Back to Journals » Journal of Inflammation Research » Volume 17
Classification and Regression Tree Analysis in Adult Hemophagocytic Syndrome: Identifying Acute Heart Failure Predictors in ICU Patients
Authors Kilincer Bozgul SM , Kurtulmus IA, Yargucu Zihni F , Akad Soyer N, Yagmur B, Gunes A, Koymen G, Bozkurt D
Received 16 August 2024
Accepted for publication 19 November 2024
Published 25 November 2024 Volume 2024:17 Pages 9711—9723
DOI https://doi.org/10.2147/JIR.S491627
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Sukriye Miray Kilincer Bozgul,1 Ilkce Akgun Kurtulmus,2 Figen Yargucu Zihni,3 Nur Akad Soyer,4 Burcu Yagmur,5 Ajda Gunes,4 Gorkem Koymen,6 Devrim Bozkurt1
1Ege University, Faculty of Medicine, Department of Internal Medicine, Division of Intensive Care, Izmir, Turkey; 2Torbalı State Hospital, Izmir, Turkey; 3Ege University, Faculty of Medicine, Department of Internal Medicine, Division of Rheumatology, Izmir, Turkey; 4Ege University, Faculty of Medicine, Department of Internal Medicine, Division of Hematology, Izmir, Turkey; 5Ege University, Faculty of Medicine, Department of Cardiology, Izmir, Turkey; 6Izmir City Hospital, Department of Internal Medicine, Division of Medical Oncology, Izmir, Turkey
Correspondence: Figen Yargucu Zihni, Ege University, Faculty of Medicine, Department of Internal Medicine, Division of Rheumatology, Izmir, Turkey, Email [email protected]
Background: Hemophagocytic syndrome (HPS) is a rare but life-threatening condition often complicated by heart failure (HF). This study aimed to identify predictors of acute heart failure in HPS patients using classification and regression tree (CART) analysis.
Methods: A retrospective analysis was performed on 146 HPS patients without a diagnosis of HF. Variables such as age, cardiothoracic ratio (CTR), etiology, N-terminal pro-brain natriuretic peptide (NT-proBNP), C-reactive protein (CRP), ferritin, triglyceride, and lactate dehydrogenase (LDH) levels at diagnosis were included. CART analysis was employed to develop models predicting heart failure status. The model’s performance was evaluated using sensitivity, specificity, and overall accuracy.
Results: This study identified several key predictors of acute heart failure in HPS patients. CTR emerged as the most significant predictor, with patients exhibiting a higher ratio being at a greater risk of developing heart failure. NT-proBNP levels and CRP levels were also significant predictors, indicating cardiac stress and systemic inflammation. Age and etiology played crucial roles, with older patients and those with rheumatological causes showing a higher susceptibility to heart failure. The CART models demonstrated good accuracy, with CTR being the most important predictor.
Conclusion: This study highlights critical factors, such as CTR, NT-proBNP, CRP levels, age, and etiology in predicting acute heart failure in HPS patients. Early identification of these predictors can facilitate timely interventions, potentially improving outcomes and reducing mortality rates. These findings provide valuable insights for clinical practice and pave the way for further research on acute heart failure management in HPS.
Plain Language Summary: HPS is a serious condition that can lead to heart failure, a life-threatening complication. This study aimed to identify factors that predict heart failure in HPS patients to help doctors diagnose and treat it early. By analyzing data from 146 patients, we found that a higher cardiothoracic ratio, elevated NT-proBNP levels, and increased CRP levels were strong predictors of heart failure. These findings mean that doctors can monitor these factors in HPS patients to catch heart failure early and start treatment sooner, potentially saving lives. The study also found that women with HPS might be more likely to develop heart failure, suggesting they need closer monitoring. Overall, this research provides valuable information that can improve patient care and outcomes in HPS.
Keywords: hemophagocytic syndrome, acute heart failure, CART analysis, predictive factors, inflammation
Introduction
Hemophagocytic syndrome (HPS) is a potentially fatal systemic inflammatory disorder due to the persistent activation of cytotoxic T cells and natural killer cells, coupled with excessive activation of macrophages.1,2 The incidence of HPS increased 11% annually between 2003–2018 in England (a 4-fold increase over 16 years), and a 14% annual increase in 15–54 year-olds and a 16% annual increase in those who are 55 years and older have also been reported.3 In adults, HPS is commonly secondary to infectious, malignant, or autoimmune diseases.1 Clinical and laboratory findings are quite nonspecific, including fever, hepatosplenomegaly, cytopenia, and hyperferritinemia. Most patients require intensive care unit (ICU) admissions as a result of organ dysfunction. In patients with HPS who developed multiorgan dysfunction, mortality rates of 40%–80% have been reported.4–6 Acute liver failure, disseminated intravascular coagulation, acute respiratory failure, decreased level of consciousness, and seizures are the life-threatening clinical manifestations of HPS.7 Cardiovascular complications in HPS patients have been reported as acute heart failure, acute myocardial infarction, and circulatory failure in recent studies, with mortality rates of 25.6%–53%.8–11
The heart is a target of systemic inflammation, and the role of inflammation in the development of acute heart failure (HF) has been identified.12–14 Pro-inflammatory cytokines involved in cardiac injury are TNF-α, IFN-γ, IL-1β, IL-6, IL-17, and IL-18. Among these cytokines, elevated TNF-α levels have been associated with negative inotropic effects, ventricular dilatation, and mortality.15,16 Additionally, IL-1β has been reported as a myocardial depressant cytokine that causes the release of inducible nitric oxide.17 In sustained high-grade systemic inflammation, cardiac damage resulting from circulatory failure seems inevitable With early immunosuppressive treatment, successful management can be achieved.8,9,18
With growing knowledge of the relationship between the heart and hyperinflammation after the COVID-19 pandemic12 and since HPS is a multisystem hyperinflammatory disorder due to the rarity of the condition in adults, there is no available data to predict cardiac complications during the course of HPS.
In this retrospective study, we aimed to identify the factors that predict the development of acute heart failure in patients with HPS admitted to the ICU.
Materials and Methods
This retrospective observational study included adult critically ill patients diagnosed with HPS according to the HLH-2004 diagnostic criteria in the ICU of the Ege University Faculty of Medicine between 2012 and 2023. De novo acute HF was defined as a manual review of the patients’ electronic medical records (ICD codes), the absence of a history of HF diagnosis and medication use, and the presence of new and rapidly worsening symptoms and signs in accordance with guidelines.19,20 Patients with a known history of heart failure and missing data were excluded. The Institutional Ethical Review Board of Ege University Hospital approved the study (24–5T/35). The study was conducted in accordance with good clinical practice guidelines and adhered to the principles of the Declaration of Helsinki. Patients or their relatives provided written informed consent. According to the results of diagnostic investigations, HPS etiological diagnoses were made by a multidisciplinary team, including an intensivist, rheumatologist, and hematologist. Demographic, clinical, and laboratory parameters were retrieved from electronic medical records. The cardiothoracic ratio (CTR) was measured on a PA chest x-ray, which is the ratio of the maximal transverse dimension of the heart to the maximal transverse thoracic dimension21 and expressed as a percentage. The primary outcome was the identification of risk factors for the development of HF in patients with HPS, and the secondary outcome was ICU mortality.
Statistical Analysis
The data obtained from the study were summarized using descriptive statistics. Continuous variables were presented as mean ± standard deviation or median with minimum and maximum values, depending on their distribution. Categorical variables were summarized as numbers and percentages. The normality of the numerical variables was assessed using appropriate tests and visualizations based on the sample size and data characteristics. The Shapiro–Wilk test was used for small sample sizes (n < 50), while the Kolmogorov–Smirnov and Anderson-Darling tests were used for larger samples (n ≥ 50). The Kolmogorov–Smirnov test accommodates larger sample sizes, whereas the Anderson-Darling test is more sensitive to deviations in the tails of the distribution. Additionally, visual tools, such as histograms and Q-Q plots, were used to evaluate the assumption of normality.
For comparing categorical variables between groups, the Pearson chi-square test was employed in 2×2 tables with expected cell counts of 5 or more. In 2×2 tables with expected counts less than 5, Fisher’s exact test was preferred. For RxC tables with expected counts below 5, the Fisher-Freeman-Halton test was utilized.
When comparing the two independent groups, an independent samples t-test was used if the numerical variables followed a normal distribution. If the numerical variables did not follow a normal distribution, the Mann–Whitney U-test was chosen.
We conducted a classification and regression tree (CART) analysis to develop models predicting acute heart failure status using datasets of 146 participants, validated with 10-fold cross-validation. The independent variables for Model 1 included age, CTR, etiology, N-terminal pro-brain natriuretic peptide (NT-proBNP), C-reactive protein (CRP), triglyceride, lactate dehydrogenase (LDH), and ferritin levels at diagnosis. For Model 2, the variables were CTR, CRP, albumin, etiology, gender, and LDH. Equal prior probabilities were assigned to each class. The Gini criterion was used for node splitting, and the optimal trees were selected within one standard error of the minimum misclassification cost.
The primary rationale for preferring CART analysis over logistic regression is the assumption that nonlinear relationships and complex interactions exist within the dataset. CART enables the identification and interpretation of these complex relationships. The nonparametric nature of CART eliminates the need for assumptions about data distribution, thus enhancing model flexibility. One significant advantage is the visual interpretability of the results, which facilitates the achievement of research objectives.
Statistical analyses were performed using Jamovi (Version 2.3.28, Jamovi project, 2023, https://www.jamovi.org), JASP (Version 0.18.3, Jeffreys’ Amazing Statistics Program, 2024, https://jasp-stats.org), and Minitab (Version 21.4.2, Minitab, LLC, 2023) software packages. The level of statistical significance was set at p ≤ 0.05.
Results
Among 146 patients, 49.3% (n = 72) developed symptoms and signs of acute heart failure. The female sex ratio was higher in patients with heart failure, and this difference was statistically significant (female: 59.7%, male: 40.3%, p = 0.031). Additionally, the CTR was significantly higher in patients with acute heart failure (p < 0.001). Regarding etiology, a significant difference was observed (p = 0.007). Rheumatological causes were more prevalent in patients with acute heart failure, while infectious etiology was more common in those without heart failure. Age, length of hospital stay, time from diagnosis to treatment, time from diagnosis to death, and mortality rates did not show significant differences between patients with and without acute heart failure (p > 0.05 for each) (Table 1).
 |
Table 1 Clinical Characteristics and Outcomes Based on Acute Heart Failure Status |
The levels of NT-proBNP, triglycerides, direct bilirubin, and CRP were significantly higher in patients with acute heart failure (p < 0.05 for each). Conversely, no significant differences were observed in other laboratory results between patients with and without heart failure (p > 0.05 for each) (Supplementary Table 1).
Model 1: CART Results
Binary Response Information
The response variable, heart failure status, had two classes: present (acute heart failure), with 72 cases (49.32%), and absent (acute heart failure), with 74 cases (50.68%).
CART Analysis
The analysis identified CTR and age as the most significant predictors, resulting in a decision tree with four terminal nodes. The primary splits were based on CTR ≤ 52.5 and age ≤ 60. The subsequent splits further differentiated patients based on etiology. In patients with a CTR below 52.5, the probability of not having heart failure was 75.4%. In patients with a CTR above 52.5, the probability of acute heart failure was 69.1%, with age being the determining factor in these patients. The probability of acute heart failure was 84.3% in patients with a CTR above 52.5 and aged 60 years or less. Patients with a CTR above 52.5 and those aged above 60 years were differentiated according to etiology. Patients with an etiology due to infection, malignancy, or other causes had a 69.6% probability of not having heart failure, while patients with an etiology due to rheumatological causes had an 85.7% probability of having acute heart failure (Figure 1: Tree diagram for Model 1).
 |
Figure 1 Decision Tree for Model 1 Predicting Heart Failure Status Based on Cardiothoracic Ratio, Age, and Etiology. |
Model Performance
The confusion matrix used to evaluate the model’s performance shows that, in the training data, 49 out of 72 cases of acute heart failure were correctly predicted, resulting in an accuracy of 68.1%. Similarly, 65 out of 74 cases without heart failure were correctly predicted, with an accuracy of 87.8%, leading to an overall accuracy of 78.1%. In the test data, 43 out of 72 cases of acute heart failure were correctly predicted (59.7% accuracy), and 59 out of 74 cases without heart failure were correctly predicted (79.7% accuracy), with an overall accuracy of 69.9%. The model’s sensitivity was 68.1% in the training data and 59.7% in the test data. The false positive rate was 12.2% in the training data and 20.3% in the test data; the false negative rate was 31.9% in the training data and 40.3% in the test data; and the true negative rate (specificity) was 87.8% in the training data and 79.7% in the test data (Table 2).
 |
Table 2 Confusion Matrix and Statistics for Model 1 from CART Analysis |
Statistical Measures
The mean log-likelihood values were 0.517 for the training data and 1.139 for the test data. The area under the ROC curve (AUC) values were 0.788 (95% CI, 0.275 to 1) for training and 0.704 (95% CI, 0.613 to 0.794) for testing (Figure 2: ROC curves for Model 1 test and training data). Lift values were calculated at 1.723 for training and 1.319 for testing. Misclassification costs were 0.441 for training and 0.606 for testing. These measurements indicate that the model performs better on training data than on test data. While the decrease in performance indicators on the test data suggests some limitations in the model’s generalizability, the obtained values are still within acceptable limits.
 |
Figure 2 Receiver Operating Characteristic (ROC) Curve for Model 1 with Area Under the Curve (AUC) Values for Training (0.7881) and Test (0.7038) Data. |
Variable Importance
The relative importance of predictors was expressed as a percentage, with the most effective predictor rated at 100%. According to our analysis, CTR had the highest importance at 100%, followed by age at 41.9%, NT-proBNP at 31.4%, and etiology at 31.0%. Other variables included CRP (20.2%), ferritin at diagnosis (8.3%), triglyceride (8.3%), and LDH (4.8%) (Figure 3: Importance of variables for Model 1).
 |
Figure 3 Relative Variable Importance for Model 1, Highlighting Cardiothoracic Ratio as the Most Significant Predictor. |
Model 2: CART Results
Binary Response Information
The response variable, heart failure status, had two classes: present (acute heart failure), with 72 cases (49.32%), and absent (acute heart failure), with 74 cases (50.68%).
CART Analysis
The analysis identified CTR and CRP as the most significant predictors, resulting in a decision tree with five terminal nodes. The primary splits were based on CTR ≤ 52.5 and CRP ≤ 34.57. Subsequent splits further differentiated patients based on etiology and CRP levels. The probability of not developing heart failure in patients with a CTR below 52.5 was 75.8% (Node 2). In this group, the probability of not having heart failure in patients with a CRP level equal to or below 34.57 was 79.4%. Nevertheless, heart failure was identified in all patients with a CRP level above 34.57. In patients with a CTR above 52.5, the etiology was found to be an important determinant. In these patients, the probability of acute heart failure was 57.1% in those with an etiology due to infection, malignancy, or other causes. In patients with a CTR above 52.5, an etiology of infection, malignancy, or other causes, and a CRP level equal to or below 8.735, the probability of acute heart failure was 27.8%. In patients with a CRP level above 8.735, the probability of acute heart failure was 74.2%. In patients with a CTR above 52.5 and a rheumatological etiology, the probability of acute heart failure was 90.3% (Figure 4: Tree diagram for Model 2).
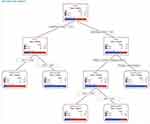 |
Figure 4 Decision Tree for Model 2 Predicting Heart Failure Status Based on Cardiothoracic Ratio, CRP Levels, and Etiology. |
Model Performance
The confusion matrix used to evaluate the model’s performance shows that in the training data, 54 out of 72 cases of heart failure were correctly predicted, resulting in an accuracy of 75.0%. Similarly, 63 out of 74 cases without heart failure were correctly predicted, with an accuracy of 85.1%, leading to an overall accuracy of 80.1%. In the test data, 49 out of 72 cases of acute heart failure were correctly predicted (68.1% accuracy), and 57 out of 74 cases without heart failure were correctly predicted (77.0% accuracy), with an overall accuracy of 72.6%. The model’s sensitivity was 75.0% in the training data and 68.1% in the test data. The false positive rate was 14.9% in the training data and 23.0% in the test data; the false negative rate was 25.0% in the training data and 31.9% in the test data; and the true negative rate (specificity) was 85.1% in the training data and 77.0% in the test data (Table 3).
 |
Table 3 Confusion Matrix and Statistics for Model 2 from CART Analysis |
Statistical Measures
The mean log-likelihood values were 0.481 for the training data and 1.677 for the test data. The area under the ROC curve (AUC) values were 0.8259 (95% CI, 0.428 to 1) for training and 0.7102 (95% CI, 0.622 to 0.798) for testing (Figure 5: ROC curves for the Model 2 test and training data). Lift values were calculated at 1.872 for training and 1.472 for testing. Misclassification costs were 0.399 for training and 0.550 for testing. These measurements indicate that the model performs better on training data than on test data. While the decrease in performance indicators on the test data suggests some limitations in the model’s generalizability, the obtained values are still within acceptable limits.
 |
Figure 5 Receiver Operating Characteristic (ROC) Curve for Model 2 with Area Under the Curve (AUC) Values for Training (0.8259) and Test (0.7102) Data. |
Variable Importance
The relative importance of predictors was expressed as a percentage, with the most effective predictor rated at 100%. According to our analysis, CTR had the highest importance at 100%, followed by CRP at 77.9%, albumin at 39.5%, and etiology at 37.5%. Other variables included gender (24.9%) and LDH (7.4%) (Figure 6: Importance of variables for Model 2).
 |
Figure 6 Relative Variable Importance for Model 2, Highlighting Cardiothoracic Ratio as the Most Significant Predictor. |
Discussion
Heart failure is a complex clinical syndrome characterized by a range of signs and symptoms resulting from structural or functional defects that lead to reduced cardiac output or elevated intracardiac pressure. Acute heart failure is usually defined as the rapid onset of new or worsening signs and symptoms of HF. Our study aimed to identify predictors of acute heart failure as a prominent organ dysfunction in patients with HPS using CART analysis. The results highlight several key factors that play a crucial role in predicting acute heart failure within this patient population. CTR was found to be the most significant predictor, indicating that patients with a higher CTR are at a substantially increased risk of developing heart failure. A CTR value of > 0.5 is considered abnormal and may indicate cardiomegaly.22 Although it can be difficult to obtain true enlargement, CTR determination can provide beneficial parameters for patient assessment, especially in intensive care units. Our findings underscore valuable information on the monitoring of CTR in managing patients with HPS.
Additionally, elevated levels of NT-proBNP were strongly associated with heart failure, corroborating its utility as a biomarker for cardiac stress and dysfunction. CRP, an inflammatory marker, also emerged as a significant predictor, highlighting the role of systemic inflammation in the progression of heart failure. Elevated B-type natriuretic peptide or NT-proBNP levels in HPS patients with cardiac involvement have been reported in the current literature.23 Additionally, temporal changes in NT-proBNP were closely related to mortality.11 CRP in HPS patients has been related to overall survival in a recent study,24 but it has not been well established in HPS patients with cardiac involvement. Previous studies have highlighted the importance of biomarkers, such as NT-proBNP and inflammatory markers, in predicting heart failure, but their role in HPS remains underexplored. This study addresses this gap by using CART analysis to identify the key predictors of acute heart failure in HPS patients. The findings of the present study suggest that integrating NT-proBNP, which is a tool for predicting patient mortality in both heart failures with reduced or preserved ejection fraction and CRP levels into routine clinical assessments, could enhance the early detection and management of acute heart failure in these patients.
Age and the etiology of HPS further influenced the risk of acute heart failure. Older patients and those with rheumatological causes of HPS were particularly susceptible to developing acute heart failure. Frapard et al reported acute circulatory failure in 34 hPS patients, the leading etiology was hematological malignancies, and older patients had worse outcomes.9 Fewer reported etiologies appear as case reports but are limited for comparison with our study. Cardiac involvement in systemic inflammatory diseases has been reported in many different clinical presentations.25 Some manifestations, such as pericarditis, are particularly well known; however, better knowledge and awareness of heart failure in systemic inflammatory diseases are critical because of high mortality and morbidity. Although subgroup analyses of rheumatological diseases could not be performed in the present study due to the small number of patients, our results suggest that personalized treatment strategies based on age and underlying etiology could be beneficial in mitigating the risk of heart failure.
The study also found a higher prevalence of acute heart failure among female patients, indicating potential gender-specific factors that warrant further investigation. Combining the fact that rheumatologic diseases are more common in women related to hormonal factors26 and that heart failure was more common in rheumatologic diseases in this study suggests that the gender difference in heart failure is because of rheumatologic etiology. Understanding these gender differences could lead to more tailored and effective treatment approaches.
The identification of these predictors has significant clinical implications. Early recognition of patients at higher risk for heart failure can lead to timely interventions, potentially improving outcomes. For example, regular monitoring of the cardiothoracic ratio and NT-proBNP levels in patients with HPS could help in the early detection and management of acute heart failure.
Our findings raise several important questions for future research. First, the role of inflammatory markers, such as CRP, in the progression of heart failure warrants further investigation. Understanding the mechanisms linking systemic inflammation to cardiac dysfunction could uncover new therapeutic targets. Second, the influence of different etiologies of HPS on heart failure risk needs more detailed exploration. Large-scale studies could help clarify how specific underlying conditions contribute to heart failure development. Lastly, the observed gender differences in heart failure prevalence among our cohort suggest that gender-specific factors should be considered in risk assessment and management strategies. When considering that heart failure with preserved ejection fraction patients are more often women,27 the heart failure that develops in these patients also raises the question of whether this is the group with preserved ejection fraction. Future studies should aim to elucidate these differences to optimize care for both men and women with HPS.
Future research should focus on the following areas: investigate the biological pathways linking inflammation to heart failure in HPS; conduct long-term follow-up studies to understand the progression of heart failure in patients with different etiologies of HPS; explore gender differences in heart failure risk and outcomes to develop tailored interventions; and test the effectiveness of early interventions based on the identified predictors in reducing heart failure incidence and improving survival.
Although our study provides valuable insights into the predictors of heart failure in patients with HPS, several limitations must be acknowledged. First, the study’s retrospective design may introduce inherent biases, such as selection bias and information bias. The reliance on existing medical records may result in incomplete data, potentially affecting the accuracy and generalizability of our findings. This study’s findings are specific to ICU-admitted patients and may not be fully generalizable to HLH patients treated in regular wards. The sample size, though adequate for initial analysis, may not be sufficient to capture the full spectrum of variability within the patient population. A larger multicenter study would be necessary to validate our results and ensure that they are applicable to a broader demographic.
Another limitation is the potential for confounding variables that were not accounted for in our analysis. Factors such as socioeconomic status, comorbid conditions, and variations in treatment protocols could influence the outcomes and should be considered in future studies. Additionally, the cross-sectional nature of some data points limits our ability to establish causality. Longitudinal studies would provide a clearer understanding of the temporal relationships between the identified predictors and the development of acute heart failure.
The use of CART analysis, while robust in handling complex interactions and non-linear relationships, may also have limitations. The results of CART models can be sensitive to the specific data set and may not generalize well to other populations. It is crucial to replicate these findings using different analytical approaches and diverse patient cohorts to confirm their validity.
Furthermore, the study did not explore the potential impact of genetic factors on the risk of acute heart failure in patients with HPS. Genetic predispositions may play a significant role and should be investigated in future research.
Lastly, although we identified several key predictors, the study did not evaluate the effectiveness of specific interventions based on these predictors. Future research should focus on interventional trials to determine the best strategies for preventing heart failure in high-risk patients, as identified by our models.
In summary, while our study advances the understanding of acute heart failure predictors in HPS, the limitations highlight the need for further research to validate and expand on these findings. Addressing these limitations will be essential in developing comprehensive and effective management strategies for this vulnerable patient population.
Conclusion
In conclusion, our findings emphasize the need for a comprehensive and multifaceted approach to managing patients with HPS. By identifying high-risk individuals through key predictors, such as cardiothoracic ratio, NT-proBNP, CRP, age, and etiology, clinicians can implement early interventions aimed at preventing the onset of acute heart failure. These strategies have the potential to significantly improve patient outcomes and reduce mortality rates associated with HPS and acute heart failure. Expanding the sample size in future studies would significantly enhance the generalizability of these findings, allowing for a more comprehensive understanding of predictors in HPS patients and improving the applicability of results across diverse patient populations.
Abbreviations
HPS, hemophagocytic syndrome; HF, heart failure; CART, classification and regression tree; CTR, cardiothoracic ratio; NT-proBNP, N-terminal pro-brain natriuretic peptide; CRP, C-reactive protein; LDH, lactate dehydrogenase; ICU, intensive care unit; AUC, area under curve.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Informed Consent
All participants or their relatives provided informed consent prior to study inclusion. This study was approved by the the Institutional Ethical Review Board of Ege University Hospital (24-5T/35). The study was conducted in accordance with good clinical practice guidelines and adhered to the principles of the Declaration of Helsinki.
Acknowledgments
We would like to extend our gratitude to Gökhan Karakoç, Co-Founder of the Company, and the editors at the Model Statistics and Clinical Trials Center for their invaluable assistance with the statistical analysis of our data.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors have no relevant financial or non-financial interests to disclose.
References
1. Henter JI, Horne A, Aricó M, et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48(2):124–131. doi:10.1002/pbc.21039
2. Dalal BI, Vakil AP, Khare NS, Wang SY, Richards MJ, Chen LY. Abnormalities of the lymphocyte subsets and their immunophenotype, and their prognostic significance in adult patients with hemophagocytic lymphohistiocytosis. Ann Hematol. 2015;94(7):1111–1117. doi:10.1007/s00277-015-2350-y
3. West J, Stilwell P, Liu H, et al. Temporal trends in the incidence of hemophagocytic lymphohistiocytosis: a nationwide cohort study from England 2003-2018. Hemasphere. 2022;6(11):e797. doi:10.1097/HS9.0000000000000797
4. Parikh SA, Kapoor P, Letendre L, Kumar S, Wolanskyj AP. Prognostic factors and outcomes of adults with hemophagocytic lymphohistiocytosis. Mayo Clin Proc. 2014;89(4):484–492. doi:10.1016/j.mayocp.2013.12.012
5. Buyse S, Teixeira L, Galicier L, et al. Critical care management of patients with hemophagocytic lymphohistiocytosis. Intensive Care Med. 2010;36(10):1695–1702. doi:10.1007/s00134-010-1936-z
6. Otrock ZK, Eby CS. Clinical characteristics, prognostic factors, and outcomes of adult patients with hemophagocytic lymphohistiocytosis. Am J Hematol. 2015;90(3):220–224. doi:10.1002/ajh.23911
7. Jordan MB, Allen CE, Weitzman S, Filipovich AH, McClain KL. How I treat hemophagocytic lymphohistiocytosis. Blood. 2011;118(15):4041–4052. doi:10.1182/blood-2011-03-278127
8. Abdelhay A, Mahmoud A, Mostafa M, et al. Delay in treatment of adult hemophagocytic lymphohistiocytosis is associated with worse in-hospital outcomes. Ann Hematol. 2023;102(11):2989–2996. doi:10.1007/s00277-023-05271-w
9. Frapard T, Darmon M, Fadllalah J, Mariotte E, Valade S. Acute circulatory failure in critically ill patients with hemophagocytic syndrome. J Crit Care. 2022;70:154064. doi:10.1016/j.jcrc.2022.154064
10. Chizinga M, Kalra SS, Innabi A, Rackauskas M, Ataya A, Emtiazjoo A. Macrophage activating syndrome causing decompensated right heart failure. Respir Med Case Rep. 2021;33:101409. doi:10.1016/j.rmcr.2021.101409
11. Bozkurt D, Bozgul SMK, Emgin O, et al. Mortal interaction between hemophagocytic syndrome and newly developed heart failure. Arq Bras Cardiol. 2021;116(3):395–401. doi:10.36660/abc.20190642
12. Costa IB, Bittar CS, Rizk SI, et al. The heart and COVID-19: what cardiologists need to know. Arq Bras Cardiol. 2020;114(5):805–816. doi:10.36660/abc.20200279
13. Shirazi LF, Bissett J, Romeo F, Mehta JL. Role of inflammation in heart failure. Curr Atheroscler Rep. 2017;19(6):27. doi:10.1007/s11883-017-0660-3
14. Castillo EC, Vázquez-Garza E, Yee-Trejo D, García-Rivas G, Torre-Amione G. What is the role of the inflammation in the pathogenesis of heart failure? Curr Cardiol Rep. 2020;22(11):139. doi:10.1007/s11886-020-01382-2
15. Levine B, Kalman J, Mayer L, Fillit HM, Packer M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med. 1990;323(4):236–241. doi:10.1056/NEJM199007263230405
16. Kubota T, Miyagishima M, Alvarez RJ, et al. Expression of proinflammatory cytokines in the failing human heart: comparison of recent-onset and end-stage congestive heart failure. J Heart Lung Transplant. 2000;19(9):819–824. doi:10.1016/S1053-2498(00)00173-X
17. Van Tassell BW, Seropian IM, Toldo S, Mezzaroma E, Abbate A. Interleukin-1beta induces a reversible cardiomyopathy in the mouse. Inflamm Res. 2013;62(7):637–640. doi:10.1007/s00011-013-0625-0
18. Seegobin K, Alhaj Moustafa M, Majeed U, et al. Macrophage activation led acute heart failure managed successfully with immunosuppression. J Blood Med. 2021;12:1037–1043. doi:10.2147/JBM.S340361
19. Authors/Task Force Members; McDonagh TA, Metra M, Adamo M, et al. “2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC”. European J Heart Failure. 2022;24(1):4–131. doi:10.1002/ejhf.2333
20. Arrigo M, Jessup M, Mullens W, et al. Acute heart failure. Nat Rev Dis Primers. 2020;6(1):16. doi:10.1038/s41572-020-0151-7
21. Danzer CS. The Cardiothoracic Ratio. Am J Med Sci. 1919;157(4):513–554. doi:10.1097/00000441-191904000-00007
22. Kearney MT, Fox KA, Lee AJ, et al. Predicting death due to progressive heart failure in patients with mild-to-moderate chronic heart failure. J Am Coll Cardiol. 2002;40(10):1801–1808. doi:10.1016/S0735-1097(02)02490-7
23. de La Rochefoucauld J, Anguel N, Schmidt J, Noel N, Monnet X, Lambotte O. Heart involvement: a neglected manifestation of haemophagocytic syndrome associated with high mortality. J Crit Care. 2024;80:154498. doi:10.1016/j.jcrc.2023.154498
24. Zhang Q, Lin Y, Bao Y, Jin Y, Ye X, Tan Y. Analysis of prognostic risk factors and establishment of prognostic scoring system for secondary adult hemophagocytic syndrome. Curr Oncol. 2022;29(2):1136–1149. doi:10.3390/curroncol29020097
25. Knockaert DC. Cardiac involvement in systemic inflammatory diseases. Eur Heart J. 2007;28(15):1797–1804. doi:10.1093/eurheartj/ehm193
26. Juárez-Melchor D, Munguía-Realpozo P, Mendoza-Pinto C, et al. Genetic component of autoimmune rheumatological diseases. Reumatol Clin. 2022;18(10):614–620. doi:10.1016/j.reuma.2021.08.003
27. Kapelios CJ, Shahim B, Lund LH, Savarese G. Epidemiology, clinical characteristics and cause-specific outcomes in heart failure with preserved ejection fraction. Card Fail Rev. 2023;9:e14. doi:10.15420/cfr.2023.03
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

