Back to Journals » Journal of Inflammation Research » Volume 18
Combining Preoperative and Postoperative Prognostic Nutritional Index as an Improved Prognostic Factor for Overall Survival in Patients with Colorectal Cancer
Received 13 April 2025
Accepted for publication 1 July 2025
Published 8 July 2025 Volume 2025:18 Pages 8935—8944
DOI https://doi.org/10.2147/JIR.S529218
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Fatih Türker
Jong Min Lee,1 Jeonghyun Kang2
1Department of Surgery, Yongin Severance Hospital, Yonsei University College of Medicine, Yongin, Republic of Korea; 2Department of Surgery, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Republic of Korea
Correspondence: Jeonghyun Kang, Department of Surgery, Gangnam Severance Hospital, Yonsei University College of Medicine, 211 Eonju-ro, Gangnam-gu, Seoul, 06273, Republic of Korea, Tel +82-2-2019-3303, Fax +82-2-3462-5994, Email [email protected]
Purpose: While preoperative prognostic nutritional index (PNI) is a well-established prognostic marker in colorectal cancer (CRC), and postoperative PNI has gained attention, their combined prognostic value remains largely unexplored.
Patients and Methods: We analyzed patients who underwent curative surgery for stage I–III CRC between March 2004 and February 2014. The pre- and postoperative PNI, measured within 1 month before and 3– 8 weeks after surgery, were combined to create “change-PNI” The Cox proportional hazards model was used to assess the prognostic significance, and the C-index was compared across values.
Results: The optimal pre- and postoperative PNI cutoff values predicting 5-year overall survival (OS) were 48.05 and 43.65, respectively. The patients were categorized into four groups based on their pre- and postoperative values: pre-low + post-low (G1), pre-low + post-high (G2), pre-high + post-low (G3), and pre-high + post-high (G4). A multivariable Cox proportional hazards model demonstrated that patients in G2, G3, and G4 had significantly lower mortality risks than those in G1 (HR [95% CI] vs G1: G2, 0.341 [0.186– 0.625]; G3, 0.457 [0.222– 0.941]; G4, 0.222 [0.123– 0.401]). The C-index of change-PNI (0.671, 95% CI 0.617– 0.720) was superior to that of preoperative PNI (0.609, 95% CI 0.563– 0.654) (bootstrap mean difference: 0.062, 95% CI 0.029– 0.099) and postoperative PNI (0.622, 95% CI 0.581– 0.664) (bootstrap mean difference: 0.049, 95% CI 0.014– 0.085).
Conclusion: Change-PNI serves as a more effective independent immuno-nutritional marker than pre- or postoperative PNI in predicting OS in patients undergoing surgery for non-metastatic colorectal cancer.
Keywords: colorectal cancer, prognostic nutritional index, postoperative outcomes, overall survival
Graphical Abstract:

Introduction
The accurate prediction of cancer patient prognosis plays a crucial role in developing treatment strategies, evaluating responses, and conducting consultations with patients and their families.1 For colorectal cancer (CRC), tumor-node-metastasis (TNM) staging based on clinical and pathological evaluations is widely used as the standard for prognostic assessment.2 However, this tumor-based prediction method has limitations in accurately predicting disease progression. For example, a survival paradox can occur in which some early stage III patients may exhibit better survival outcomes than those with advanced stage II disease.3 To overcome these limitations, efforts are underway to modify the existing prediction models and identify new prognostic factors.4
Many investigators have identified the prognostic value of nutritional and inflammatory host-related biomarkers in patients with CRC, including the Glasgow Prognostic Score (GPS), neutrophil-lymphocyte ratio (NLR), platelet-lymphocyte ratio (PLR), and prognostic nutritional index (PNI).5,6 The PNI, calculated from the lymphocyte count and serum albumin level, reflects a patient’s nutritional and immune status. These immuno-nutritional markers are considered important preoperative prognostic factors associated with delayed wound healing, muscle weakness, and immune dysfunction. Furthermore, they are linked to cancer progression, and several studies have identified the molecular pathways involved in cancer-related inflammation.7,8
Significant immunological changes were observed in these patients during the perioperative period. An immunosuppressive state in response to acute inflammation and surgical stress can promote micrometastases and negatively affect oncological outcomes.9 The postoperative immune-nutritional status, such as PNI levels, has gained attention as a key determinant of prognosis in patients with cancer,10,11 raising the question of whether postoperative status affects survival independently or merely reflects preoperative conditions.
However, few studies have compared the importance of pre- versus postoperative immune-nutritional status, as most have focused on the preoperative period alone.11,12 This study investigated the prognostic impact of a combined pre- and postoperative PNI and compared its predictive efficacy with either one alone.
Materials and Methods
Study Patients
This retrospective cohort study examined patients who underwent curative resection for stage I–III colorectal adenocarcinoma and had available records of both pre- and postoperative PNI at Gangnam Severance Hospital, Yonsei University College of Medicine, between March 2004 and February 2014. We initially selected 1697 patients who underwent surgical resection of colorectal tumors during this period. Patients were excluded if they had histologically defined neuroendocrine or gastrointestinal stromal tumors (n = 112); appendiceal or anal cancers (n = 19); CRC tumors stage 0, IV, or missing stage information (n = 223); hereditary nonpolyposis CRC or familial adenomatous polyposis-associated cancers (n = 6); preoperative treatment (n = 112); emergent surgery (n = 4); inflammatory bowel disease-associated cancers (n = 2); of double primary or synchronous cancers (n = 20). Additionally, patients without available PNI data or blood test results within one month prior to surgery (n = 52) and those without PNI data collected between 3 and 8 weeks after surgery (n = 482) were excluded. Finally, 665 patients were included in this study. Postoperative PNI was assessed between 3 and 8 weeks after surgery based on our institution’s standard protocol. Most patients are discharged within 7 days and return for follow-up at 3 weeks for pathology review and treatment planning. Adjuvant chemotherapy typically begins between 6 and 8 weeks postoperatively. To minimize the influence of surgery and chemotherapy, we set 8 weeks as the upper limit for postoperative PNI measurement. Details of the inclusion process are presented in Supplementary Figure S1. The study protocol followed the ethical standards of the institutional and national research committees as well as the 1964 Helsinki Declaration and its later amendments.
Follow-Up
All patients underwent surgical resection and were followed up every 3–6 months to monitor for tumor recurrence. Blood tests were conducted at each visit and chest and abdominopelvic computed tomography scans were performed every 6–12 months. Adjuvant chemotherapy was primarily recommended for patients with high-risk stage II or stage III CRC, in accordance with the National Comprehensive Cancer Network guidelines.13 Colonoscopies were generally scheduled for patients at 1, 3, and 5 years postoperatively.
Determination of Cutoff Values for Pre- and Postoperative PNI, and Grouping Based on Combined PNI
The PNI value was calculated using the following formula: 10 × serum albumin (g/dL) + 0.005 × total peripheral lymphocyte count (cells/mm3). To establish optimal cutoff values for both pre- and postoperative PNI, we employed the X-tile program, an open-source tool specifically designed for cutoff selection in biomarker studies. Overall survival (OS) was used as the primary outcome to determine the cutoff values. The X-tile software evaluates various ways to divide the data into two groups (low vs high PNI) by analyzing their relationship with survival outcomes. Log rank tests were used to compare the survival curves between the groups and identify significant differences in survival. Additionally, chi-squared (χ²) statistics were used to assess how effectively each cutoff separated patients into distinct prognostic groups, determining the division with the strongest correlation to survival differences. This dual analysis yielded the most accurate high and low PNI cutoff values for both the pre- and postoperative periods, ensuring precise prognostic stratification. The patients were then categorized into four groups (change-PNI) based on their preoperative (pre-PNI) and postoperative PNI (post-PNI) values: G1: pre-low + post-low; G2: pre-low + post-high; G3: pre-high + post-low; and G4: pre-high + post-high.
Statistical Analyses
All statistical analyses were performed using R version 4.1.0 (R-project, Institute for Statistics and Mathematics, Vienna, Austria). Categorical variables were compared using a chi-squared test or Fisher’s exact test for two groups, and ANOVA was used for comparisons between multiple groups. A Mann–Whitney U-test was used for continuous variables for the two groups, while a Kruskal–Wallis test was used for comparisons across multiple groups. OS was defined as the time from the date of surgery to death from any cause. Patients with OS periods longer than 5 years were censored.
Multivariate Cox regression analysis was conducted to identify independent risk factors for OS. Owing to the potential multicollinearity between pre-, post-, and change-PNI, we excluded pre- and post-PNI from the multivariable model and retained change-PNI as the representative variable. Additionally, stages I and II were grouped and compared against stage III due to the relatively small number of stage I patients and the low number of events (deaths) in that subgroup, which limited statistical power for separate analysis. The Kaplan–Meier method with the Log rank test was used to compare OS between the patient groups. The concordance index (C-index) of pre-, post-, and change-PNI for OS prediction was compared using bootstrapped differences to evaluate the relative predictive performance of each variable. Statistical significance was defined as P < 0.05. However, for pairwise comparisons, the Bonferroni correction was applied, adjusting the significance threshold to P < 0.0083 (0.05/6) to account for multiple testing.
Results
The cutoff values for pre- and post-PNI were determined to be 48.05 and 43.65, respectively (Supplementary Figure S2). Based on these values, 665 patients were categorized into the following change-PNI groups: 37 (5.6%) in G1, 159 (23.9%) in G2, 41 (6.2%) in G3, and 428 (64.3%) in G4.
Patient Characteristics According to Change-PNI Group
Patient characteristics were compared between the four change-PNI groups (Table 1, Supplementary Table S1). The mean age varied significantly between the groups, with patients in G1 being the oldest (71.9 years) and those in G4 being the youngest (60.0 years). G1 had the highest percentage of American Society of Anesthesiologists Physical Status Classification (ASA) III and IV patients (13.5%), whereas G4 had the lowest percentage (5.1%). Regarding tumor location, G3 had the highest proportion of rectal cancer cases (70.7%), whereas G1 (83.8%) and G2 (87.4%) were predominantly colon cancer cases. Tumor size was significantly larger in G2 (66.7% of tumors being ≥5 cm) than in G3 (43.9%) and G4 (34.3%). Complications were less frequent in patients in the G2 (18.2%) and G4 (18.0%) groups than in the G1 and G3 groups, while chemotherapy treatment was more common in G2 (86.8%) and G4 (88.8%) group patients. The pre- and post-PNI values varied significantly between the groups according to the definition of each group.
 |
Table 1 Patient Characteristics According to Change-PNI Groups |
Predictive Factors Associated with OS
Age ≥ 70 years, CEA ≥ 5 ng/mL, tumor size ≥ 5 cm, postoperative complications, lymphovascular invasion (LVI), stage III, adjuvant chemotherapy, pre-PNI, post-PNI, and change-PNI were significant predictors of OS in a univariable analysis (Table 2). A multivariable Cox regression model adjusted for age, CEA, tumor size, postoperative complications, LVI, tumor stage, and adjuvant chemotherapy demonstrated that patients in groups G2, G3, and G4 had significantly lower mortality risks than those in group G1 (Table 3). The hazard ratios (HRs) and 95% confidence intervals (CIs) were as follows (vs G1): G2, 0.341 (0.186–0.625); G3, 0.457 (0.222–0.941); and G4, 0.222 (0.123–0.401).
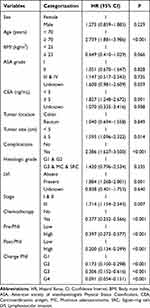 |
Table 2 Univariable Analysis of Factors Associated with Overall Survival |
 |
Table 3 Multivariable Analysis of Factors Associated with Overall Survival |
Survival Probability According to Change-PNI Groups
Kaplan–Meier survival analysis revealed significant differences in OS among the change-PNI groups (Figure 1). The patients in G4 (89.4%) had a significantly better OS than those in all other groups (G1: 37.6%, P < 0.001; G2: 81.0%, P = 0.005; G3: 70.7%, P < 0.001). Patients in the G1 group had the worst survival outcomes compared with patients in the other groups (G2, P < 0.001; G3, P = 0.005; G4, P < 0.001). However, the outcomes in patients in the G2 and G3 groups were not significantly different (P = 0.09).
C-Index Comparison between Change-PNI, Pre-PNI, and Post-PNI
The C-index of change-PNI (0.671, 95% CI 0.617–0.720) was superior to that of pre-PNI (0.609, 95% CI 0.563–0.654), with a bootstrap mean difference of 0.062 (95% CI 0.029–0.099). In addition, the C-index of change-PNI was superior to post-PNI (0.622, 95% CI 0.581–0.664), with a bootstrap mean difference of 0.049 (95% CI 0.014–0.085) (Table 4).
 |
Table 4 Comparison of Concordance Index Between Change-, Pre-, and Post-PNI |
Discussion
This study demonstrated that the integrated categorization of pre- and postoperative PNI offers a better prediction of OS than either pre- or post-PNI alone in patients with stage I–III CRC. Although a few studies have briefly described survival curves using similar groupings,13,14 to our knowledge, this is the first study demonstrating that combined pre-and post-PNI provides superior risk stratification for predicting OS compared to pre- and post-PNI alone.
There is evidence that systemic inflammatory markers are associated with mortality in various types of cancers.8 Markers such as LMR, NLR, and PLR are well-known indicators of inflammatory response; however, recent studies have suggested that controlling nutritional status score and PNI, incorporating both serum albumin and lymphocyte counts, could more accurately predict patient prognosis.15,16 The hypoalbuminemia in patients with CRC may result from increased metabolic demand, anorexia, or bowel obstruction. A poor nutritional status may lead to impaired immune surveillance and decreased responsiveness to cancer treatments.17 Lymphopenia may also affect the prognosis by suppressing adaptive immune responses, enhancing tumor immune evasion, and promoting a tumor-favoring, inflammatory, and immunosuppressive microenvironment.18,19 However, research on systemic markers has traditionally focused on baseline values prior to the initiation of cancer treatment.
Recently, the prognostic value of systemic inflammatory markers during the post-treatment period has gained attention.14,20–22 Tamai et al demonstrated that postoperative PNI, measured before adjuvant chemotherapy, was an independent predictor of OS in high-risk patients with stage II and III CRC. In their study, patients with a low PNI at recurrence had worse survival outcomes than those with a high PNI.14 C-reactive protein (CRP), another systemic inflammatory marker, has been shown to predict oncological outcomes, and several studies have reported that postoperative CRP levels significantly affect survival.20,22 Although the cutoff values for postoperative CRP differ between studies, elevated postoperative CRP has been consistently identified as an independent risk factor for recurrence in patients with CRC.
The relationship between pre- and postoperative inflammatory markers and their prognostic value remains unclear. The pre- and postoperative levels of systemic inflammatory markers are generally correlated, but significant changes are observed in a substantial proportion of patients following surgery. Interestingly, Guthrie et al observed in 206 patients undergoing CRC resection that among those with preoperative modified GPS of 2 (indicative of poor prognosis), 68% shifted to a score of 0 or 1 postoperatively, whereas only 32% remained at a score of 2.23 This aligns with our findings, where 80.8% of patients initially classified as having a low pre-PNI shifted to the high post-PNI group.
Several studies have reported that postoperative inflammatory markers are strong predictors of cancer prognosis.24,25 In this study, the differences in OS based on post-PNI levels (low vs high: 55.0% vs 87.2%) were more pronounced than those based on pre-PNI levels (low vs high: 72.8% vs 87.8%) (Supplementary Figure S3). Notably, there was no significant difference (P = 0.092) in OS between patients with low post-PNI only (G3) and those with low pre-PNI (G2), suggesting that patients with low post-PNI may require additional attention compared with those with low pre-PNI (Figure 1).
The cause of persistently low postoperative PNI levels following CRC resection remains unclear, but it may be related to the chronic dysregulation of immune and inflammatory responses triggered by micrometastatic disease or non-malignant tissue injury/necrosis.23 Plausible mediators include pro-inflammatory cytokines, such as interleukin (IL)-6 and IL-8, which are known to be elevated in patients with CRC.26 These cytokines, especially IL-6 and IL-8, are moderately associated with systemic inflammation markers and contribute to tumor survival, growth, and metastasis by promoting angiogenesis and chemotaxis of monocytes to the tumor site.27,28 In addition, postoperative nutritional deterioration—such as sarcopenia induced by surgical stress or poor oral intake due to gastrointestinal dysfunction—may also contribute to sustained low PNI levels.29,30 These factors can impair immune recovery and delay return to homeostasis, thereby potentially worsening long-term oncologic outcomes.
Unsurprisingly, patients with consistently high (G4) or low (G1) PNI levels both pre- and postoperatively exhibited the best and worst survival outcomes, respectively. However, caution is warranted when comparing outcomes between patients with a low PNI at only one time point, either preoperatively (G2) or postoperatively (G3). Although G2 patients (low pre-PNI and high post-PNI) were more likely to have larger (>5 cm) colon tumors, they experienced fewer postoperative complications and received adjuvant chemotherapy more frequently than G3 patients (high pre-PNI and low post-PNI). Further studies are needed to elucidate the prognostic differences between these groups.
This study had some limitations. First, although the sample size was large, the retrospective nature of the study and its single-institution setting limited the generalizability of the findings. Second, the cutoff values for PNI are not standardized and vary across studies; in this study, we determined the cutoff values based on the characteristics of the included patients, which may have influenced the results and prevented the direct comparison with previous literature. Third, there was variability in the timing of PNI measurements among patients. Preoperative blood tests were performed within 8 weeks of surgery, following our institution’s policy for preoperative anesthetic evaluation, and postoperative measurements were obtained 3–8 weeks after surgery. This variability may affect the accuracy of the prognostic assessments. Fourth, microsatellite instability status (MSI) was not routinely assessed during the study period and could not be incorporated into the survival analysis. Given the known prognostic relevance of MSI, particularly in stage II–III CRC, this omission may have limited the precision of our survival modeling. Finally, certain pathological predictors of prognosis (eg, surgical margins) and treatment-related factors such as chemotherapy regimens (eg, FOLFOX) or surgical techniques were not included in the analysis.
Conclusion
In conclusion, our results suggest that the combined pre- and postoperative PNI value serves as an independent prognostic factor for non-metastatic CRC and offers a more accurate prediction of OS compared to pre- or post-PNI alone. Therefore, postoperative PNI should be routinely assessed in the management of patients with CRC to guide personalized treatment and enhance patient outcomes.
Ethics Statements
The Gangnam Severance Hospital Institutional Review Board approved this study (approval number: 3-2024-0313) and waived the requirement for written informed consent owing to the retrospective nature of the study. All clinical data were fully anonymized before access and analysis. No personally identifiable information was collected or stored, and all patient records were handled in compliance with institutional data protection policies and the Declaration of Helsinki.
Acknowledgments
We would like to thank Editage (www.editage.co.kr) for the English language editing. The abstract of this paper was presented at the 2025 American Society of Colon and Rectal Surgeons Annual Scientific Meeting as a Quick Shot presentation (session: Quick Shot: Colorectal Cancer and Quality and Cost; presentation ID: QS200) on Monday, May 12, 2025. The abstract is available at: https://ascrs25.eventscribe.net/fsPopup.asp?PresentationID=1555571&mode=presInfo.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2022R1F1A1074811).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Nunez JJ, Leung B, Ho C, et al. Predicting the survival of patients with cancer from their initial oncology consultation document using natural language processing. JAMA Network Open. 2023;6:e230813. doi:10.1001/jamanetworkopen.2023.0813
2. Tong G-J, Zhang G-Y, Liu J, et al. Comparison of the eighth version of the American Joint Committee on Cancer manual to the seventh version for colorectal cancer: a retrospective review of our data. World J Clin Oncol. 2018;9:148–161. doi:10.5306/wjco.v9.i7.148
3. Kim MJ, Jeong S-Y, Choi S-J, et al. Survival paradox between stage IIB/C (T4N0) and stage IIIA (T1-2N1) colon cancer. Ann Surg Oncol. 2015;22:505–512. doi:10.1245/s10434-014-3982-1
4. Yang Y, Yang Z, Lyu Z, et al. Pathological-features-modified TNM staging system improves prognostic accuracy for rectal cancer. Dis Colon Rectum. 2024;67:645–654. doi:10.1097/DCR.0000000000003034
5. Tokunaga R, Sakamoto Y, Nakagawa S, et al. Comparison of systemic inflammatory and nutritional scores in colorectal cancer patients who underwent potentially curative resection. Int J Clin Oncol. 2017;22:740–748. doi:10.1007/s10147-017-1102-5
6. Yan L, Nakamura T, Casadei-Gardini A, et al. Long-term and short-term prognostic value of the prognostic nutritional index in cancer: a narrative review. Ann Transl Med. 2021;9(21):1630. doi:10.21037/atm-21-4528
7. Schneider SM, Veyres P, Pivot X, et al. Malnutrition is an independent factor associated with nosocomial infections. Br J Nutr. 2004;92:105–111. doi:10.1079/BJN20041152
8. An S, Shim H, Kim K, et al. Pretreatment inflammatory markers predicting treatment outcomes in colorectal cancer. Ann Coloproctol. 2022;38:97–108. doi:10.3393/ac.2021.01004.0143
9. Bezu L, Akçal Öksüz D, Bell M, et al. Perioperative immunosuppressive factors during cancer surgery: an updated review. Cancers. 2024;16:2304. doi:10.3390/cancers16132304
10. McSorley ST, watt DG, Horgan PG, et al. Postoperative systemic inflammatory response, complication severity, and survival following surgery for colorectal cancer. Ann Surg Oncol. 2016;23(9):2832–2840. doi:10.1245/s10434-016-5204-5
11. Shibutani M, Maeda K, Nagahara H, et al. The prognostic significance of the postoperative prognostic nutritional index in patients with colorectal cancer. BMC Cancer. 2015;15:521. doi:10.1186/s12885-015-1537-x
12. Lee S-Y, Lee SI, Min B-W, et al. Prognostic implication of systemic inflammatory markers in young patients with resectable colorectal cancer. Ann Surg Treat Res. 2021;100(1):25–32. doi:10.4174/astr.2021.100.1.25
13. National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology, Colon Cancer. Version 5.2024; 2024.
14. Tamai M, Kiuchi J, Kuriu Y, et al. Clinical impact of postoperative prognostic nutritional index in colorectal cancer patients undergoing adjuvant chemotherapy. Am J Cancer Res. 2021;11:4947–4955.
15. Proctor MJ, Morrison DS, Talwar D, et al. A comparison of inflammation-based prognostic scores in patients with cancer. A Glasgow inflammation outcome study. Eur J Cancer. 2011;47:2633–2641. doi:10.1016/j.ejca.2011.03.028
16. Kim H, Shin D-M, Lee J-H, et al. Combining prognostic nutritional index (PNI) and controlling nutritional status (CONUT) score as a valuable prognostic factor for overall survival in patients with stage I-III colorectal cancer. Front Oncol. 2023;13:1026824. doi:10.3389/fonc.2023.1026824
17. Zhang L, Wang K, Kuang T, et al. Low geriatric nutritional risk index as a poor prognostic biomarker for immune checkpoint inhibitor treatment in solid cancer. Front Nutr. 2023;10:1286583. doi:10.3389/fnut.2023.1286583
18. Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi:10.1038/nature01322
19. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi:10.1016/j.cell.2010.01.025
20. Yamamoto M, Saito H, Uejima C, et al. Prognostic value of the combination of pre- and postoperative C-reactive protein in colorectal cancer patients. Surg Today. 2018;48:986–993. doi:10.1007/s00595-018-1689-9
21. Kocak MZ, Coban S, Araz M, et al. Prognostic biomarkers in metastatic colorectal cancer: delta prognostic nutritional index, delta neutrophil to lymphocyte ratio, and delta platelet to lymphocyte ratio. Support Care Cancer. 2023;31(6):357. doi:10.1007/s00520-023-07829-w
22. Matsubara D, Arita T, Nakanishi M, et al. The impact of postoperative inflammation on recurrence in patients with colorectal cancer. Int J Clin Oncol. 2020;25:602–613. doi:10.1007/s10147-019-01580-1
23. Guthrie GJ, Roxburgh CS, Farhan-Alanie OM, et al. Comparison of the prognostic value of longitudinal measurements of systemic inflammation in patients undergoing curative resection of colorectal cancer. Br J Cancer. 2013;109(1):24–28. doi:10.1038/bjc.2013.330
24. McMillan DC, Canna K, McArdle CS. Systemic inflammatory response predicts survival following curative resection of colorectal cancer. Br J Surg. 2003;90:215–219. doi:10.1002/bjs.4038
25. Jamieson NB, Glen P, McMillan DC, et al. Systemic inflammatory response predicts outcome in patients undergoing resection for ductal adenocarcinoma head of pancreas. Br J Cancer. 2005;92:21–23. doi:10.1038/sj.bjc.6602305
26. Kantola T, Klintrup K, Väyrynen JP, et al. Stage-dependent alterations of the serum cytokine pattern in colorectal carcinoma. Br J Cancer. 2012;107:1729–1736. doi:10.1038/bjc.2012.456
27. Park JW, Chang HJ, Yeo HY, et al. The relationships between systemic cytokine profiles and inflammatory markers in colorectal cancer and the prognostic significance of these parameters. Br J Cancer. 2020;123:610–618. doi:10.1038/s41416-020-0924-5
28. Jain SM, Deka D, Das A, et al. Role of interleukins in inflammation-mediated tumor immune microenvironment modulation in colorectal cancer pathogenesis. Dig Dis Sci. 2023;68(8):3220–3236. doi:10.1007/s10620-023-07972-8
29. Trejo-Avila M, Bozada-Gutiérrez K, Valenzuela-Salazar C, et al. Sarcopenia predicts worse postoperative outcomes and decreased survival rates in patients with colorectal cancer: a systematic review and meta-analysis. Int J Colorectal Dis. 2021;36(6):1077–1096. doi:10.1007/s00384-021-03839-4
30. Liu S, Zhang S, Li Z, et al. Insufficient post-operative energy intake is associated with failure of enhanced recovery programs after laparoscopic colorectal cancer surgery: a prospective cohort study. Front Nutr. 2021;8:768067. doi:10.3389/fnut.2021.768067
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


