Back to Journals » Journal of Pain Research » Volume 17
Clinical Trial Study Protocol: A Prospective Blinded, Randomized, Controlled Clinical Trial Protocol to Assess the Efficacy of Ultrasound-Guided Transversus Abdominis Plane Block on Postoperative Analgesia and Recovery Quality in Laparoscopic Donor Hepatectomy
Authors Liu S , Song B, Zhang L, Li X, Cui L
Received 6 May 2024
Accepted for publication 18 October 2024
Published 22 October 2024 Volume 2024:17 Pages 3401—3408
DOI https://doi.org/10.2147/JPR.S476966
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Karina Gritsenko
Shen Liu, Bijia Song, Liang Zhang, Xiuliang Li, Lingli Cui
Department of Anesthesiology, Beijing Friendship Hospital, Capital Medical University, Beijing, 100050, People’s Republic of China
Correspondence: Lingli Cui, Email [email protected]
Introduction: Liver transplantation is considered an effective treatment for end-stage liver disease. Laparoscopic donor hepatectomy (LDH) has become a new standard procedure. And it is important to minimize the pain of the donor. Good postoperative analgesia can reduce the occurrence of postoperative complications and promote the early recovery of the donor. Ultrasound-guided transversus abdominis plane (TAP) block can provide effective analgesia for liver donors and reduce postoperative opioid consumption. This study aims to use ultrasound-guided TAP block for LDH to improve postoperative analgesia for donors while reducing opioid consumption and improving patient rehabilitation quality.
Methods/Analysis: This study is a prospective blinded, randomized, controlled clinical trial with a concealed allocation of patients (living liver donors) scheduled to receive laparoscopic partial hepatectomy 1:1 to receive local infiltration anesthesia or TAP block. This study will recruit a total of 80 patients. The primary outcome is the dosage of opioids within 24 hours after surgery.
Ethics and Dissemination: This trial has been approved by the Institutional Review Board of Beijing Friendship Hospital of China Capital University. This trial study protocol was approved on 8 May 2023. The trial will start recruiting patients after being registered on the Chinese Clinical Trial Registry.
Trial Registration Number: ChiCTR2300071694.
Keywords: liver transplantation, liver donor, laparoscopic hepatectomy, TAP block, postoperative analgesia
Introduction
Liver failure is the most common severe liver disease syndrome in clinics. Liver transplantation is an important treatment for patients with acute liver failure, end-stage liver disease, and primary liver cancer.1,2 The shortage of donated organs is a limited factor in liver transplantation, with many patients dying during the waiting process.3 Living donor liver transplantation (LDLT) has become a way to expand the source of donors, and an effective method to replace cadaver liver transplantation in areas with insufficient cadaver liver supply.4 The United States has been undergoing adult Laparoscopic donor hepatectomy (LDH) for over 20 years, with over 4500 patients underwent the surgery.5 In Asian countries, living donors are predominant over cadaveric donors for liver transplants.6 Since donors are originally healthy and active, they should get a fast and safe recovery after surgery.
The traditional liver hepatectomy is an open surgery, which involves making a “J” incision in the right abdomen. The operation is traumatic, and moderate anxiety and severe pain may occur after the surgery.7 With the advancement of laparoscopic technology, LDH is increasingly used for living donor liver transplantation and has become a new standard procedure for obtaining living donor liver.8,9 Laparoscopy has improved traditional open surgery, which is beneficial to reducing complications and promoting rehabilitation, this surgical improvement meets the appearance and functional needs of liver donors. Previous study showed that patients undergoing LDH experience had less pain and less analgesics when compared to traditional open living donor hepatectomy.10,11 But severe postoperative pain is still frequently observed in donors.12 As with all major abdominal surgery, good pain management is required after LDH. Therefore, a safe and effective analgesic method is needed during the perioperative period of LDH to improve donor postoperative analgesia and promote early recovery. Postoperative analgesia is also an important component of Enhanced Recovery after Surgery (ERAS) after partial hepatectomy.13,14
Opioid analgesics as a foundational option for the treatment of postoperative pain remains to be the most effective. However, liver transplantation donors may experience liver dysfunction after surgery, which can lead to adverse reactions such as accumulation of opioids, excessive sedation, and respiratory depression.15 Meanwhile, opioid analgesics may cause common side effects such as nausea, vomiting, intestinal obstruction, and consciousness problems, which limit its use after donor surgery and may even inhibit donor postoperative recovery.16 Multimodal analgesia, which combines different techniques and medications to reduce opioid consumption and achieve sufficient postoperative pain relief, is crucial in pain management for living liver donors. Regional block analgesia is a part of multimodal analgesia, including epidural analgesia, paravertebral block, erector spinae block, transverse abdominal plane block, and external oblique intercostal block, which have shown good therapeutic effects in reducing pain and promoting early recovery. Epidural analgesia can provide excellent postoperative analgesia for traditional open partial hepatectomy.17 However, due to the concerns about nerve injury and postoperative coagulation dysfunction, the proportion of epidural analgesia after hepatectomy is relatively low in clinical practice.18,19 The use of epidural analgesia after living donor liver surgery is still controversial. Studies have found that both paraspinal nerve block and erector spinae plane block can provide good postoperative analgesia for liver surgery, but there are also risks of hematoma and pneumothorax.20,21 The process of paravertebral nerve block and erector spinae plane block require the patient to change their position and the whole operation is relatively complex. The external oblique interventional (EOI) block is a relatively new block technique. Studies have shown that it has good analgesic effects on upper lateral abdominal wall.22,23 It has been reported that EOI block has good analgesic and recovery effect on liver surgery.24 However, LDH is different from general liver surgery. It has a larger incision in the lower abdomen, which makes it difficult for EOI block to achieve good analgesia and has the risk of internal organ damage. Local infiltration anesthesia is often used as the basis for multimodal analgesia in laparoscopic surgery, especially in laparoscopic gallbladder surgery, which can provide good analgesia.25 However, local infiltration can only provide partial incision analgesia, which is not effective for visceral pain.
Ultrasound-guided transversus abdominis plane (TAP) block has been increasingly used to provide analgesic coverage for the anterior abdominal wall due to its simple operation, clear anatomy, and high safety.26,27 TAP block, as a multimodal analgesic method, is commonly used for postoperative pain after various abdominal surgeries.28–31 Some studies have shown that TAP block can reduce opioid consumption after open living donor hepatectomy and promote postoperative recovery in patients.1,32,33 However, these studies focus on open living donor hepatectomy and pay insufficient attention to postoperative analgesia for LDH. Previous study have confirmed that bilateral TAP block in laparoscopic hepatectomy can provide effective analgesia for patients with liver tumors.34 Due to the pain tolerance of living liver donors is poorer than that of tumor patients,35 whether TAP block can provide effective analgesia after laparoscopic living donor hepatectomy is still uncertain. This study performed bilateral TAP block on patients undergoing LDH, with the aim of assessing the impact of TAP block on postoperative analgesia and recovery of LDH.
Methods and Analysis
Trial Design
This study is a prospective blinded, randomized, controlled clinical trial with a concealed allocation of patients (living liver donors) scheduled to receive laparoscopic partial hepatectomy 1:1 to receive local infiltration anesthesia or TAP block. The study will recruit a total of 80 patients and started in July 2023. The recruiting period will be 24–36 months. The trial will start recruiting patients after being registered on the Chinese Clinical Trial Registry.
Eligibility Criteria
All enrolled patients must fulfill the inclusion criteria and do not include any exclusion criteria. In addition, patients can withdraw from the trial at any time if they refuse to sign informed consent, lose follow-up, do not report efficacy and safety data, or are requested by investigators.
Inclusion criteria: gender unlimited, aged 18–65, ASA I–II, planned to undergo LDH.
Exclusion criteria: Intraoperative conversion to open surgery; Intraoperative massive bleeding; Serious adverse events such as local anesthetic poisoning occurred; Unplanned transfer to intensive care unit (ICU) with tracheal catheter after surgery; Reoperation within 48 hours after surgery; History of severe cardiovascular disease; Infection at the puncture site; History of local anesthesia allergy; History of chronic pain and long-term use of analgesics; history of drug or alcohol abuse; Refusal to participate in observation.
Participant Eligibility and Consent
Eligible patients will be selected based on the listed criteria. They will receive written and oral information notifications and will be included in the study after signing written informed consent.
Randomization and Blinding
Before the start of the trial, random serial numbers of the study subjects will be generated online by the computer based on the sample size. Patients will be divided into two groups based on the generated random serial numbers: TAP group (T group) and local infiltration group (C group), with 40 patients in each group. TAP block will be performed by experienced regional anesthesia specialists, each has performed over 30 such blocks, while local infiltration anesthesia will be performed by the same group of surgeons. The random serial number was stored in the envelope, which will be opened by an anesthesiologist after the surgeon sutured the abdominal fascia, and the corresponding intervention will be performed immediately after the surgery. The patients and the postoperative follow-up team members (outcome assessors) are unaware of the grouping.
Interventions
All enrolled patients will be assigned to one of the following two study groups:
►TAP group (T group): Patients will receive standard anesthesia, and undergo bilateral TAP block after surgery while before extubation.
►Local infiltration group (C Group): Patients will receive standard anesthesia and undergo incision local infiltration anesthesia during surgical sutures.
All patients receive routine monitoring, including electrocardiogram (ECG), heart rate (HR), non-invasive blood pressure (NIBP), pulse oxygen saturation (SpO2), and anesthesia depth monitoring (BIS). Both groups receive radial artery puncture and central vein puncture.
Intravenous Induction of General Anesthesia and Intubation
Methylprednisolone 40 mg will be injected intravenously before general anesthesia induction in both groups.
General anesthesia induction: sufentanil 0.4 ug/kg, propofol 2 mg/kg, cis-atracurium 0.2 mg/kg, tracheal intubation after 3 minutes.
Tracheal intubation: After exposing the glottis, insert a single lumen tracheal tube (male 7.5#, female 7#) for intubation. Mechanical ventilation parameter settings: tidal volume 6–8 mL/kg, respiratory rate 12–15 breaths/min, respiratory ratio (I:E) 1:2, inhaled oxygen concentration (FiO2) 60%, positive end-expiratory pressure (PEEP) 5 cmH2O.
Anesthesia maintenance: Continuous intravenous infusion of propofol, remifentanil, and cis-atracurium to maintain BIS values between 40 and 60.
Intravenous injection of tropisetron 5 mg and tramadol 100 mg 30 minutes before the end of surgery. After the patient fully awake, intravenous injection of atropine 0.5 mg and neostigmine 1 mg completely reversed muscle relaxation. Then, the tracheal catheter will be removed and the patient will be sent to the postanesthesia care unit (PACU) for observation. After reaching the PACU standard, the patient will be transferred to the ICU.
Relevant Complications of Management During Anesthesia
During anesthesia, intravenous injection of 50–100ug deoxyadrenaline to increase blood pressure and maintain it at ± 20% of baseline. If the heart rate decreased to less than 45 times/min, intravenous atropine 0.5 mg.
Intraoperative Analgesia: Ultrasound-Guided Transversus Abdominis Plane (TAP) Block and Local Infiltration Anesthesia
TAP block: after routine abdominal disinfection, a high-frequency 8–13mhz ultrasonic probe was placed between the costal arch and iliac crest (navel level), perpendicular to the midaxillary line, to determine the neurovascular fascia plane and abdominal wall muscle layer. Using the in-plane needle insertion method, the needle will be injected from the anterior axillary line to the area between the internal oblique abdominis and the transversus abdominis muscle. 2 mL of 0.9% sodium chloride solution will be injected to separate the plane and confirm the needle tip position again. After no blood was drawn back, 0.333% ropivacaine 25–30 mL will be administered. And the same method is used for the other side.
Local infiltration anesthesia: According to the routine surgical plan of our center, the surgical incision of LDH is shown in Figure 1. 0.333% ropivacaine 25 mL will be used for local infiltration of the larger incision in the lower abdomen and 5 mL will be used local infiltration in each of the five smaller Trocar incisions.
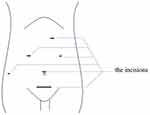 |
Figure 1 Surgical incisions of LDH. |
Postoperative Analgesia Treatment
Both groups will be treated with intravenous analgesia pump for postoperative analgesia. The analgesic pump formulation is a mixture of sufentanil 200 mg and ondansetron 20 mg of 200 mL, background dose infusion 2 mL/h, with a single push infusion of 2 mL and a locking time of 10 minutes.
The anesthesia nurse of PACU will perform a VAS score every 5 minutes. If the resting VAS score is still greater than 4 points after pressing the analgesic pump, remedial analgesia will be given (such as intravenous sufentanil 5ug in the PACU, intravenous tramadol 100 mg after transfer to the ward, until the VAS score is less than 4 points).
Pain Management in the Surgery Ward
For surgical pain management, in addition to continuous PCIA analgesia, flurbiprofen axetil will be administered intravenously twice a day (50 mg/dose). When the patient’s resting VAS score is ≥4, if necessary, an additional injection of morphine hydrochloride 10 mg should be administered.
Outcome Measures
Primary Outcome Measure
The cumulative opioid consumption (converted into intravenous morphine equivalents) within 24 hours postoperatively.
Secondary Outcomes Measures
- General information of patients, intraoperative anesthesia and surgical condition.
- Preoperative VAS score; resting VAS score and exercise VAS score, Aono’s four-point scale (AFPS) restlessness score,36 Ramsay sedation score at extubation, entering and exiting PACU.
- Resting VAS score and exercise VAS score at 2, 4, 16, 24, 48 hours after surgery; time of first use of opioids; effective compressions and total compressions of the analgesic pump at 48 hours after surgery; consumption of opioids and amount of remedial analgesics at 48 hours after surgery;
- Quality of Recovery-15 (QOR-15)37 at 24 and 48 hours after surgery; first anal exhaust time and first ambulation time, sleep quality, time of removing urinary tube and drainage tubes time, postoperative hospitalization days;
- Adverse reactions such aAs postoperative nausea, vomiting, dizziness, skin itching, and adverse events related to nerve block.
- Select donors from T group who underwent right hepatectomy and collect blood samples through central venous catheters at 10, 20, 30, 60, 120 minutes and 24 hours after TAP block to monitor the plasma concentration of ropivacaine.
- Record blood pressure, heart rate, respiratory rate, and oxygen saturation at the time of entering the operating room, end of the surgery, extubation, entering PACU, exiting PACU, 2 hours, 4 hours, 16 hours, 24 hours and 48 hours after surgery.
- Collect liver enzyme indicators (AST, ALT, LDH, bilirubin) and coagulation indicators (PT, INR, APTT) after entering the ICU, on the first and second day after surgery.
Statistical Analysis and Sample Size Calculation
This study will use SPSS 23.0 software for data statistical analysis. The data will be represented in terms of mean ± SD, frequency (percentage), and median (IQR). Student’s t-test will be used to compare normally distributed continuous variables and a Rank sum test will be used to compare skewed distribution variables. Categorical variables will be analyzed using the Pearson χ2 test or Fisher’s exact test. The primary outcome is the total intravenous morphine equivalent doses consumed in the first 24 hours after surgery. According to the pre-trial data, the consumption of opioids within 24 hours after surgery in the T Group was 44.8 ± 14.7 mg, while in C Group was 68.3 ± 23.9 mg. According to the mean superiority test of the two independent samples, the clinically significant difference between the two groups was 10 mg of morphine. The Type I error and Type II error of the hypothesis test were set to 0.05 and 0.2, respectively, and the proportion of sample size between the two groups was 1. The sample size of each group was 35 cases calculated by using PASS software. It is expected that the dropout rate will be at least 10%, and 40 patients will be included in each group, with a total of 80 patients in both groups.
Ethical Considerations, Amendments and Dissemination
This trial is conducted in accordance with the principles of the Declaration of Helsinki. The informed consent procedure is in accordance with the ethical principles and limitations therein. This trial has been approved by the Institutional Review Board of Beijing Friendship Hospital of China Capital University. Any modifications to the study protocol that may affect the potential benefits or safety of the patients, including the changes in study objectives, study design, patient population, sample size, research process or observation indicators, must be submitted to the local medical ethics committee for approval, and the protocol must be formally revised. Regarding privacy protection measures for patients, we will use anonymous data in reports and articles to avoid identifying specific subjects. If data needs to be shared, we will use patient numbers instead of personal identification information. Only authorized personnel are allowed to access shared data to prevent patient privacy exposure. If the study protocol is changed, we will notify all participants and sign informed consent again. The revised protocol will be updated on the Trial Register website to ensure transparency. Regardless of the outcome, the results of this trial will be publicly published in scientific journals or scientific conferences.
Trial Status
The trial study protocol was approved by the Institutional Review Board of Beijing Friendship Hospital of China Capital University on 8 May 2023. The trial began recruiting patients after being registered at the Chinese Clinical Trial Registry.
Discussion
Currently, LDH is increasingly used for living donor liver transplantation.8,9 Although this surgical improvement meets the appearance and functional needs of the liver donor, severe postoperative pain is still frequently observed in the donor.12 In recent years, there has been increasing interest in TAP block as a multimodal analgesic approach to reduce opioid consumption and related side effects after laparoscopic and open surgery.38–40 At present, some trials have begun to study the effect of TAP block on postoperative analgesia after living donor hepatectomy. Kitlik et al performed ultrasound-guided TAP block on donors with a right abdominal “J” incision at the conclusion of surgery and skin closure, with bilateral single point injection of bupivacaine. And the results showed that TAP block can reduce the cumulative morphine consumption after living donor hepatectomy.33 Erdogan et al performed preoperative TAP block with the same method on the same type surgery of donors. The results mentioned that TAP effectively reduced the dose of opioids during surgery and shorten the hospital stay of donors.32 In addition, Amundson et al performed postoperative TAP block using multi-injection liposomal bupivacaine on donors underwent open living donor hepatectomy, and reported that this operation can improve initial postoperative analgesia and improve time to solid food intake and bowel activity while enhanced recovery.41 Maeda et al performed continuous subcostal TAP block on living liver donors with “T” shape incision after surgery, and the results showed no difference in hospital stay. However, continuous TAP block effectively reduced the consumption of postoperative opioids.1 Although the above studies demonstrated the effect of TAP block on opioid consumption, the donors in these trials underwent a more traumatic right abdominal “J” incision or “T” incision. There are no prospective studies observing the effects of TAP block on LDH. In addition, most studies have focused on the effect of TAP block on opioid consumption during surgery. Our study is a prospective blinded, randomized controlled clinical trial to evaluate the effect of tap block on the improvement of postoperative analgesia and recovery after LDH. We hope that TAP block will improve the quality of donor recovery, reduce postoperative pain scores and decrease postoperative opioid use and side effects. The advantage of our trial design is that TAP block is safer when performed under ultrasound guidance. Only one puncture on each side to minimize trauma. During TAP block, there is no need for the patient to turn over to reduce operation time. Ultrasound-guided block has low requirements for ultrasonic machines, simple and easy to identify sound images. At the same time, ultrasound-guided TAP block is easy to operate and has a short learning curve, which is easier to promote clinically. At present, the degree of postoperative pain in donors is not clear. The follow-up of the postoperative pain in donors can provide more experience and information for the pain management in the future.
Although the trial was carefully designed, there were still some limitations. First, most postoperative questionnaires are subjective and influenced by the patient’s impression of pain. As a result, they may not be able to directly measure the analgesic effect, which can lead to subjective bias. In order to reduce this bias, researchers conducted training before the start of the trial. They will explain the questionnaire filling method in detail to patients to minimize bias as much as possible. Meanwhile, the postoperative pain of the patients should be objectively evaluated in this trial, including the time of postoperative relief analgesia and the duration of postoperative analgesia, etc, to ensure the accuracy of the data. Second, postoperative TAP block may lead to a lack of blinding in this trial. To solve this problem, we tried to perform TAP block immediately after surgery when the patient was not awake. Separate the follow-up doctor (outcome assessors) from the surgeon and anesthesiologist, and the outcome assessors will not be informed of the grouping. This effectively eliminates the limitations of this trial. Third, our trial used bilateral tap blockade, with a maximum ropivacaine dosage of 200 mg, which may constitute a potential risk of toxicity. Studies have shown that the use of 3 mg/kg of ropivacaine for TAP block can exhibit potential toxicity in venous plasma concentration.42 However, in the study by Borglum et al, 60ml0.375% ropivacaine was given TAP block, and the plasma concentration of ropivacaine was measured to be much lower than the potential toxicity threshold.43 Our trial detected the plasma concentration of ropivacaine after surgery to observe whether there was a risk of toxicity. Although reports suggest that TAP blockade is a safe analgesic regimen, its impact on postoperative chronic pain and long-term prognosis in liver donor patients is still difficult to evaluate.
Overall, this trial is an important attempt to evaluate the effect of TAP block on postoperative analgesia and prognosis in liver donors. If the tap block achieves positive results, it will greatly help the postoperative analgesic and prognosis of liver donors. The reduction of postoperative pain and improvement in prognosis will bring collective benefits to future patients. However, there are still some challenges in this trial. It is uncertain whether TAP is beneficial for the long-term prognosis of donors and further research is needed.
Data Sharing Statement
All data used or analyzed in this study can be obtained from the corresponding authors.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Maeda A, Shibata SC, Wada H, et al. The efficacy of continuous subcostal transversus abdominis plane block for analgesia after living liver donation: a retrospective study. Journal of Anesthesia. 2016;30(1):39–46. doi:10.1007/s00540-015-2085-x
2. Aguirre-Valadez J, Torre A, Vilatobá M, et al. Indications for liver transplant. Rev Invest Clin. 2014;66(6):534–546.
3. Bachir NM, Larson AM, Palmer BF. Adult liver transplantation in the United States. Am J Med Sci. 2012;343(6):462–469. doi:10.1097/MAJ.0b013e3182308b66
4. Nadalin S, Capobianco I, Panaro F, et al. Living donor liver transplantation in Europe. Hepatobiliary Surgery and Nutrition. 2016;5(2):159–175. doi:10.3978/j.issn.2304-3881.2015.10.04
5. Abu-Gazala S, Olthoff KM. Status of adult living donor liver transplantation in the United States: results from the adult-to-adult living donor liver transplantation cohort study. Gastroenterology Clinics of North America. 2018;47(2):297–311. doi:10.1016/j.gtc.2018.01.004
6. de Villa VH, Lo C-M, Chen C-L. Ethics and rationale of living-donor liver transplantation in Asia. Transplantation. 2003;75(Supplement):S2–5. doi:10.1097/01.TP.0000046532.44975.57
7. Hardman MI, Olsen DA, Amundson AW. Multimodal analgesia decreases postoperative opioid consumption in living liver donation. Mayo Clinic Proceedings: Innovations, Quality & Outcomes. 2021;5(3):583–589.
8. Hong SK, Choi G-S, Han J, et al. Pure laparoscopic donor hepatectomy: a multicenter experience. Liver Transpl. 2021;27(1):67–76. doi:10.1002/lt.25848
9. Soubrane O, Eguchi S, Uemoto S, et al. Minimally invasive donor hepatectomy for adult living donor liver transplantation: an international, multi-institutional evaluation of safety, efficacy and early outcomes. Annals of Surgery. 2022;275(1):166. doi:10.1097/SLA.0000000000003852
10. Bekheit M, Khafagy P-A, Bucur P, et al. Donor safety in live donor laparoscopic liver procurement: systematic review and meta-analysis. Surg Endosc. 2015;29(11):3047–3064. doi:10.1007/s00464-014-4045-1
11. Xu J, Hu C, Cao H-L, et al. Meta-analysis of laparoscopic versus open hepatectomy for live liver donors. PLoS One. 2016;11(10):e0165319. doi:10.1371/journal.pone.0165319
12. Jeong JS, Wi W, Chung YJ, et al. Comparison of perioperative outcomes between pure laparoscopic surgery and open right hepatectomy in living donor hepatectomy: propensity score matching analysis. Sci Rep. 2020;10(1):5314. doi:10.1038/s41598-020-62289-0
13. Yip VS, Dunne DFJ, Samuels S, et al. Adherence to early mobilisation: key for successful enhanced recovery after liver resection. Eur J Surg Oncol. 2016;42(10):1561–1567. doi:10.1016/j.ejso.2016.07.015
14. Joshi GP, Kehlet H. Postoperative pain management in the era of ERAS: an overview. Best Pract Res Clin Anaesthesiol. 2019;33(3):259–267. doi:10.1016/j.bpa.2019.07.016
15. Yong BH, Tsui SL, Leung CC, et al. Management of postoperative analgesia in living liver donors. Transplantation Proceedings. 2000;32(7):2110. doi:10.1016/S0041-1345(00)01592-X
16. Simpson JC, Bao X, Agarwala A. Pain management in enhanced recovery after surgery (ERAS) protocols. Clinics in Colon and Rectal Surgery. 2019;32(2):121–128. doi:10.1055/s-0038-1676477
17. Koul A, Pant D, Rudravaram S, et al. Thoracic epidural analgesia in donor hepatectomy: an analysis. Liver Transpl. 2018;24(2):214–221. doi:10.1002/lt.24989
18. Tzimas P, Prout J, Papadopoulos G, et al. Epidural anaesthesia and analgesia for liver resection. Anaesthesia. 2013;68(6):628–635. doi:10.1111/anae.12191
19. Rosero EB, Cheng GS, Khatri KP, et al. Evaluation of epidural analgesia for open major liver resection surgery from a US inpatient sample. Proc (Bayl Univ Med Cent). 2014;27(4):305–312. doi:10.1080/08998280.2014.11929141
20. Dieu A, Huynen P, Lavand’homme P, et al. Pain management after open liver resection: procedure-specific postoperative pain management (PROSPECT) recommendations. Reg Anesth Pain Med. 2021;46(5):433–445. doi:10.1136/rapm-2020-101933
21. Huang X, Wang J, Zhang J, et al. Ultrasound-guided erector spinae plane block improves analgesia after laparoscopic hepatectomy: a randomised controlled trial. Br J Anaesth. 2022;129(3):445–453. doi:10.1016/j.bja.2022.05.013
22. Elsharkawy H, Kolli S, Soliman LM, et al. The external oblique intercostal block: anatomic evaluation and case series. Pain Med. 2021;22(11):2436–2442. doi:10.1093/pm/pnab296
23. Doymus O, Ahiskalioglu A, Kaciroglu A, et al. External oblique intercostal plane block versus port-site infiltration for laparoscopic sleeve gastrectomy: a randomized controlled study. Obes Surg. 2024;34(5):1826–1833. doi:10.1007/s11695-024-07219-z
24. Liotiri D, Diamantis A, Papapetrou E, et al. External oblique intercostal (EOI) block for enhanced recovery after liver surgery: a case series. Anaesth Rep. 2023;11(1):e12225. doi:10.1002/anr3.12225
25. Loizides S, Gurusamy KS, Nagendran M, et al. Wound infiltration with local anaesthetic agents for laparoscopic cholecystectomy. Cochrane Database of Systematic Reviews. 2014;2014(3):CD007049. doi:10.1002/14651858.CD007049.pub2
26. Abrahams MS, Horn JL, Noles LM, Aziz MF, et al. Evidence-based medicine: ultrasound guidance for truncal blocks. Regional Anesthesia and Pain Medicine. 2010;35(2 Suppl):S36–S42. doi:10.1097/AAP.0b013e3181d32841
27. PETERSEN PL, MATHIESEN O, TORUP H, et al. The transversus abdominis plane block: a valuable option for postoperative analgesia? A topical review. Acta anaesthesiologica Scandinavica. 2010;54(5):529–535. doi:10.1111/j.1399-6576.2010.02215.x
28. De Oliveira GJ, Castro-Alves LJ, Nader A, et al. Transversus abdominis plane block to ameliorate postoperative pain outcomes after laparoscopic surgery: a meta-analysis of randomized controlled trials. Anesth Analg. 2014;118(2):454–463. doi:10.1213/ANE.0000000000000066
29. Kawahara R, Tamai Y, Yamasaki K, et al. The analgesic efficacy of ultrasound-guided transversus abdominis plane block with mid-axillary approach after gynecologic laparoscopic surgery: a randomized controlled trial. J Anaesthesiol Clin Pharmacol. 2015;31(1):67–71. doi:10.4103/0970-9185.150547
30. Grape S, Kirkham KR, Akiki L, et al. Transversus abdominis plane block versus local anesthetic wound infiltration for optimal analgesia after laparoscopic cholecystectomy: a systematic review and meta-analysis with trial sequential analysis. Journal of Clinical Anesthesia. 2021;75:110450. doi:10.1016/j.jclinane.2021.110450
31. El-Dawlatly AA, Turkistani A, Kettner SC, et al. Ultrasound-guided transversus abdominis plane block: description of a new technique and comparison with conventional systemic analgesia during laparoscopic cholecystectomy. British Journal of Anaesthesia. 2009;102(6):763–767. doi:10.1093/bja/aep067
32. Erdogan MA, Ozgul U, Uçar M, et al. Effect of transversus abdominis plane block in combination with general anesthesia on perioperative opioid consumption, hemodynamics, and recovery in living liver donors: the prospective, double-blinded, randomized study. Clin Transplant. 2017;31(4). doi:10.1111/ctr.12931
33. Kitlik A, Erdogan MA, Ozgul U, et al. Ultrasound-guided transversus abdominis plane block for postoperative analgesia in living liver donors: a prospective, randomized, double-blinded clinical trial. J Clin Anesth. 2017;37:103–107. doi:10.1016/j.jclinane.2016.12.018
34. Zhang J, Liu T, Zhou H, et al. The safety and efficacy of ultrasound-guided bilateral dual transversus abdominis plane (BD-TAP) block in ERAS program of laparoscopic hepatectomy: a prospective, randomized, controlled, blinded, clinical study. Drug Des Devel Ther. 2020;14:2889–2898. doi:10.2147/DDDT.S255385
35. Cywinski JB, Parker BM, Xu M, Irefin SA, et al. A comparison of postoperative pain control in patients after right lobe donor hepatectomy and major hepatic resection for tumor. Anesth Analg. 2004;99(6):1747–1752. doi:10.1213/01.ANE.0000136423.17446.5D
36. Cho SA, Ahn S-M, Kwon W, et al. Comparison of remimazolam and desflurane in emergence agitation after general anesthesia for nasal surgery: a prospective randomized controlled study. Korean J Anesthesiol. 2024;77(4):432–440. doi:10.4097/kja.23953
37. Stark PA, Myles PS, Burke JA. Development and psychometric evaluation of a postoperative quality of recovery score: the QoR-15. Anesthesiology. 2013;118(6):1332–1340. doi:10.1097/ALN.0b013e318289b84b
38. Belavy D, Cowlishaw PJ, Howes M, et al. Ultrasound-guided transversus abdominis plane block for analgesia after Caesarean delivery. British Journal of Anaesthesia. 2009;103(5):726–730. doi:10.1093/bja/aep235
39. Finnerty O, Sharkey A, Mc Donnell JG. Transversus abdominis plane block for abdominal surgery. Minerva anestesiologica. 2013;79(12):1415.
40. Bhatia N, Arora S, Jyotsna W, et al. Comparison of posterior and subcostal approaches to ultrasound-guided transverse abdominis plane block for postoperative analgesia in laparoscopic cholecystectomy. J Clin Anesth. 2014;26(4):294–299. doi:10.1016/j.jclinane.2013.11.023
41. Amundson AW, Olsen DA, Smith HM, et al. Acute benefits after liposomal bupivacaine abdominal wall blockade for living liver donation: a retrospective review. Mayo Clin Proc Innov Qual Outcomes. 2018;2(2):186–193. doi:10.1016/j.mayocpiqo.2018.03.003
42. Griffiths JD, Barron FA, Grant S, et al. Plasma ropivacaine concentrations after ultrasound-guided transversus abdominis plane block. Br J Anaesth. 2010;105(6):853–856. doi:10.1093/bja/aeq255
43. Borglum J, Jensen K, Christensen AF, et al. Distribution patterns, dermatomal anesthesia, and ropivacaine serum concentrations after bilateral dual transversus abdominis plane block. Reg Anesth Pain Med. 2012;37(3):294–301. doi:10.1097/AAP.0b013e31824c20a9
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

