Back to Journals » Degenerative Neurological and Neuromuscular Disease » Volume 14
Cold Inducible RNA-Binding Protein Promotes the Development of Alzheimer’s Disease Partly by Inhibition of uPA in Astrocytes
Authors Li Z , Liu JP, Yao FH, Cao Y, Li SC, Liu YY, Wen SX, Liu YX, Liu AJ
Received 27 August 2024
Accepted for publication 10 December 2024
Published 27 December 2024 Volume 2024:14 Pages 143—155
DOI https://doi.org/10.2147/DNND.S490526
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Thomas Müller
Ze Li,1,* Jing Peng Liu,2,* Feng Hua Yao,3,* Yang Cao,1 Shou Chun Li,1 Yuan Yang Liu,1 Su Xin Wen,1 Yu Xiao Liu,1 Ai Jun Liu1
1Department of Neurosurgery, First Medical Center of the Chinese PLA General Hospital, Beijing, 100853, People’s Republic of China; 2Department of Traditional Chinese Medical Science, Sixth Medical Center of the Chinese PLA General Hospital, Beijing, 100037, People’s Republic of China; 3Department of Nephrology, First Medical Center of Chinese PLA General Hospital, Beijing, 100853, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Ai Jun Liu; Yu Xiao Liu, Department of Neurosurgery, First Medical Center of the Chinese PLA General Hospital, 28 Fuxing Road, Haidian District, Beijing, 100853, People’s Republic of China, Tel +86 1066848351 ; +86 1066848354, Email [email protected]; [email protected]
Background: Cold inducible RNA-binding protein (CIRP) is an important danger-associated molecular pattern involved in tissue-specific and systemic inflammation related to inflammation and Alzheimer’s disease (AD). However, the precise roles and mechanism of CIRP in the functional changes in astrocytes during the development of AD are still unknown. This study aimed to assess gene expression alterations in astrocytes after they overexpress CIRP (oe-CIRP) and to explore the relationship between abnormal CIRP expression and AD.
Methods: We created astrocyte cell lines with a CIRP or control vector expression using three human glioma cell lines U87, U251 and H4, and analyzed the mRNA expression profiles of 3 pairs of cells via microarray. Bioinformatics identified differentially expressed mRNAs between CIRP-overexpressing (ov-CIRP) and control groups, validated by q-PCR and Western blotting (WB). Finally, the effect of CIRP overexpression in astrocytes on neurons was observed in a coculture system.
Results: We identified 119 mRNAs with obvious fold changes between the ov-CIRP and control groups for all 3 pairs of human glioma cell lines. The biological functional analysis indicated that urokinase plasminogen activator (uPA), a gene whose expression significantly decreased after CIRP overexpression, was closely associated with AD. WB and q-PCR confirmed that CIRP overexpression significantly inhibited uPA at both mRNA and protein levels in U87, U251 and H4 cells. Moreover, compared with those cocultured with control astrocytes, SH-SY5Y cells cocultured with CIRP-overexpressing astrocytes exhibited a significant increase in the expression of amyloid-β (Aβ)1– 42 and the hyperphosphorylated microtubule-associated protein tau (Tau).
Conclusion: CIRP overexpression in astrocytes inhibits uPA expression, promoting Aβ 1-42 production and tau phosphorylation in neurons, thereby increasing AD risk. These results suggest that the overexpression of CIRP in astrocytes contributes to the development of AD.
Keywords: cold-inducible RNA-binding protein, Alzheimer disease, astrocyte, neuron
Background
AD is one of the most common diseases of neurodegenerative dementia which acts as the sixth leading cause of death in the US.1 In recent years, a large number of new candidate drugs on AD have failed in clinical trials, which led to the pessimistic prospects for AD drug development. Neuronal injury and degeneration is the major feature of AD, which is specifically manifested as synaptic damage in the hippocampus and the defect of learning and memory in neocortex regions.2 Thus, deep exploring of the pathophysiology of synaptic damage during the development of AD is important to discover new therapeutic methods to protect the synapses from irreversible neuron degeneration.
As one of the most common cell types in the brain, astrocytes are vital to sustain neuron health, they not only supply structural support to neurons but also maintain the balance of brain micro-environments. The interactions between astrocytes and neurons are crucial for the survival and function of dopaminergic neurons. Previous studies proved that the dysfunction of astrocytes was able to cause the degenerative alteration of dopaminergic neurons.3,4 For example, Cheng et al reported that astrocytes which derived from induced pluripotent stem cells could alleviate dopaminergic neurodegeneration in vitro.5 Kuter et al proved that astrocytes played a crucial role in early degeneration of nigrostriatal neurons.6 However, the mechanism by which astrocytes affect synaptic function during neurodegeneration is still unclear.
Cold-inducible RNA-binding protein (CIRP) comprise an amino-terminal consensus sequence RNA binding domain and a carboxyl-terminal glycine-rich domain.7 Current researches have shown that CIRP is not only involved in the regulation of cellular stress responses such as UV irradiation, hypoxia and hypothermia, but also regarded as a damage associated molecular pattern (DAMP) which could bind to the TLR4 and promote inflammation by activating nuclear transcription factor nuclear factor-κB.8,9 Moreover, several studies reported CIRP was capable of regulating the neuronal dysfunction via the IL-6Rα/STAT3/Cdk5 pathway and promoting the neuronal calpain activity, which was closely associated with pathological change of neurons from AD patients.10,11 Of note, a recent study reported CIRP may be a potential target of alcohol-induced AD, because alcohol-induced CIRP from microglial contributed to the development of AD.12 So, it is interesting to find whether abnormal expression of CIRP in astrocytes was a risk factor for AD.
In this article, we aimed to explore the effects of CIRP over-expression on the expression profiles of mRNAs in human astrocytes and identify whether and how CIRP regulate the AD-associated genes in astrocytes. The results from micro-array revealed there were close correlations between CIRP over-expression and AD in 3 different human glioma astrocyte cell lines. In addition, it revealed for the first time that over-expression of CIRP could inhibit the expression of uPA, the favorable factors of AD, in astrocytes. Furthermore, astrocytes-neuron co-culture experiments in vitro elucidated that over-expression of CIRP in astrocytes promoted expression of BACE, Aβ1-42 and phosphorylated tau protein (p-Tau) in neuron cells. These findings supplied a potential new mechanism of CIRP in pathologies of AD and confirmed eCIRP as a promising therapeutic target for the neural degenerative disease.
Materials and Methods
Cell Culture
Human glioma cell lines including U87, U251, H4 and human neuroblastoma SH-SY5Y cells were brought from Shanghai GeneChem Co. Ltd (Shanghai, China). The cells were cultured in 6-well plates in DMEM or RPMI-1640 medium (Thermo Fisher Scientific) containing 10% fetal bovine serum and 1% penicillin/streptomycin (Thermo Fisher Scientific), at 37°C and 5% CO2.
Establishment of Human Glioma Cell Lines CIRP Which Stably Over-Expressed CIRP
CIRP lentiviral particles which contain the green fluorescent protein (GFP) and full length of CIRP gene (over-expressed CIRP group) and control lentiviral particles (control group) were purchased from Shanghai Genechem Co., Ltd. Transfection was carried out according to the instructions from the producer. Briefly, the cells were cultured with lentiviral particles and polybrene (5ug/mL) in medium for 12 h. Then the medium containing lentiviral particles was removed, the cells were washed and added with complete medium. The GFP gene expression was observed by fluorescence microscopy at 3 days after transfection.
A Co-Culture System
SH-SY5Y cells were cultured with complete medium containing 10 μM retinoic acid (Sigma-Aldrich) for 7 days to be differentiated into neurons, as previously reported.13 Then a co-culture system was established by plating SH-SY5Y cells and astrocytes in a Trans-well system (0.4 μm pore size, Corning, NY, USA). The oe-CIRP or control human astrocytes were cultured onto the lower chamber (LC) of the trans-well system after LPS treatment, and the neurons were cultured onto the upper chamber (UC). After 24 h, the culture medium with or without uPA (20 ng/mL) was added to the upper and lower chambers. After they were co-cultured for 48 h, the cells were collected for the gene and protein tests.
Profiling of mRNA Expression
Core ® lncRNA + mRNA expression profile microarray was used to profile the expression of mRNA, which was performed by capitalbio Bio-tech (Beijing, China). Briefly, RNA samples from cells in different groups were purified, amplified and transcribed into fluorescent cRNAs. Next, cRNA was hybridized to the microarray. The gene expression profiles of human species were detected using Boao core LncRNA + mRNA Human Gene Expression Microarray V4.0,4x180K chip with method number AG-GE-WL 10-01-2010 and data analysis method number AG-GE-DL 00-01-2010.
Screening and Mapping of Differentially Expressed Genes
After expression spectrum chip hybridization scanning, tiff-format image data were processed using Feature Extraction lift software. Gene expression difference and statistical significance p values were calculated using GeneSpring GX software. Briefly, the tiff plots derived from chip scan were processed with Feature Extraction software to obtain the raw data files. Then, the raw data file was imported into GeneSpring software, written grouping and other parameter information. Each sample was normalized and performed QC analysis, cluster analysis and graphical presentation with Cluster3.0 software. In this study, the log fold difference based on 2, obtained by subtracting the normalized signal values, with positive values indicating higher expression and negative values indicating lower expression. The fold change between two groups were numerically equal to FC (abs). GeneSpring software is used for data normalization and statistical processing of differentially expressed lncRNA/mRNA analysis. In addition to the above, the result files also include statistical significance indicators. The p-value was calculated using the T-test unpaired method. And the p-value adjusted using the Benjamini Hochberg FDR method. The screening criteria for conventional differences mRNA are: FC (abs) > 2.0-fold and p≤0.05. Finally, differential comparisons were made to obtain differential genes according to the grouping information, and the differential mRNAs were analyzed by GO pathway analysis.
Validation of Differentially Expressed Genes
Expression of screened mRNAs was validated by qRT-PCR. Briefly, trizol reagent was used to extract total RNA from cells and cDNA was synthesized with SuperScript III reverse transcriptase. Then, the quantification of target gene expression was carried out using SLAN Real-Time PCR System (Hongshi, Shanghai, China). GAPDH was regarded as an internal control. Expression levels were calculated with the primer pairs for the amplification of target mRNAs as shown in Table 1. Data were analyzed using the comparative cycle threshold method.
 |
Table 1 The Sequence of Primers |
Western Blotting
The cells from different groups were collected, and washed with PBS. Then they were mixed with cell lysate, centrifuged at 12,000 g in 4°C for 10 min. The supernatant of mixture was gathered for protein extraction with the Qproteome Mammalian Protein Prep kit. The concentration of protein was examined using the BCA Protein Assay Kit. Then, the protein was isolated by polyacrylamide gels, and transferred onto nitrocellulose membrane by iBlot. The binds were observed by chemiluminescence using a FluorChem E system. Antibodies to Aβ1−42 (ab180956, Abcam), beta-secretase (ab183612) uPA (ab32057, Abcam) and p-Tau (Ser396) (ab32057, Abcam) were used to determine the protein expression. β-Actin was regarded as the internal control.
Statistical Analysis
The data were shown as the mean ± standard error of mean (SD). Statistical analyses were performed by GraphPad Prism 9. One- or two-way analysis of variance (ANOVA) was used to analyze significant differences among the groups, and Student’s t-test was used to assess the differences between the two groups. P values less than 0.05 were regarded as statistically significant.
Results
Establishment of Astrocyte Cell Lines Which Over-Expressed CIRP
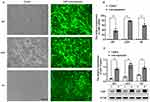
The cells was observed by the fluorescence microscope on the third day after they were transfected with control-GFP or CIRP-GFP lentiviral vectors. As shown in Figure 1A, more than 80% of transfected cells expressed GFP, which implied that the efficiency of the infection was above 80%. The results from q-PCR assay demonstrated that the mRNA levels of CIRP were significantly promoted in ov-CIRP groups compared with those in control groups with approximately 59.66-fold, 78.11-fold, and 38.09-fold increases in U87, U251, and H4 from oe-CIRP groups vs those from control groups, respectively (p<0.001, Figure 1B). The results of Western blotting assay confirmed that the protein expressions of CIRP were also greatly increased in oe-CIRP groups when compared with those in control groups for all 3 pairs of astrocytes (all p<0.001, Figure 1C).
Identification of mRNAs with Significant Fold Changes in Astrocytes Which Over-Expressed CIRP
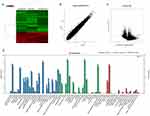
We used volcano plots and heat map to determine the variation of gene expression between ov-CIRP and control human glioma cells. In total, 119 mRNAs showed differential expression in all 3 pairs of cells. Among them, 39 mRNAs were greatly up-regulated, and 80 mRNAs were obviously down-regulated (a threshold of ≥2-fold and P<0.05, Figure 2A–C). The changed mRNAs were associated with reproductive progress, rhythmic progress, response to stimulation and so on (Figure 2D). The KEGG and GO pathway analysis showed the up- or down-regulated mRNAs was mostly enriched in FGF signaling pathway, activated NOTCH1 Transmits Signal to the Nucleus, interleukin receptor SHC signaling and so on (Figure 3A). Of note, the results of significant enriched disease terms indicated that over-expression of CIRP was involved in asthma ie, dehydroepiandrosterone, rheumatoid arthritis, autism spectrum disorder, Alzheimer’s disease and so on (Figure 3B).
CIRP Over-Expression in Astrocytes Induced the AD-Like Changes of Neurons in Co-Culture System
As shown in Figure 4A, SH-SY5Y cells were cocultured with ov-CIRP or control astrocyte cell lines (U251, U87 and H4) to examine whether CIRP over-expression in astrocytes has the effect on amyloid accumulation and hyper-phosphorylation of neural cells. The results from q-PCR showed that the mRNA levels of BACE were greatly increased in ov-CIRP groups compared with those in control groups with approximately 5.51-fold, 7.31-fold, and 3.41-fold decreases in U87, U251, and H4 from ov-CIRP groups compared with those from control groups, respectively (p<0.001, Figure 4B). The results from WB assay indicated that the expression levels of beta-secretase, Aβ1-42 and p-Tau (Ser396) were significantly increased in the SH-SY5Y cells from ov-CIRP groups in comparison to these from control groups (Aβ1-42: group effect F (1, 12) = 346.8; p-Tau: F (1, 12) = 524.7; beta-secretase: F (1, 12) = 158.7; all p<0.001, Figure 4C).
uPA Was Significantly Down-Regulated After CIRP Over-Expression
To further explore the relationship between CIRP and Alzheimer’s disease, the top ten changed mRNAs after CIRP over-expression are listed in Table 2, which include WNT5B, FGFR3, PSD4, UNC93B1, IFI44, uPA, LBH, BNIP3, LIF and KYNU.
 |
Table 2 The Top 10 Changed mRNAs |
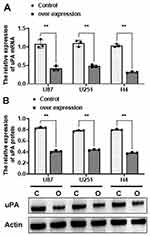
A lot of evidence proved the tissue-type plasminogen activator (tPA) and urokinase-type plasminogen activator (uPA) play the important roles in the pathogenesis of AD. Thus, we verified the mRNA and protein expression of uPA in all 3 pairs of cells by q-PCR and WB assays. The results showed that the mRNA levels of uPA were greatly decreased in ov-CIRP groups compared with those in control groups with approximately 38.09-fold, 78.11-fold, and 59.66-fold decreases in U87, U251, and H4 from oe-CIRP groups vs those from control groups respectively (p<0.001, Figure 5A). Accordingly, the protein expressions of uPA were also significantly decreased in oe-CIRP groups when compared with those in control groups for all 3 pairs of astrocytes (group effect F (1, 12) = 277.3, p<0.001, Figure 5B).
uPA Alleviated Ov-CIRP Astrocytes Induced Dysfunction of Neuron Cells
To analyze the effect of uPA on ov-CIRP astrocytes induced dysfunction of neuron cells, the SH-SY5Y cells were co-cultured with ov-CIRP astrocyte cell lines which were treated with or without uPA (Figure 6A). Then we found that, when ov-CIRP astrocytes lines (U87, U251, and H4) were treated with uPA, the promoting levels of beta-secretase, Aβ1-42 protein and p-Tau in SH-SY5Y induced by ov-CIRP astrocytes were significantly inhibited after uPA treatment (ov-CIRP+uPA vs ov-CIRP groups: Aβ1-42, approximately 40%, 41%, and 30% decrease in U87, U251, and H4, respectively; p-Tau, approximately 50%, 61%, and 45% decrease in U87, U251, and H4, respectively; beta-secretase, approximately 47%, 30%, and 29% decrease in U87, U251, and H4, respectively; all p<0.001, Figure 6B).
Discussion
As one of the most common neurodegenerative diseases, AD is characterized by the aggregation of beta-amyloid (Aβ), the hyperphosphorylation of tau and the promoted activities of acetylcholinesterase (AchE) enzyme.14–19
A lot of researchers believed that deeper studies on novel molecular mechanisms and pathological changes of AD are crucial to finding new drug targets. However, the mechanism of how CIRP take actions in the development of AD was still unclear.
To gain novel insights to the functions of CIRP in the pathogenesis of astrocyte, we comprehensively analyzed the mRNA profiling data from 3 pairs of control and ov-CIRP human astrocytoma cell lines. We identified the significantly changed mRNAs in all 3 pairs of cells and annotated their function. Previous studies reported that CIRP was widely involved with the biological processes including circadian modulation, the proliferation and survival of cells, telomere maintenance, cellular stress response, inflammation and cancer.20 Here, the data from micro-array also identified that the changes mRNAs induced by CIRP over-expression were implicated in response to stimulus, inflammatory bowel disease, cell growth and so on, which were in accordance with previous studies (Figures 2 and 3). Moreover, our work revealed a comprehensive transcripts network implying the abnormal expression of CIRP in astrocytes was associated with a series of nervous system diseases such as Autism spectrum disorder, Parkinson’s disease (motor and cognition), Bipolar disorder and schizophrenia, AD and so on (Table 3).
 |
Table 3 The Relative Disease of CNS After CIRP Over-Expression |
It is well known that the pathological damage of AD is related to the accumulation of extracellular senile plaques which are composed of aggregates of Aβ peptides, and the hyper-phosphorylation of Tau.21 Wang et al reported that eCIRP activated STAT3 via IL-6Rα and speculated that eCIRP derived micro-glial may be an important mediator of neuronal tau phosphorylation after exposure to alcohol.10 Here, in order to explore the function of CIRP in astrocyte induced neuronal injury, the phosphorylation of p-Tau and the expression of BACE, Aβ1-42 in neuron cells were measured after they were co-cultured with ov-CIRP or control astrocytes lines. Then our data revealed for the first time that over-expression of CIRP in astrocytes could greatly promote the expression levels of p-Tau (Ser396), Aβ1-42, and BACE in neurons from co-culture system. These results proved that CIRP in astrocytes could act as a vital mediator of AD.
At last, we explore the molecular mechanisms on how CIRP in astrocytes regulate the neuronal tau phosphorylation. The results from micro-array identified three mRNA in astrocytes as candidates of AD after CIRP over-expression. Among them, uPA gained our attention. It is well known that uPA encodes a secreted serine protease that converts plasminogen to plasmin, mutation of uPA is closely associated with the onset of Alzheimer’s disease. Thus, we identified the expression levels of uPA in control and ov-CIRP astrocytoma cells by q-PCR and WB assays. The results confirmed a significant decrease of uPA in ov-CIRP astrocyte compared with control astrocytes at both mRNA and protein levels.
Merino et al reported that uPA and its receptor were mainly expressed in neuronal extensions, growth cones and a subpopulation of astrocytes in the mature brain, and the release of uPA in the mature central nervous system could improve the axonal and synaptic function after injury.22,23 Moreover, the increasing evidence proved that the plasminogen activator urokinase could protect neurons from amyloid β triggered synaptic injury.24,25 On the basis of this evidence, we speculated the neuronal damage induced by CIRP over-expression in astrocytes may be involved with the decreasing expression of uPA. Thus, we added uPA to astrocyte neuron co-culture system and found uPA treatment significantly alleviated ov-CIRP astrocytes induced dysfunction of neuron, as evidenced by the decreased Aβ1-42 accumulation and tau hyper-phosphorylation in ov-CIRP+uPA groups. These results implied that over-expression of CIRP in astrocytes could cause AD-like alteration of neurons partly depending on the down-regulation of uPA. Of note, previous studies mainly focused on the function of neuronal uPA in AD, here we revealed for the first time that abnormal expression of uPA in astrocytes was another cause of neuron dysfunction. Our data confirmed the significant role of CIRP in the development of AD, supplied the understanding of molecular signaling in the CIRP induced neuron damage, and provided a possible new therapeutic target to alleviate cognitive decline during AD. However, the current work only explores the roles of CIRP in astrocytes at cell levels and the deeper studies should be carried out in animals. Moreover, the mechanism on how CIRP affects the levels of uPA is still unclear.
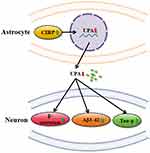
In summary, this study revealed that over-expression of CIRP in astrocyte exerts a harmful impact on the neurons by elevating the expression levels of BACE, Aβ1-42 and p-Tau (Ser396) in neuron cells partly via down-regulation of uPA (Figure 7). Thus, down-regulation of CIRP expression may be a useful method to alleviate synaptic dysfunction during AD.
However, there were some limitations in this article. Firstly, there are some differences between glioma cell lines and astrocytic function, although U87, U251, and H4 originate from astrocytes. Due to oncogenic background glioma cell lines, they can only reflect some features of astrocytes. Secondly, in this article we did not assess whether CIRP-overexpressing astrocytes affect SH-SY5Y cells through altering the secretion of additional cytokines or neurotoxic factors or through changing the other inflammatory or stress-response pathways in astrocytes which can contribute to AD pathogenesis. Finally, we only explored the role of uPA in CIRP-induced changes, but did not explore the specific pathways in these effects. So further studies are needed to explore the accurate mechanisms.
Data Sharing Statement
All the datasets in manuscript or additional file are available from the corresponding authors on reasonable request.
Ethics Approval and Consent to Participate
The experiments were approved by the Ethical Committee of the General Hospital of Chinese PLA, Beijing, China.
Consent for Publication
All authors approve of the publication of the final manuscript.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The present work was supported by grants from the PLA General Hospital Youth Independent Innovation Science Fund project (22QNFC106,22QNFC003).
Disclosure
All authors declare that they have no conflicts of interest in this work.
References
1. 2020 Alzheimer’s disease facts and figures. Alzheimers Dement. 2020;16:391–460. PMID: 32157811. doi:10.1002/alz.12068
2. Sanabria-Castro A, Alvarado-Echeverria I, Monge-Bonilla C. Molecular pathogenesis of Alzheimer’s disease: an update. Ann Neurosci. 2017;24(1):46–54. doi:10.1159/000464422
3. Booth HDE, Hirst WD, Wade-Martins R. The role of astrocyte dysfunction in Parkinson’s disease pathogenesis. Trends Neurosci. 2017;40(6):358–370. doi:10.1016/j.tins.2017.04.001
4. Bantle CM, Hirst WD, Weihofen A, Shlevkov E. Mitochondrial dysfunction in astrocytes: a role in Parkinson’s disease? Front Cell Dev Biol. 2020;8(608026). doi:10.3389/fcell.2020.608026
5. Cheng XY, Biswas S, Li J, et al. Human iPSCs derived astrocytes rescue rotenone-induced mitochondrial dysfunction and dopaminergic neurodegeneration in vitro by donating functional mitochondria. Transl Neurodegener. 2020;9(1):13. doi:10.1186/s40035-020-00190-6
6. Kuter K, Olech L, Glowacka U. Prolonged dysfunction of astrocytes and activation of microglia accelerate degeneration of dopaminergic neurons in the rat substantia Nigra and block compensation of early motor dysfunction induced by 6-OHDA. Mol Neurobiol. 2018;55(4):3049–3066. doi:10.1007/s12035-017-0529-z
7. Nishiyama H, Itoh K, Kaneko Y, Kishishita M, Yoshida O, Fujita J. A glycine-rich RNA-binding protein mediating cold-inducible suppression of mammalian cell growth. J Cell Biol. 1997;137(4):899–908. doi:10.1083/jcb.137.4.899
8. Murao A, Tan C, Jha A, Wang P, Aziz M. Exosome-mediated eCIRP release from macrophages to induce inflammation in sepsis. Front Pharmacol. 2021;12:791648. doi:10.3389/fphar.2021.791648
9. Zhong P, Zhou M, Zhang J, Peng J, Zeng G, Huang H. The role of cold-inducible RNA-binding protein in respiratory diseases. J Cell Mol Med. 2022;26(4):957–965. doi:10.1111/jcmm.17142
10. Sharma A, Brenner M, Jacob A, Marambaud P, Wang P. Extracellular CIRP activates the IL-6Ralpha/STAT3/Cdk5 pathway in neurons. Mol Neurobiol. 2021;58(8):3628–3640. doi:10.1007/s12035-021-02368-z
11. Sharma A, Sari E, Lee Y, et al. Extracellular CIRP induces calpain activation in neurons via PLC-IP(3)-dependent calcium pathway. Mol Neurobiol. 2023;60(6):3311–3328. doi:10.1007/s12035-023-03273-3
12. Sharma A, Brenner M, Wang P. Potential role of extracellular CIRP in alcohol-induced Alzheimer’s disease. Mol Neurobiol. 2020;57(12):5000–5010. doi:10.1007/s12035-020-02075-1
13. Mendsaikhan A, Takeuchi S, Walker DG, Tooyama I. Differences in gene expression profiles and phenotypes of differentiated SH-SY5Y neurons stably overexpressing mitochondrial ferritin. Front Mol Neurosci. 2018;11:470. doi:10.3389/fnmol.2018.00470
14. Rai SN, Singh C, Singh A, Singh MP, Singh BK. Mitochondrial dysfunction: a potential therapeutic target to treat Alzheimer’s disease. Mol Neurobiol. 2020;57(7):3075–3088. doi:10.1007/s12035-020-01945-y
15. Srivastava P, Tripathi PN, Sharma P, et al. Design and development of some phenyl benzoxazole derivatives as a potent acetylcholinesterase inhibitor with antioxidant property to enhance learning and memory. Eur J Med Chem. 2019;163:116–135. doi:10.1016/j.ejmech.2018.11.049
16. Tripathi PN, Srivastava P, Sharma P, et al. Biphenyl-3-oxo-1,2,4-triazine linked piperazine derivatives as potential cholinesterase inhibitors with anti-oxidant property to improve the learning and memory. Bioorg Chem. 2019;85:82–96. doi:10.1016/j.bioorg.2018.12.017
17. Singh M, Agarwal V, Pancham P, et al. A comprehensive review and androgen deprivation therapy and its impact on Alzheimer’s disease risk in older men with prostate cancer. Degener Neurol Neuromuscul Dis. 2024;14:33–46. doi:10.2147/DNND.S445130
18. Tripathi PN, Lodhi A, Rai SN, et al. Review of pharmacotherapeutic targets in Alzheimer’s disease and its management using traditional medicinal plants. Degener Neurol Neuromuscul Dis. 2024;14:47–74. doi:10.2147/DNND.S452009
19. Ramakrishna K, Karuturi P, Siakabinga Q, et al. Indole-3 carbinol and diindolylmethane mitigated beta-amyloid-induced neurotoxicity and acetylcholinesterase enzyme activity: in silico, in vitro, and network pharmacology study. Diseases. 2024;12(8):184. doi:10.3390/diseases12080184
20. Han J, Zhang Y, Ge P, et al. Exosome-derived CIRP: an amplifier of inflammatory diseases. Front Immunol. 2023;14:1066721. doi:10.3389/fimmu.2023.1066721
21. DeTure MA, Dickson DW. The neuropathological diagnosis of Alzheimer’s disease. Mol Neurodegener. 2019;14(1):32. doi:10.1186/s13024-019-0333-5
22. Merino P, Diaz A, Jeanneret V, et al. Urokinase-type plasminogen activator (uPA) binding to the uPA Receptor (uPAR) promotes axonal regeneration in the central nervous system. J Biol Chem. 2017;292(7):2741–2753. doi:10.1074/jbc.M116.761650
23. Merino P, Diaz A, Manrique LG, Cheng L, Yepes M. Urokinase-type plasminogen activator (uPA) promotes ezrin-mediated reorganization of the synaptic cytoskeleton in the ischemic brain. J Biol Chem. 2018;293(24):9234–9247. doi:10.1074/jbc.RA118.002534
24. Diaz A, Merino P, Guo JD, et al. Urokinase-type plasminogen activator protects cerebral cortical neurons from soluble abeta-induced synaptic damage. J Neurosci. 2020;40(21):4251–4263. doi:10.1523/JNEUROSCI.2804-19.2020
25. Yepes M. The plasminogen activating system in the pathogenesis of Alzheimer’s disease. Neural Regen Res. 2021;16(10):1973–1977. doi:10.4103/1673-5374.308076
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Mechanisms of Cancer-Induced Bone Pain
Wang X, Li L, Wang Y
Journal of Pain Research 2025, 18:315-326
Published Date: 20 January 2025