exp Med. 2023;23(8):4107–4122. doi:10.1007/s10238-023-01180-4
17. Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13(3):159–175. doi:10.1038/nri3399
18. Mutua V, Gershwin LJ. A Review of Neutrophil Extracellular Traps (NETs) in disease: potential Anti-NETs therapeutics. Clinical Reviews in Allergy & Immunology. 2021;61(2):194–211. doi:10.1007/s12016-020-08804-7
19. Merza M, Hartman H, Rahman M, et al. Neutrophil extracellular traps induce trypsin activation, inflammation, and tissue damage in mice with severe acute pancreatitis. Gastroenterology. 2015;149(7):1920–1931.e8. doi:10.1053/j.gastro.2015.08.026
20. Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447(7146):869–874. doi:10.1038/nature05877
21. Serhan CN, Hong S, Gronert K, et al. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196(8):1025–1037. doi:10.1084/jem.20020760
22. Serhan CN, Bäck M, Chiurchiù V, et al. International Lipids in Parenteral Nutrition Summit 2022 Experts. Expert consensus report on lipid mediators: role in resolution of inflammation and muscle preservation. FASEB j. 2024;38(10):e23699. doi:10.1096/fj.202400619R
23. Gobbetti T, Dalli J, Colas RA, et al. (2017). Protectin D1n-3 DPA and resolvin D5n-3 DPA are effectors of intestinal protection.
24. Cunha EF, Rocha MD, Pereira FP, Blasbalg R, Baroni RH. Walled-off pancreatic necrosis and other current concepts in the radiological assessment of acute pancreatitis. Radiologia brasileira. 2014;47(3):165–175. doi:10.1590/0100-3984.2012.1565
25. Hu JX, Zhao CF, Wang SL, et al. Acute pancreatitis: a review of diagnosis, severity prediction and prognosis assessment from imaging technology, scoring system and artificial intelligence. World J Gastroenterol. 2023;29(37):5268–5291. doi:10.3748/wjg.v29.i37.5268
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




