Back to Journals » Journal of Inflammation Research » Volume 17
Clinical and Diagnostic Value of High-Density Lipoprotein-Based Inflammatory Indices and Lipid Ratios in Young Adults with Schizophrenia
Authors Chen L , Zheng C, Luan H, Chen X
Received 12 June 2024
Accepted for publication 7 September 2024
Published 14 September 2024 Volume 2024:17 Pages 6363—6374
DOI https://doi.org/10.2147/JIR.S473528
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Liling Chen,1 Cunqing Zheng,1 Honglin Luan,2 Xinyuan Chen3
1Department of Clinical Laboratory, Wenzhou Seventh People’s Hospital, Wenzhou, Zhejiang Province, People’s Republic of China; 2Department of Psychiatry, Wenzhou Seventh People’s Hospital, Wenzhou, Zhejiang Province, People’s Republic of China; 3Department of Clinical Laboratory, Wenzhou Central Hospital, Wenzhou, Zhejiang Province, People’s Republic of China
Correspondence: Xinyuan Chen, Department of Clinical Laboratory, Wenzhou Central Hospital, Bailidonglu Street, Lucheng District, Wenzhou, 325000, People’s Republic of China, Tel +8615858805715, Fax +86057788070190, Email [email protected]
Purpose: This study aimed to assess High-density Lipoprotein (HDL)-based Inflammatory Indices and lipid profile changes in antipsychotic-naive first-episode schizophrenia (AN-FES) patients, chronic schizophrenia (CS) patients, and explore the clinical and predictive value of these parameters for schizophrenia.
Patients and Methods: The study cohort included 52 AN-FES patients, 46 CS patients, and 52 healthy controls (HCs), with an average age of 24 years. Upon admission, patients underwent complete blood count and lipid profile analyses. Various ratios were calculated, including neutrophil-to-HDL (NHR), monocyte-to-HDL (MHR), lymphocyte-to-HDL (LHR), and platelet-to-HDL (PHR), as well as lipid ratios like triglycerides/HDL, non-HDL/HDL, total cholesterol/HDL, and low-density lipoprotein/HDL. For the AN-FES group, these evaluations were repeated after two months of treatment with atypical antipsychotics. Statistical analyses included correlation analysis, Receiver Operating Characteristic (ROC) curve analysis, and univariate and multivariate regression.
Results: Compared to HCs, CS patients exhibited significantly higher MHR and NHR values, while AN-FES patients showed elevated levels of PHR, MHR, and NHR. No significant differences were observed in LHR or lipid ratios across the three groups. In the AN-FES cohort, MHR correlated positively with neutrophil counts, and NHR with monocyte counts. Additionally, white blood cell counts were positively associated with both MHR and NHR. Following treatment, NHR levels decreased, whereas TG/HDL ratios increased, with MHR and PHR remaining elevated. ROC analysis highlighted NHR as the most diagnostically valuable parameter (AUC = 0.799), with 86.5% specificity at an optimal cutoff of 3.534, outperforming MHR and PHR. Regression analyses recognized NHR (OR=2.225) as an independent risk factor for schizophrenia, even after adjusting for confounders.
Conclusion: HDL-based inflammatory indices, particularly NHR, may serve as valuable diagnostic and prognostic markers in young adults with schizophrenia, even though significant alterations in lipid ratios were not observed in this demographic.
Keywords: neutrophil-to-HDL ratio, NHR, monocyte-to-HDL ratio, MHR, platelet-to-HDL ratio, PHR, lipid ratios, schizophrenia, inflammatory indices
Introduction
Schizophrenia (SCZ) is a severe mental disorder that affects millions of people globally. It is characterized by cognitive disturbances, perceptual anomalies, and emotional blunting. Despite extensive research, the etiology of SCZ remains unclear, involving complex interactions between immune, environmental, and genetic factors.1,2 Recent studies indicate that inflammation and immune dysfunction, both within the central nervous system and peripheral immune responses,3,4 along with oxidative stress, play significant roles in the pathophysiology of SCZ.5,6 Individuals with chronic schizophrenia (CS) or those experiencing their first episode of schizophrenia may exhibit systemic inflammation and disruptions in lipid metabolism.7–11 Abnormal lipid metabolism may contribute to inflammation in various diseases,12 highlighting the importance of investigating the relationship between inflammatory and metabolic pathways in SCZ.13–15
High-density lipoprotein cholesterol (HDL) is a crucial component of the blood lipid profile recognized for its anti-thrombotic, anti-inflammatory, and antioxidant properties, as well as its regulation of activated neutrophil functions.16–18 Decreased HDL levels were shown to be related to inflammation.19 Inflammatory responses can cause fluctuations in the levels of neutrophils, monocytes, lymphocytes, and platelets, all of which are easily measured through a complete blood count.20 Consequently, combined markers derived from peripheral blood cell measures and biochemical assays, such as the neutrophil-to-HDL ratio (NHR), lymphocyte-to-HDL ratio (LHR), monocyte-to-HDL ratio (MHR), and platelet-to-HDL ratio (PHR), are under scrutiny as potential indicators of inflammation across various diseases.21–23 Among these, MHR has recently emerged as a novel inflammatory marker in SCZ patients.24,25 A large retrospective study has also identified elevated NHR, LHR, MHR, and PHR values in SCZ patients,26 suggesting that these ratios could be valuable biomarkers for assessing inflammatory status in SCZ.
The diagnosis of SCZ primarily relies on clinical signs and symptoms, with a lack of established biomarkers for accurate diagnosis. Additionally, those evaluating inflammatory and lipid metabolic biomarkers over time in antipsychotic-naive first-episode schizophrenic (AN-FES) patients, are limited. The impact of antipsychotic treatments on these biomarkers, especially in newly diagnosed patients, is not well understood. This study aims to compare various peripheral HDL-based Inflammatory Indices (including MHR, NHR, PHR, and LHR) and novel lipid ratios (such as triglycerides/HDL, non-HDL/HDL, total cholesterol /HDL, low-density lipoprotein cholesterol /HDL) in young AN-FES patients, CS patients, and healthy controls (HCs). Additionally, it also evaluates the potential of these indices as innovative inflammatory markers in AN-FES individuals and their responsiveness to short-term, two-month treatment regimens. A deeper understanding of the interplay between lipid profiles, inflammation, and SCZ could enhance the early detection and comprehension of the disorder.
Materials and Methods
Participants and Study Design
This single-center, prospective, observational study was conducted at Wenzhou Seventh People’s Hospital from January 2020 to December 2023. Informed consent was obtained from all participants. The study protocol was approved by the Institutional Ethics Committee of Wenzhou Seventh People’s Hospital, adhering to the Declaration of Helsinki and relevant national and international guidelines. Diagnoses of SCZ were confirmed via the Structured Clinical Interview based on DSM-IV criteria. The participants were Han Chinese individuals in the acute phase of illness requiring hospitalization. AN-FES patients were those experiencing their first episode, not previously exposed to antipsychotics, and with an illness duration of less than or equal to one year. CS patients had a history of medication non-compliance, had not received antipsychotic depot injections for at least one month, and had an illness duration of three years or more.
Exclusion criteria encompassed co-morbid psychiatric conditions, significant medical illnesses (eg, infections, head injuries, autoimmune diseases, epilepsy, heart failure, tumors), pregnancy, lactation, recent use of certain medications (immunosuppressants, antioxidants, or anti-inflammatories), and other conditions affecting inflammatory or immune status. HCs were demographically matched community members screened by psychiatrists to ensure they had no psychiatric history and were subject to the same exclusion criteria as the patient groups.
Laboratory Analysis
Blood samples for laboratory assessments were collected from both CS and AN-FES patients on the day following admission, typically during the acute phase of their illness. For the AN-FES group, a second blood sample was obtained after two months of treatment with atypical antipsychotics, including olanzapine (n=30), risperidone (n=10), quetiapine (n=6), aripiprazole (n=3), clozapine (n=2), and amisulpride (n=1). This sampling aimed to evaluate the effects of these medications on various biomarkers.
Venous blood was collected after a 12-hour fast into dry and EDTA tubes. Dry tubes were processed for biochemical assays, while EDTA samples facilitated complete blood counts utilizing a Sysmex XN-1000 automatic hematology analyzer (Sysmex Corporation, Kobe, Japan). This produced white blood cell (WBC), neutrophil (NEU), lymphocyte (LYM), platelet (PLT), and monocyte (MON) counts. Concurrently, lipid profile testing for triglycerides (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL), and HDL was conducted via an AU 5800 analyzer (Beckman Coulter Inc., Brea, CA, USA). Subsequently, inflammatory, and lipid-related ratios were calculated for comparison across groups. Specifically, MHR was calculated by dividing the MON count by the HDL level; NHR by dividing the NEU count by the HDL level; PHR by dividing the PLT count by the HDL level; and LHR by dividing the LYM count by the HDL level. Non-HDL cholesterol was calculated by subtracting HDL from TC, with the non-HDL/HDL ratio determined accordingly.
Statistical Analysis
Statistical analyses were performed using SPSS software version 27 (SPSS Inc., Chicago, IL, USA). The Kolmogorov–Smirnov test was employed to ascertain the normality of variable distributions. Variables adhering to normal distribution were presented as mean ± standard deviation (SD), whereas those deviating from normalcy were described using the median and interquartile range (IQR), and categorical data were expressed as percentages. Group comparisons for continuous and categorical variables utilized the Mann–Whitney and Chi-square tests, respectively. Multigroup analysis hinged on one-way ANOVA, with two-group comparisons via the independent t-test. Correlation assessments were conducted using Spearman’s rank correlation coefficient, excluding covariant indicators from analyses (eg, MHR was excluded from correlation analysis with monocytes and HDL-C). In logistic regression analyses for AN-FES, variables with P < 0.1 in univariate models were adjusted for confounders in multivariate analyses. MHR as a continuous variable, was transformed (x10^8/mmol) for regression. Receiver Operating Characteristic (ROC) curve analysis identified optimal cut-off values via the Youden index. A two-tailed P < 0.05 indicated statistical significance, denoted as follows: ***p < 0.001, **p < 0.01, *p < 0.05.
Results
Analysis of Clinicodemographic Characteristics and Laboratory Indicators in AN-FES, CS, and HC Groups
A total of 150 participants were involved in the study, consisting of 52 individuals with AN-FES, 46 with CS, and 52 HCs. Gender distribution was comparable across the groups without significant differences. Median ages were 25.0 years for the AN-FES group, 23.0 years for the CS group, and 25.1 years for the HCs group, with no significant variations observed (Table 1).
 |
Table 1 Comparison of Demographic and Laboratory Variables Among an-FES, CS, and HCs Groups Upon Admission |
Cell counts analysis via ANCOVA revealed significant differences in WBC, Neu, Mon, Eos, and PLT counts among the three groups. Post hoc comparisons indicated that the AN-FES group had higher WBC, Neu, and Mon counts but lower Eos counts compared to HCs; the CS group demonstrated similarly increased Neu and Mon but decreased Eos. Additionally, PLT counts were significantly elevated in the AN-FES group when contrasted with the CS group (Table 1).
Significant differences were detected in the serum concentrations of TC and HDL across groups. The AN-FES group exhibited lower TC and HDL concentrations compared to HCs. The CS group also showed reduced HDL levels, though the difference between AN-FES and CS groups was not statistically significant (p = 0.319). Serum parameter distributions among study participants are further detailed in Table 1.
Comparisons of HDL-Based Inflammatory Indices and Lipid Ratios Among AN-FES, CS, and HC Groups
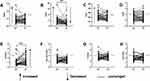
As depicted in Figures 1A–C, MHR, NHR, and PHR were significantly elevated in the AN-FES group ([MHR] 0.403 [0.298, 0.526], [NHR] 4.157 [2.963, 5.625], [PHR] 206.015 [174.286, 242.500]) compared to HCs ([MHR] 0.269 [0.214, 0.350], [NHR] 2.200 [1.638, 2.953], [PHR] 171.538 [128.859, 193.125]). In the CS group, MHR and NHR were also elevated, with respective values of [MHR] 0.391 [0.244, 0.455] and [NHR] 2.991 [2.025, 4.432] when compared to HCs. However, no significant difference in PHR was found between CS patients (170.339 [143.571, 214.000]) and HCs, indicating increases of 45% and 36% in MHR and NHR, respectively, within the CS group. There were no significant differences in MHR, NHR, and PHR between the AN-FES and CS groups. In contrast, LHR values did not vary significantly among the groups, with medians of 1.550 (AN-FES group), 1.380 (CS group), and 1.430 (HCs group) respectively, as shown in Figure 1D.
Analyses of lipid ratios, such as TG/HDL, non-HDL/HDL, TC/HDL, and LDL/HDL, displayed no substantial divergence across groups, as depicted in Figures 1E-H.
Correlation Analysis of MHR, NHR, and PHR with Other Indicators in AN-FES Patients
The relationships between MHR, NHR, and PHR with other indicators were analyzed using Spearman correlation in AN-FES patients. Significant correlations were observed between MHR (r = 0.470, p < 0.001) (Figure 2B) and NHR (r = 0.846, p < 0.001) (Figure 2A) with WBC counts. Furthermore, a positive correlation was noted between NHR and Mon counts (r = 0.405, p = 0.003) (Figure 2C), while MHR demonstrated a significant association with Neu counts (r = 0.451, p = 0.001) (Figure 2D).
Predictive Factors for an-FES Occurrence: Univariate and Multivariate Logistic Regression Analysis
To investigate the potential contribution of HDL-based indices to AN-FES occurrence, a multivariable logistic regression analysis was conducted. This approach adjusted for covariates including WBC, EOS, and TC, which differed between control and AN-FES groups. Variables with a P-value below 0.1 in the univariate analysis, such as WBC, EOS, TC, MHR, NHR, and PHR, were incorporated into the multivariable model. Notably, NHR was independently associated with AN-FES occurrence, doubling the risk (OR=2.225, P < 0.001) (Table 2).
 |
Table 2 Univariate and Multivariate Logistic Regression Analysis for the Influencing Factors for the Occurrence of an-FES |
Diagnostic Potential of MHR, NHR, and PHR for an-FES
ROC curve analysis evaluated the diagnostic utility of MHR, NHR, and PHR for AN-FES, with AUC values detailed in Table 3. NHR was the most effective discriminator between AN-FES patients and HCs, showing an AUC of 0.799 (95% CI 0.713–0.886, P < 0.001), a sensitivity of 63.50%, and a specificity of 86.5% at the optimal threshold of 3.534. MHR and PHR also demonstrated respectable diagnostic value, with AUCs above 0.7 and sensitivity values of 76.90%.
 |
Table 3 ROC Curve Analysis of MHR, NHR, and PHR for an-FES |
Changes in Hematological Parameters and Lipid Profile Following Antipsychotic Administration Intervention in an-FES Patients
After a two-month treatment with atypical antipsychotics, AN-FES patients exhibited significant reductions in WBC, NEU, MON, and PLT counts, whereas EOS count increased. LYM and BAS counts remained unchanged. Additionally, a significant increase in the concentration of TG was observed (Table 4).
 |
Table 4 The Changes of Peripheral Hematological Parameters and Lipid Profile Before and After Atypical Antipsychotic Treatment in Patients with AN-FES |
Regarding HDL-based inflammatory Indices and lipid ratios, there was a notable decrease in NHR levels from 2.908 (2.216, 4.578) to a value trending towards normal at 2.200 (1.638, 2.953) (Figure 3B). However, MHR and PHR levels remained elevated (Figure 3A and C). No significant changes were found in the LHR (Figure 3D), non-HDL/HDL, TC/HDL, and LDL/HDL (Figure 3F-H), while the TG/HDL ratio significantly increased from 0.783 (0.559, 1.389) to 1.216 (0.680, 1.871) (Figure 3E).
Discussion
This study investigated HDL-based inflammatory indices and unconventional lipid ratios in young patients with AN-FES and CS. We found that MHR, NHR, and PHR were significantly elevated in the AN-FES group, while only MHR and NHR showed similar elevation in the CS group compared to controls. Notably, NHR levels decreased significantly following a two-month antipsychotic intervention in AN-FES patients, while MHR and PHR remained elevated. The predictive capability of these markers in diagnosing AN-FES was evaluated using ROC analysis, indicating that NHR, MHR, and PHR effectively predicted AN-FES, with NHR exhibiting the highest AUC and specificity. Moreover, NHR emerged as an independent factor for predicting the onset of AN-FES. Unconventional lipid ratios at baseline showed no significant difference between AN-FES, CS patients, and HCs, except for a slight elevation in TG/HDL following antipsychotic treatment in AN-FES patients. Collectively, these results highlight the complex interplay between immune markers and lipid metabolism in SCZ.
MHR is a novel inflammatory marker representing the ratio of Mon counts to HDL values.27 Its level has been revealed to be related to hypertensive complications,28 coronary heart disease,29 fatty liver disease,30 acute ischemic stroke,31 and Sleep Apnea Syndrome.32 MHR reflects the balance between inflammatory responses and lipid metabolism, both implicated in the pathophysiology of SCZ.24,26,33 Monocytes, key players in the innate immune response, secrete pro-inflammatory cytokines essential for immune defense during infection and inflammatory diseases.34 Previous studies have highlighted increased macrophage/monocyte inflammatory activation patterns in SCZ.35,36 Elevated MON counts and MHR values have also been reported in patients with SCZ.24,37 Our study extends these findings by revealing consistent dysregulation of MON counts and HDL levels across different illness stages, showing increased monocytes, HDL, and MHR observed in both AN-FES and CS groups. The significant correlations between MHR and WBC/NEU count in AN-FES indicate a potential link between systemic inflammation and immune cell profile levels in the early stages of the SCZ.
NHR serves as a biomarker of systemic inflammatory response, calculated by dividing the absolute number of neutrophils by HDL levels.27 Neutrophils, the most abundant type of white blood cell, are crucial in the innate immune system’s initial defense against infections. Studies have shown a close relationship between HDL and neutrophils, where HDL can hinder the activation, adhesion, movement, and migration of neutrophils, while activated neutrophils can impact HDL function.38,39 NHR emerges as a robust biomarker due to its amalgamation of HDL’s anti-inflammatory and antioxidant effects with neutrophils’ pro-inflammatory actions.40 In our study, we observed increased NEU counts and NHR values in both AN-FES and CS patients compared to HCs. At an optimal cutoff of 3.534, NHR exhibited a high specificity of 86.5% for diagnosing AN-FES, highlighting its utility in clinical settings. Supporting our findings, a retrospective study by Wei et al also identified an independent association between NHR and SCZ risk, though with a lower cutoff of 2.47 and a specificity of 51.05%.26 Our findings support the use of NHR as a valuable inflammatory marker in evaluating inflammation in AN-FES patients, reinforcing the hypothesis that inflammation and immune responses contribute to SCZ pathogenesis.
PHR is determined by dividing the PLT value by the HDL value. Platelets play a key role in the inflammatory response, regulating endothelial cell permeability and the recruitment of neutrophils, macrophages, and their effectors.41 Activated platelets have inflammatory functions in several physiological and pathological conditions. Previous studies have suggested a potential link between platelets and mental diseases through inflammatory responses.42,43 Wei et al have demonstrated that SCZ patients exhibit higher PHR values and lower PLT counts compared to healthy controls.26 Another study indicated significantly higher PLT and PHR values in the aggressive group than in the non-aggressive group.33 In our study, PHR levels were also elevated in AN-FES patients compared to HCs, while no significant differences were observed between CS and HCs. Interestingly, the AN-FES group had higher PLT counts than the CS group, suggesting a protective role for platelets in CS. This could be related to the regulation of 5-hydroxy tryptamine by platelets, which plays a key role in SCZ episodes.
LHR is a calculated metric derived from the LYM count divided by the HDL level and is often considered a potential inflammation indicator. Previous research reported higher LHR values in SCZ groups compared to healthy controls, although it was not independently associated with SCZ in multivariate analysis.26 In our study, we did not observe significant differences in LYM levels or LHR among the studied groups. This lack of difference may be attributed to the physiological role of lymphocytes in inflammation. Unlike neutrophils, which are the primary responders to inflammatory stimuli and exhibit marked fluctuations, lymphocytes are primarily involved in the adaptive immune response, resulting in more stable levels. Such stability may limit the sensitivity of LHR in detecting inflammatory changes, especially in acute conditions. Our study focused on patients in the acute phase of SCZ, where rapid inflammatory changes are more common, suggesting that LHR may not effectively capture these changes. Another consideration is the relatively small sample size and the variability in clinical characteristics, which may have reduced the statistical power to detect subtle differences in LHR, potentially masking true associations.
The short-term changes of HDL-based inflammatory Indices following antipsychotic treatment in our study exhibited a significant decrease in NHR, supporting the inflammation hypothesis of SCZ, which suggests that inflammatory processes play a critical role in the disease’s development. However, the persistent elevation of MHR and PHR levels post-treatment indicates that while some inflammatory markers respond positively to antipsychotic medications, others remain elevated, reflecting ongoing immune dysregulation in individuals with SCZ. This finding highlights the complexity of immune responses in SCZ and suggests that antipsychotic treatment alone may not fully address the inflammatory component of the disorder. Consequently, these results underscore the necessity for sustained interventions targeting inflammation in SCZ, such as adjunctive anti-inflammatory therapies or tailored lifestyle modifications, to effectively manage persistent immune dysregulation in patients.
Another important finding of our study was the alteration of lipid metabolism in SCZ. Specifically, when compared to HCs, TC and HDL were found to be lower in the acute-onset young AN-FES group. However, there were not any significant variances in lipid ratios between AN-FES and HC groups at the baseline, contrasting with certain previous reports. For instance, a study conducted in Taiwan involving participants with acute-phase SCZ indicated lower HDL, higher LDL, and increased ratios of TC/HDL and LDL/HDL compared to HCs.44 Another study reported similar TC and TG levels in drug-naive psychosis patients compared to HCs, with slightly lower HDL levels45 Discrepancies in findings could be attributed to differences in the studied population and sample sizes.
The changes in lipid metabolism seen in individuals with SCZ, especially those receiving treatment with atypical antipsychotics, present a notable clinical concern. In our study of AN-FES patients, we observed a significant increase in TG levels and the TG/HDL ratio following 2-month atypical antipsychotics, with 58% of patients receiving olanzapine. This finding supports the hypothesis that atypical antipsychotics can disrupt normal lipid homeostasis, likely through mechanisms such as insulin resistance, altered appetite regulation, and direct effects on lipid metabolism.46 Consistent with our findings, A previous meta-analysis by Pillinger et al similarly reported that quetiapine, olanzapine, and clozapine were associated with elevated TG levels.47 These results underscore the necessity for vigilant monitoring of lipid profiles in patients receiving atypical antipsychotics, given the potential for rapid and clinically significant metabolic alterations.
Studies comparing CS patients with healthy individuals have yielded varying results. While some research shows no significant differences in TC and LDL levels,48 other studies indicate higher levels of TC, LDL-C, and TC/HDL-C without significant changes in TG and HDL concentrations.49 An investigation focusing on male inpatients with SCZ found lower TC and LDL levels but no significant difference in HDL.50 Conversely, a retrospective study revealed reduced levels of TC, HDL, and LDL in SCZ patients.26 Our study observed only a decrease in HDL levels in the CS group compared to HCs, possibly influenced by the younger age of our patient group. We postulate that different inflammatory processes might be at play in younger individuals with AN-FES versus those with CS.
While interpreting the findings, it is essential to acknowledge certain limitations and exercise caution. The relatively small sample size may limit the generalizability of results. Potential confounding factors such as comorbidities or concomitant medications not accounted for could influence outcomes. Additionally, the short duration of antipsychotic treatment and lack of long-term follow-up data to assess the sustainability of changes in HDL-based Indices are notable limitations. Despite these limitations, our results support the inflammatory hypothesis of schizophrenia, suggesting MHR, NHR, and PHR as potential biomarkers with clinical utility in SCZ patients. Further research in this area may offer insights into personalized therapeutic strategies targeting immune-inflammatory pathways in SCZ management.
Conclusion
In conclusion, our study identified significant increases in the MHR by 45% and the NHR by 36% in individuals with CS compared to HCs. More notably, AN-FES patients exhibited a 50% increase in MHR, an 89% increase in NHR, and a 20% increase in PHR relative to HCs. Among these biomarkers, NHR demonstrated the highest specificity and emerged as an independent risk factor for the onset of AN-FES, highlighting its potential utility in early diagnosis and risk assessment. Notably, after a 2-month course of antipsychotic treatment, NHR levels decreased significantly, while MHR and PHR remained elevated, suggesting persistent low-grade inflammation in individuals with SCZ. Despite the lack of significant baseline differences in lipid ratios between AN-FES patients and HCs, antipsychotic treatment led to substantial increases in TG levels and the TG/HDL ratio. These findings highlight the complex interplay between lipid metabolism, inflammation, and schizophrenia, emphasizing the need for further research into how immune-inflammatory pathways could inform the clinical management of SCZ.
Ethics Approval and Consent to Participate
The study was in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of the Wenzhou Seventh People’s Hospital (approval number: KY2023-09). All participants provided written informed consent.
Acknowledgments
We thank all those who supported and participated in the study, including patients, and their family members.
Author Contributions
All authors made a significant contribution to the work reported, whether it was in the conception, study design, execution, data acquisition, analysis, and interpretation, or all these areas. All authors took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; agreed on the journal to which the article has been submitted; and agreed to be accountable for all aspects of the work.
Funding
This study was supported by the Wenzhou Science and Technology Bureau Project, Wenzhou, Zhejiang Province, China (Y20220838).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Petronis A. The origin of schizophrenia: genetic thesis, epigenetic antithesis, and resolving synthesis. Biol Psychiatry. 2004;55(10):965–970. doi:10.1016/j.biopsych.2004.02.005
2. Comer AL, Carrier M, Tremblay M, et al. The inflamed brain in schizophrenia: the convergence of genetic and environmental risk factors that lead to uncontrolled neuroinflammation. Front Cell Neurosci. 2020;14:274.
3. Müller N. Inflammation in schizophrenia: pathogenetic aspects and therapeutic considerations. Schizophr Bull. 2018;44(5):973–982. doi:10.1093/schbul/sby024
4. Vallée A. Neuroinflammation in schizophrenia: the key role of the WNT/β-catenin pathway. Int J Mol Sci. 2022;23(5):2810. doi:10.3390/ijms23052810
5. Sawa A, Sedlak TW. Oxidative stress and inflammation in schizophrenia. Schizophr Res. 2016;176(1):1–2. doi:10.1016/j.schres.2016.06.014
6. Zhang M, Zhao Z, He L, et al. A meta-analysis of oxidative stress markers in schizophrenia. Sci China Life Sci. 2010;53(1):112–124. doi:10.1007/s11427-010-0013-8
7. Hepgul N, Pariante CM, Dipasquale S, et al. Childhood maltreatment is associated with increased body mass index and increased C-reactive protein levels in first-episode psychosis patients. Psychol Med. 2012;42(9):1893–1901. doi:10.1017/S0033291711002947
8. Pillinger T, Beck K, Stubbs B, et al. Cholesterol and triglyceride levels in first-episode psychosis: systematic review and meta-analysis. Br J Psychiatry. 2017;211(6):339–349. doi:10.1192/bjp.bp.117.200907
9. Russell A, Ciufolini S, Gardner-Sood P, et al. Inflammation and metabolic changes in first episode psychosis: preliminary results from a longitudinal study. Brain Behav Immun. 2015;49:25–29. doi:10.1016/j.bbi.2015.06.004
10. Baumeister D, Russell A, Pariante CM, et al. Inflammatory biomarker profiles of mental disorders and their relation to clinical, social and lifestyle factors. Social Psychiatry Psychiatric Epidemiol. 2014;49(6):841–849. doi:10.1007/s00127-014-0887-z
11. Di Nicola M, Cattaneo A, Hepgul N, et al. Serum and gene expression profile of cytokines in first-episode psychosis. Brain Behav Immun. 2013;31:90–95. doi:10.1016/j.bbi.2012.06.010
12. Zhang J, Dai W, Chen Y. Editorial: the roles of lipids in immunometabolism: the crosstalk between lipid metabolisms and inflammation. Frontiers Cardiovasc Med. 2022;9:938535
13. Nettis MA, Pergola G, Kolliakou A, et al. Metabolic-inflammatory status as predictor of clinical outcome at 1-year follow-up in patients with first episode psychosis. Psychoneuroendocrinology. 2019;99:145–153. doi:10.1016/j.psyneuen.2018.09.005
14. Goldsmith DR, Massa N, Miller BJ, et al. The interaction of lipids and inflammatory markers predict negative symptom severity in patients with schizophrenia. NPJ Schizophrenia. 2021;7(1):50. doi:10.1038/s41537-021-00179-8
15. Chen S, Broqueres-You D, Yang G, et al. Relationship between insulin resistance, dyslipidaemia and positive symptom in Chinese antipsychotic-naive first-episode patients with schizophrenia. Psychiatry Res. 2013;210(3):825–829. doi:10.1016/j.psychres.2013.08.056
16. Zimetti F, Adorni MP, Marsillach J, et al. Connection between the altered HDL antioxidant and anti-inflammatory properties and the risk to develop alzheimer’s disease: a narrative review. Oxid Med Cell Longev. 2021;2021(1):6695796. doi:10.1155/2021/6695796
17. Barter PJ, Nicholls S, Rye KA, et al. Antiinflammatory properties of HDL. Circulation Res. 2004;95(8):764–772. doi:10.1161/01.RES.0000146094.59640.13
18. Shih CM, Lin FY, Yeh JS, et al. Dysfunctional high density lipoprotein failed to rescue the function of oxidized low density lipoprotein-treated endothelial progenitor cells: a novel index for the prediction of HDL functionality. Translational Res. 2019;205:17–32. doi:10.1016/j.trsl.2018.09.005
19. Murphy AJ, Woollard KJ, Hoang A, et al. High-density lipoprotein reduces the human monocyte inflammatory response. Arteriosclerosis Thrombosis Vasc Biol. 2008;28(11):2071–2077. doi:10.1161/ATVBAHA.108.168690
20. Goossens J, Morrens M, Coppens V. The potential use of peripheral blood mononuclear cells as biomarkers for treatment response and outcome prediction in psychiatry: a systematic review. Molecul Diagn & Therapy. 2021;25(3):283–299. doi:10.1007/s40291-021-00516-8
21. Wang Y, Zhang J, Li H, et al. Prognostic value of leucocyte to high-density lipoprotein-cholesterol ratios in COVID-19 patients and the diabetes subgroup. Front Endocrinol. 2021;12:727419. doi:10.3389/fendo.2021.727419
22. Song Y, Zhao Y, Shu Y, et al. Combination model of neutrophil to high-density lipoprotein ratio and system inflammation response index is more valuable for predicting peripheral arterial disease in type 2 diabetic patients: a cross-sectional study. Front Endocrinol. 2023;14:1100453. doi:10.3389/fendo.2023.1100453
23. Korkmaz ŞA, Kızgın S. Neutrophil/high-density lipoprotein cholesterol (HDL), monocyte/HDL and platelet/HDL ratios are increased in acute mania as markers of inflammation, even after controlling for confounding factors. Curr Med Res Opin. 2023;39(10):1383–1390. doi:10.1080/03007995.2023.2260302
24. Kılıç N, Tasci G, Yılmaz S, et al. Monocyte/HDL cholesterol ratios as a new inflammatory marker in patients with schizophrenia. J Pers Med. 2023;13(2):276. doi:10.3390/jpm13020276
25. Pak A, Sci IM, Muneer A, et al. Investigation of the monocyte to high density lipoprotein ratio as an inflammatory marker in schizophrenia. Annals of PIMS-Shaheed Zulfiqar Ali Bhutto Med Univers. 2023;19(3):251–255. doi:10.48036/apims.v19i3.839
26. Wei Y, Wang T, Li G, et al. Investigation of systemic immune-inflammation index, neutrophil/high-density lipoprotein ratio, lymphocyte/high-density lipoprotein ratio, and monocyte/high-density lipoprotein ratio as indicators of inflammation in patients with schizophrenia and bipolar disorder. Front Psychiatry. 2022;13:941728. doi:10.3389/fpsyt.2022.941728
27. Gkantzios A, Tsiptsios D, Karapepera V, et al. Monocyte to HDL and neutrophil to HDL ratios as potential ischemic stroke prognostic biomarkers. Neurol Int. 2023;15(1):301–317. doi:10.3390/neurolint15010019
28. Kaplan IG, Kaplan M, Abacioglu OO, et al. Monocyte/HDL ratio predicts hypertensive complications. Bratisl Lek Listy. 2020;121(2):133–136. doi:10.4149/BLL_2020_018
29. Guo J, Chen M, Hong Y, et al. Comparison of the predicting value of neutrophil to high-density lipoprotein cholesterol ratio and monocyte to high-density lipoprotein cholesterol ratio for in-hospital prognosis and severe coronary artery stenosis in patients with ST-segment elevation acute myocardial infarction following percutaneous coronary intervention: a retrospective study. J Inflamm Res. 2023;16:4541–4557. doi:10.2147/JIR.S425663
30. Wang L, Dong J, Xu M, et al. Association between monocyte to high-density lipoprotein cholesterol ratio and risk of non-alcoholic fatty liver disease: a cross-sectional study. Front Med Lausanne. 2022;9:898931. doi:10.3389/fmed.2022.898931
31. Zehir R, Sarak T, Barutcu S, et al. Monosit sayısı/yüksek yoğunluklu lipoprotein oranı, STEMI’de enfarktla ilişkili arterin oklüzyonunu öngörür. Turkish J Clin Lab. 2017;8(3):91–96.
32. Acat M, Yazıcı O. The monocyte/HDL cholesterol ratio in obstructive sleep apnea syndrome. Meandros Med Dental J. 2018. doi:10.4274/meandros.63935
33. Cheng N, Ma H, Zhang K, et al. The predictive value of monocyte/high-density lipoprotein ratio (MHR) and positive symptom scores for aggression in patients with schizophrenia. Medicina. 2023;59(3). doi:10.3390/medicina59030503
34. Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11(11):762–774. doi:10.1038/nri3070
35. Bergink V, Gibney SM, Drexhage HA. Autoimmunity, inflammation, and psychosis: a search for peripheral markers. Biol Psychiatry. 2014;75(4):324–331. doi:10.1016/j.biopsych.2013.09.037
36. Drexhage RC, van der Heul-Nieuwenhuijsen L, Padmos RC, et al. Inflammatory gene expression in monocytes of patients with schizophrenia: overlap and difference with bipolar disorder. A study in naturalistically treated patients. Int J Neuropsychopharmacol. 2010;13(10):1369–1381. doi:10.1017/S1461145710000799
37. Sahpolat M, Ayar D, Ari M, et al. Elevated monocyte to high-density lipoprotein ratios as an inflammation markers for schizophrenia patients. Clin Psychopharmacol Neurosci. 2021;19(1):112–116. doi:10.9758/cpn.2021.19.1.112
38. Murphy AJ, Woollard KJ. High-density lipoprotein: a potent inhibitor of inflammation. Clin Exp Pharmacol Physiol. 2010;37(7):710–718. doi:10.1111/j.1440-1681.2009.05338.x
39. Nazir S, Jankowski V, Bender G, et al. Interaction between high-density lipoproteins and inflammation: function matters more than concentration! Adv Drug Delivery Rev. 2020;159:94–119. doi:10.1016/j.addr.2020.10.006
40. Kong Y, Chen Z, Zhang J, et al. Neutrophil to high-density lipoprotein ratio (NHR) as a potential predictor of disease severity and survival time in Creutzfeldt-Jakob disease. BMC Neurol. 2023;23(1):34. doi:10.1186/s12883-023-03076-y
41. Shao Y, Li W, Wang D, et al. Prognostic value of preoperative lymphocyte-related systemic inflammatory biomarkers in upper tract urothelial carcinoma patients treated with radical nephroureterectomy: a systematic review and meta-analysis. World J Surgical Oncol. 2020;18(1):273. doi:10.1186/s12957-020-02048-7
42. Wei Y, Feng J, Ma J, et al. Characteristics of platelet-associated parameters and their predictive values in Chinese patients with affective disorders. BMC Psychiatry. 2022;22(1):150. doi:10.1186/s12888-022-03775-9
43. Shan M, Yang Z, Sun Z, et al. Association between platelet to lymphocyte ratio and depression and symptom severity among adults in the United States: a cross-sectional study. Heliyon. 2023;9(9):e20127. doi:10.1016/j.heliyon.2023.e20127
44. Huang TL, Chen JF. Serum lipid profiles and schizophrenia: effects of conventional or atypical antipsychotic drugs in Taiwan. Schizophr Res. 2005;80(1):55–59. doi:10.1016/j.schres.2005.05.001
45. Petrikis P, Tigas S, Tzallas AT, et al. Parameters of glucose and lipid metabolism at the fasted state in drug-naïve first-episode patients with psychosis: evidence for insulin resistance. Psychiatry Res. 2015;229(3):901–904. doi:10.1016/j.psychres.2015.07.041
46. Li R, Zhang Y, Zhu W, et al. Effects of olanzapine treatment on lipid profiles in patients with schizophrenia: a systematic review and meta-analysis. Sci Rep. 2020;10(1):17028. doi:10.1038/s41598-020-73983-4
47. Pillinger T, McCutcheon RA, Vano L, et al. Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: a systematic review and network meta-analysis. Lancet Psychiatry. 2020;7(1):64–77. doi:10.1016/S2215-0366(19)30416-X
48. Chow V, Reddel C, Pennings G, et al. Global hypercoagulability in patients with schizophrenia receiving long-term antipsychotic therapy. Schizophr Res. 2015;162(1–3):175–182. doi:10.1016/j.schres.2014.12.042
49. Mhalla A, Bel Hadj Salah W, Mensi R, et al. Lipid profile in schizophrenia: case control study. Tunis Med. 2018;96(1):22–29.
50. Alyoubi R, Mahassni S. Schizophrenia is linked to dyslipidemia with minimal alterations in the inflammatory biomarkers - An experience from Saudi Arabia. BioMedica. 2022;38(3):134–142. doi:10.51441/BioMedica/5-791
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.