Back to Journals » Clinical Ophthalmology » Volume 19
Comparing The Existing Myopic Keratorefractive Lenticule Extraction (KLEx) Platforms: A Narrative Review
Authors Miller SM, Sitto MM , Moin KA, Hoopes PC , Moshirfar M
Received 3 May 2025
Accepted for publication 17 June 2025
Published 9 July 2025 Volume 2025:19 Pages 2189—2202
DOI https://doi.org/10.2147/OPTH.S532742
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Sabrina M Miller,1 Mina M Sitto,2,3 Kayvon A Moin,2,4 Phillip C Hoopes,2 Majid Moshirfar2,5,6
1Department of Neurology and Ophthalmology, Michigan State University College of Osteopathic Medicine, East Lansing, MI, USA; 2Hoopes Vision Research Center, Hoopes Vision, Draper, UT, USA; 3Department of Ophthalmology, Wayne State University School of Medicine, Detroit, MI, USA; 4Department of Ophthalmology, Nassau University Medical Center, East Meadow, NY, USA; 5John A. Moran Eye Center, University of Utah School of Medicine, Salt Lake City, UT, USA; 6Utah Lions Eye Bank, Murray, UT, USA
Correspondence: Majid Moshirfar, Hoopes Vision Research Center, Hoopes Vision, Draper, UT, USA, Tel +1 801-568-0200, Fax +1 801-563-0200, Email [email protected]
Abstract: Kerato-refractive lenticule extraction (KLEx) is an evolving technique in corneal refractive surgery. The objective of this narrative review is to compare laser settings, visual outcomes, and higher order aberrations (HOAs) across the five currently available myopic KLEx platforms, specifically SmartSight, corrective lenticule extraction for advanced refractive correction (CLEAR), smooth incision lenticular keratomileusis (SILKTM), small incision lenticule extraction (SMILE®), and SMILE Pro®. A comprehensive literature search was conducted in April 2025 using the PubMed, Scopus, and Embase databases, focusing on publications related to SmartSight, CLEAR, SILK, and SMILE Pro. For SMILE, a representative set of sources was selected due to the volume of available data. In total, 26 articles were included for this review. At the 1-day follow-up, 79% of eyes using SmartSight achieved an uncorrected distance visual acuity of 20/20, compared to 63% using SILK. These findings suggest that low pulse energy and high pulse frequency contribute to improved early visual outcomes, though the lenticule cut pattern also plays an important role. At 6 months, CLEAR, SILK, SMILE, and SMILE Pro showed high predictability, with over 85% of eyes achieving a spherical equivalent within ± 0.50 D. All platforms demonstrated excellent cylindrical correction, as over 87% of eyes achieved outcomes within ± 0.50 D. HOAs increased across all platforms, with significant induction of vertical coma. Overall, the five platforms demonstrated safety, efficacy, and refractive stability. This narrative review provides an overview of the current KLEx platforms and their clinical performance to compare differences in laser parameters and the factors influencing visual recovery, astigmatism correction, and HOA induction.
Keywords: ReLEx, myopia, femtosecond laser, SILK, SMILE, CLEAR
Introduction
Refractive surgery has undergone significant advancements over the past few decades. The introduction of a picosecond laser in 1999 marked an initial breakthrough for lenticule extraction technology, which further evolved with the advent of the femtosecond laser. In 2007, Carl Zeiss (Meditec, Germany) introduced the VisuMax femtosecond laser, enabling the femtosecond lenticule extraction (FLEx) procedure. FLEx required an initial corneal flap prior to lenticule removal, leading to the development of a flapless alternative known as refractive lenticule extraction (ReLEx). Subsequently, Zeiss introduced the VisuMax 500 in 2016, coining the term “Small Incision Lenticule Extraction” or “SMILE®” for their kerato-refractive procedure.1 Following SMILE’s introduction, several manufacturers have developed their own femtosecond lasers for lenticule extraction. These include ATOS SmartSight by SCHWIND® EyeTech Solutions (Kleinostheim, Germany),2 Corrective Lenticule Extraction for Advanced Refractive Correction (CLEAR) with the LDV Z8 FS laser by Ziemer Ophthalmics (Bellmund, Bern, Switzerland),3 and Smooth Incision Lenticular Keratomileusis (SILKTM) ELITATM by Johnson & Johnson (Milpitas, CA, USA).4 With the emergence of new femtosecond laser platforms, the general term “kerato-refractive lenticule extraction” or “KLEx” has been adopted in ophthalmic literature to collectively refer to the use of femtosecond lasers for lenticule extraction.5,6
Among these newer platforms, SmartSight, CLEAR, and SILK have not obtained FDA approval for use in the United States. The SmartSight femtosecond laser entered the market in mid-2020 and received the European approval through the Conformité Européenne (CE) mark that same year.7 The CLEAR system was introduced in April 2020 and received CE approval later that year.8,9 In January 2023, Johnson & Johnson received CE and Asian market approval for the ELITA FS laser for SILK procedures in select Asian and European markets for the treatment of myopia and astigmatism.4
Although femtosecond lasers for lenticule extraction have advanced significantly, ophthalmic literature lacks a comprehensive comparison of currently available myopic KLEx platforms. Each manufacturer has integrated distinct laser parameters into their platforms, yet these differences have also not been compared. Moreover, existing studies utilizing these platforms report varying patient outcomes, which differ in methodology, outcome measures, reporting standards, and patient demographics. This makes it challenging to compare these platforms accurately and fairly. This narrative review aims to address these current gaps by introducing, synthesizing, and comparing existing literature on preoperative laser settings, system characteristics, and postoperative outcomes across the five major KLEx platforms.
Materials and Methods
Throughout this review, the authors use the name of the procedure to refer to these five major platforms, such as SmartSight, CLEAR, SILK, SMILE, and SMILE Pro.
Regarding the platforms SmartSight, CLEAR, SILK, and SMILE Pro, a comprehensive literature search was carried out using PubMed, Scopus, and Embase databases for articles published from January 1, 2020 to April 2, 2025. The starting date of 2020 was chosen for the search due to SmartSight and CLEAR being the first platforms to receive CE approval at this time. The following search terms were used: (SCHWIND ATOS OR SmartSight) OR (Ziemer CLEAR OR corneal lenticule extraction for advanced refractive correction) OR (Johnson and Johnson SILK OR smooth incision lenticule keratomileusis) OR (VisuMax 800) NOT (Review[Publication Type]) NOT (Systematic Review[Publication Type]) NOT (Meta-Analysis[Publication Type]). Inclusion criteria consisted of studies that used these laser platforms for the treatment of myopia with or without astigmatism in human subjects. Exclusion criteria consisted of studies that reported hyperopic treatment, studies using non-human subjects, studies using ten eyes or fewer, and studies not reporting visual or refractive outcomes. Manufacturer brochures and websites were also utilized to supplement available information. Two independent reviewers (SMM and MMS) conducted an independent search for the identification of relevant studies to ensure thoroughness and accuracy. Whenever there were discrepancies, a third independent reviewer (MM) resolved any discrepancies in article selection.
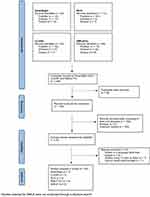
The initial search yielded 248 results (Figure 1). After a thorough screening process, a total of 165 articles were excluded due to irrelevance, 58 were duplicates, and 2 were excluded because they were written in a language other than English. Based on these criteria, a total of 21 articles were selected for inclusion: 7 for SmartSight, 3 for CLEAR, 2 for SILK, and 9 for SMILE Pro.
Since its introduction in 2008, SMILE has been reported in over 4000 studies, making it challenging to accurately compare this platform against the limited data available for the newer platforms. Therefore, the authors selected five up-to-date articles that comprehensively analyze SMILE based on study size, methodological quality, and relevance to the review’s focus. These include the Summary of Safety and Effectiveness from the Food and Drug Administration (FDA),10 given its regulatory approval, and four large retrospective analyses.11–14
A total of 26 articles were used for this literature review, including the five selected articles chosen for the SMILE platform.
Results
Patient Demographics
There were 7 studies using SmartSight, with age ranges from 18 to 55 years. The preoperative SEQ in these studies ranged from −13.75 to 0.00 D, and cylindrical astigmatism ranged from 0.00 to 5.25 D. There were 3 studies using CLEAR, with age ranges from 18 to 55 years. Preoperative SEQ for these studies ranged from −9.75 to −1.25 D. The cylindrical astigmatism in the CLEAR studies ranged from 0.0 to 3.0 D. There were 2 SILK studies with age ranges from 18 to 56 years, SEQ ranging from −8.88 to −1.38 D, and cylindrical astigmatism ranging from 0.0 to 3.0 D. The SMILE reports included ages ranging from 17 to 65 years, SEQ ranging from −13.0 to 0.0 D and cylindrical astigmatism ranging from 0.0 to 6.0 D. There were 9 studies using the SMILE Pro, which included ages ranging from 18 to 53 years. The preoperative SEQ ranged from −12.50 to 0.00 D, and preoperative cylindrical astigmatism ranged from 0.0 to 5.0 D. A summary of patient demographics from each article is presented in Table 1.
 |
Table 1 Demographics of Reviewed Studies Concerning All KLEx Platforms |
Laser Settings
Pulse Energy
The CLEAR and SILK systems employ the lowest pulse energies, with CLEAR operating below 50 nJ, though the exact value is not disclosed by the manufacturer (Table 2).8 SILK utilizes pulse energies ranging from 40–90 nJ.4 SmartSight operates within a mid-range energy level of 80–125 nJ.15,16 SMILE and SMILE Pro use higher pulse energies, ranging from 95–160 nJ and 85–160 nJ, respectively.13,24
 |
Table 2 Laser Settings and Surgical Characteristics of the Different KLEx Platforms |
Pulse Frequency
SMILE and SMILE Pro operate at the lowest pulse frequency, at 0.5 MHz and 2 MHz, respectively.24 The SmartSight system uses a mid-range frequency of up to 4 MHz.7,16 The SILK system employs higher pulse frequencies of up to 10 MHz.4 The CLEAR system operates at the highest pulse frequency, exceeding 5 MHz and reaching up to 20 MHz.8,20
Spot and Track Spacing
Regarding spot and track spacing, CLEAR is unique in that it offers overlapping spot pulses, effectively eliminating spot separation.8 The SILK system features minimal spacing, with a 1-µm spot-to-spot distance.4 The SmartSight system utilizes spot and track spacing that ranges from 2.5–4.4 µm, with an asymmetrical spot spacing pattern.32 SMILE employs spot spacing from 1.8–2.0 µm and a track spacing of 4.3–4.5 µm, whereas SMILE Pro uses spot spacing between 2.2–4.5 µm and track spacing of 2.2–4.5 µm.13,24
Laser Pulse Duration and Cut Time
SILK has the shortest pulse duration, approximately 100–200 fs.4 SmartSight has a longer pulse duration of 295 fs.33 CLEAR, SMILE and SMILE Pro have the longest pulse durations: CLEAR ranges from 200–500 fs, and SMILE and SMILE Pro both range from 220–580 fs.34,36
There does not appear to be a direct correlation between pulse duration and cut time. SMILE Pro has the fastest cut time of approximately 10 seconds.24 The SILK system also achieves a fast cut time of around 16 seconds.35 SmartSight falls within the mid-range cut time at 24 seconds,7 along with SMILE at approximately 22–30 seconds.24,26,27 CLEAR has the slowest cut time of approximately 45 seconds.8
Optical Zone and Corneal Cap Thickness
The optical zone and corneal cap thickness vary among KLEx platforms. Studies utilizing the SmartSight system reported an optical zone ranging from 5.5–7.2 mm and a corneal cap thickness of 120–150 µm.15,32 Studies using the CLEAR system had a slightly smaller optical zone of 6.0–7.0 mm, and a cap thickness of 120–140 µm.20,21 For the SILK system, studies selected a standard optical zone of 6.0 mm, and a thinner cap thickness of approximately 110 µm.4 In comparison, studies using SMILE reported optical zones ranging from 6.5–7.0 mm and 5.8–7.1 mm for the SMILE Pro.13,23,24 SMILE studies reported cap thicknesses ranging from 110–120 µm with SMILE and approximately 100–145 µm with SMILE Pro.13,23–25
Residual Stromal Bed Thickness
The minimum stromal bed thickness is 250 µm or greater in CLEAR, SILK, SMILE, and SMILE Pro.11,20,21,24 In comparison, SmartSight uses a larger minimum residual stromal thickness of 275 µm.2
Individual Differences Between KLEx Systems
Each KLEx system incorporates unique surgical features to enhance surgical outcomes. The SmartSight System includes a video-based eye registration system for centration and cyclotorsion control. Similar capabilities exist in the CLEAR, SILK, and SMILE Pro, but are absent in SMILE.8,16,22,24
The SmartSight system separates itself from the other KLEx platforms by offering a refractive progressive transition zone. This allows for a thinner lenticule without a minimum thickness requirement.16 Additionally, the system offers a central-to-peripheral cutting approach with overbending of the cap to counteract decentration. The SmartSight system also features a high numerical aperture and Quasi-Telecentric Optics to ensure uniform cutting quality across the cornea.16 Lastly, the SmartSight system attempts to minimize ocular stress with low applanation load (<250g) combined with low suction (approximately 250 mmHg).7
The CLEAR system integrates a built-in Optical Coherence Tomography for intraoperative visualization of the planned lenticule and residual stroma.20 However, unlike SMILE, the CLEAR system is unable to modify the edge of the lenticule, requiring a minimum lenticule thickness.21 The CLEAR, SMILE and SMILE Pro systems allow surgeons to create two 2.5-mm incision sites, in contrast to the singular incision site typically used with SmartSight and SILK.20,21
The SILK system was the first to introduce a biconvex lenticule shape. This is a departure from the plano-convex lenticules utilized in other KLEx systems.4
Spherical and Cylindrical Treatment Ranges
CE approval of SmartSight allows for myopic correction from −0.5 to −12.0 D and astigmatism correction up to −6.0 D.9 CLEAR permits myopic correction from −0.5 to −10.0 D, with astigmatism correction up to −5.0 D.8,9 SILK allows for myopic corrections up to −12.0 D and astigmatism corrections up to −6.0 D.4 SMILE and SMILE Pro are indicated for myopic correction of −0.5 to −12.0 D, with astigmatism treatment of −0.75 to –3.00 D; however, CE approval indicates astigmatism correction up to −5.0 D.24
Visual Outcomes
Visual outcomes were assessed by comparing cumulative uncorrected distance visual acuity (UDVA) of 20/20 or better, safety, and efficacy. Visual outcomes on postoperative day 1 (POD1) varied across the five KLEx platforms. Studies employing the SmartSight system showed differing results, with one study reporting 40% of eyes achieving 20/20 UDVA, while two other studies showed significantly higher rates (82–89%) at POD1.2,15,19 Similarly, in studies using SILK, there was a varying range of visual outcomes (46–65.9%).4,22 The percentage of eyes achieving 20/20 UDVA is not reported for SMILE Pro. However, one study reported that the mean postoperative UDVA on POD1 was logMAR 0.14 ± 0.16 (about 20/27), a statistically significant difference compared to the logMAR 0.06 ± 0.08 (about 20/22) observed in the SMILE group in the same study.28 Additionally, two different studies comparing SMILE to SMILE Pro found no statistically significant differences in visual outcomes between the two groups on POD1.29,30 The three articles that utilized CLEAR, as well as the SMILE studies, did not report POD1 outcomes.8,10–12,14,20,21
By 1 week postoperatively, visual recovery showed greater consistency across the platforms. Studies utilizing SMILE had the lowest percentage of eyes achieving 20/20 (66%),10 while those using SmartSight (80%), CLEAR (75.5%), and SILK (79–85.4%) demonstrated better results.2,4,8,22
Visual outcomes at 3 months are summarized in Table 3A.
 |
Table 3 Cumulative Snellen UDVA (%) of the Reviewed KLEx Platforms at (A) 3 Months, (B) 6 Months, and (C) 1 year Postoperatively |
At 6 months, all five platforms continued to show favorable postoperative visual outcomes. Research involving the SILK system reported the highest percentage of eyes (96%) achieving a UDVA of 20/20,4 followed by CLEAR, which showed 95.9% of eyes in one study, although another study reported only 55% of eyes.8,21 Studies using the SmartSight system maintained 92% of eyes reaching 20/20.18 Among SMILE platforms, SMILE Pro performed well, reaching 93% of eyes, while SMILE had a slightly lower rate at 84.2% of eyes (Table 3B).10,24
Long-term visual outcomes varied across platforms, with inconsistencies in follow-up duration. At 9 months, studies utilizing SILK reported excellent results, with 100% of eyes achieving a UDVA of 20/20 or better.22 One study using CLEAR demonstrated 51% of eyes reaching 20/20 at 10 months, whereas a second study reported 96.7% at 12 months.8,21 By 12 months, studies using SmartSight reached 93%, while studies employing the SMILE platform reported 93.3% (Table 3C).10,11,14,17 At the time of this review, data for 12-month outcomes are currently unavailable for the SILK or SMILE Pro platforms.
Safety was assessed by the change in preoperative to postoperative Snellen corrected distance visual acuity (CDVA). SILK demonstrated the highest safety profile at 6 months with no reports of eyes losing one or more lines of CDVA (Table 4A).4 The safety profile of SMILE also improved between the 3- and 6-month time points, with the percentage of eyes losing one or more lines of CDVA decreasing from 7.8% to 4%.10 In contrast, CLEAR and SMILE Pro demonstrated a lower safety profile at 6 months (20% and 9.8%, respectively).21,24 However, a notable percentage of eyes gained one or more lines of CDVA at 6 months postoperatively, including SMILE (20.4%), SMILE Pro (32.5%), CLEAR (26%), and SILK (59%).4,10,21,24 SMILE showed a 36.6% gain of one or more lines of CDVA, while CLEAR had a 50% gain at 1 year (Table 4B).8,10–12 Meanwhile, SmartSight had a 2% gain of one or more lines of improvement of CDVA, although 96% of eyes experienced no change in lines of CDVA.17
 |
Table 4 Change in Snellen CDVA (%) at (A) 6 Months and (B) 1 Year of the Reviewed KLEx Platforms |
Efficacy was assessed by change in UDVA postoperatively compared to preoperative CDVA. At 6 months, 77.2% of eyes treated with the SMILE Pro showed no change or gained one Snellen line.24 At 1 year, a study using the SmartSight procedure demonstrated that 95% of eyes had no change or gained one Snellen line.22 For SMILE, 78.5% of eyes had postoperative visual acuity better than or equal to preoperative CDVA at 1 year.10–12,14 For the remainder of the studies, no change in Snellen lines was reported. Instead, an Efficacy Index (EI) was reported in the study or calculated using postoperative UDVA and preoperative CDVA. For SILK, the EI was 1.08 at 6 months.4 The EI was 1.0 at 10 months for CLEAR.20
Refractive Outcomes
Predictability
Predictability was assessed across the KLEx platforms by comparing the percentage of eyes achieving a spherical equivalent (SEQ) within ± 0.50 D postoperatively. At 3 months, SILK (93.5%) and SMILE (93.2%) demonstrated high SEQ predictability.4,10,14 In contrast, SmartSight demonstrated the widest variability (60–100%) across different studies.2,7,16 SMILE Pro had a slightly lower predictability rate (81–91%).23,25,31 At 3 months, there was a 6.6% undercorrection of SEQ using SmartSight, and a tendency to undercorrect SEQ using SILK and SMILE Pro.4,7,23,25,31
At 6 months postoperatively, SMILE demonstrated high predictability, in addition to SMILE Pro (95.1%) and SILK (91.1%) (Table 5A).4,10 While still within an acceptable range, CLEAR showed lower predictability (85%) compared to the other platforms.21
 |
Table 5 (A) SEQ Accuracy and (B) Astigmatic Accuracy of the Reviewed KLEx Platforms |
By 1 year, CLEAR demonstrated significant improvement in predictability reaching 95.6% (Table 5A).8 SmartSight also showed high predictability at 1 year (90%).17 SMILE maintained high predictability from 6 months to 1 year.10 At 1 year, there was a slight undercorrection of SEQ using CLEAR and SmartSight.8,17
Residual Refractive Astigmatism
Residual refractive astigmatism among the KLEx platforms was evaluated based on the percentage of eyes within ± 0.50 D of cylinder at 6 months postoperatively (Table 5B). SmartSight demonstrated exceptional astigmatic correction at 6 months (99% of eyes), followed by SMILE (93.7%), SILK (92%), and SMILE Pro (90.8%).4,10,15 CLEAR had the lowest rate of astigmatism correction, with 88% of eyes within ± 0.50 D at 6 months postoperatively.21 In a 1-year study using CLEAR, the residual astigmatism improved to 95% of eyes with ± 0.50 D of cylinder or less.8 In comparison, SMILE showed 89.2% of eyes within ± 0.50 D of astigmatism correction at 12 months.10–12
Stability
Stability was assessed by the percentage of eyes experiencing a refractive change greater than ± 0.50 D over the reported study period, spanning from 3 months to 1 year. Overall, all KLEx platforms demonstrated good long-term stability, with minimal changes beyond the early postoperative period. SmartSight showed greater stability up to 3 months, with only 2% of eyes experiencing a refractive shift greater than ± 0.50 D.7 SILK demonstrated high stability up to 6 months, with only 1.6% of eyes experiencing a change beyond this threshold.4 SMILE Pro demonstrated 5% of eyes changing greater than ± 0.50 D from 1 to 3 months.25,26 However, a previous study showed that SMILE Pro demonstrated stable SEQ up to 6 months, indicating consistent refractive outcomes over this period.24 The CLEAR system showed less stability up to 6 months compared to other platforms, with 4% of eyes showing a change beyond ± 0.50 D.21 SMILE showed high stability reported up to 1 year, with only 4.89% of eyes experiencing a change beyond ± 0.50 D.10–12
Corneal Higher Order Aberrations (HOAs)
Corneal higher order aberrations (HOAs) were compared across the different KLEx platforms at 3 months postoperatively and measured with a 6-mm zone. In the SmartSight system, two studies reported HOAs, but the results varied. Gabric et al observed a 0.32 ± 0.26 μm increase in total corneal aberrations, a 0.17 ± 0.19 μm increase in spherical aberration, and a 0.22 ± 0.21 μm increase in mean coma.16 In contrast, Yoon et al observed a 14% increase in HOAs (0.32 to 0.37 μm), a 12% increase in spherical aberration (0.16 to 0.18 μm), and a 12% increase in mean coma (0.15 to 0.17 μm) at 3 months postoperatively.7
The CLEAR platform demonstrated a 59% increase in total HOAs (0.39 to 0.62 μm) at 1 year. There was minimal induction of spherical aberration from 0.21 to 0.30 μm at 6 months, which remained stable at 1 year. Vertical coma increased in magnitude from −0.05 to −0.06 μm, while horizontal coma increased from 0.04 to 0.12 μm. Notably, this study demonstrated that the values stabilized over 1 year and remained consistent for all HOAs.8 Another study using the CLEAR system reported greater mean induction in spherical aberration from 0.16 ± 0.60 μm preoperatively to 0.43 ± 0.16 μm at 6 months postoperatively.21
For the SILK platform, total HOAs were evaluated over 9 months and increased by 0.33 ± 0.13 μm postoperatively.22 The authors also observed a significant increase of −0.08 ± 0.19 μm in vertical coma. However, by 1 week, there were no significant changes in horizontal coma and spherical aberration.
In SMILE, total HOAs increased from 0.07 μm at 3 months to 0.09 μm at 12 months.10 Additionally, both coma and spherical aberration increased (0.12 to 0.16 μm and 0.13 to 0.16 μm, respectively). In a study comparing SMILE to SMILE Pro, postoperative total HOAs at 1 month were significantly lower in SMILE Pro than in SMILE (P = 0.036). However, there was no statistical difference between the two platforms in terms of spherical aberration or horizontal and vertical coma.30 In contrast, a contralateral study comparing SMILE and SMILE Pro found no significant differences in HOAs between the two groups at 3 months.26
Discussion
Across the five KLEx platforms, visual outcomes varied on POD1 but stabilized by 1 month postoperatively. Platforms utilizing lower pulse energy and higher pulse frequency showed faster visual recovery. Pradhan et al found that using the SmartSight platform with pulse energies of 75 to 135 nJ and pulse frequency up to 4 MHz resulted in excellent POD1 outcomes.19 Conversely, the SILK platform, which uses lower pulse energies than SmartSight at 40 to 90 nJ and higher pulse frequency than SmartSight at 10 MHz, had less favorable POD1 outcomes.4,22 We believe that early visual outcomes are influenced not only by pulse energy and pulse frequency but also by the lenticule cut pattern. SILK employs a radial cut pattern for anterior and posterior lenticule planes that crosses the corneal center multiple times, delivering a higher energy dose centrally. SmartSight, however, uses a spiral cut pattern that starts centrally and moves counterclockwise peripherally for anterior and posterior lenticule planes, reducing central energy exposure. Although higher energy may facilitate easier dissection of the lenticule, some studies have shown a disadvantage of greater energy exposure, including increased corneal stromal death and an inflammatory response, as well as early postoperative blurry vision. Previous studies have shown that lower energy levels in SMILE (100 nJ and 125 nJ, respectively) are the optimal settings to achieve better and quicker 3-month visual outcomes.13,37 The authors likely attribute this to more regular lenticule cutting, reduced gas bubble formation, better stromal interface quality, and improved healing outcomes. These reasons may help explain the superior early visual outcomes seen with SmartSight. Additionally, SmartSight’s progressive transition zone, unique in that it creates a zero-thickness lenticule edge, may preserve more corneal tissue and enhance early visual recovery.19 Therefore, while lower pulse energy and higher pulse frequency facilitate better early visual outcomes, we believe that the lenticule cut pattern also plays a critical role.
Beyond pulse energy, pulse frequency, and lenticule cut pattern, spot spacing may also indirectly play a role in postoperative outcomes for these platforms. The overlapping spot pattern in CLEAR and the absence of pulse separation (<1 µm) in SILK result in more continuous lenticule creation. This reduces the need for extensive manual dissection by the surgeon, ultimately simplifying the lenticule removal. Minimizing surgical manipulation leads to both decreased keratocyte activation and the inflammatory response, resulting in improved early postoperative visual recovery.38 Therefore, utilizing a smaller spot spacing on newer systems may theoretically improve early visual outcomes by facilitating easier lenticule removal and reducing the need for more extensive dissection. However, as found in our review, POD1 data from the CLEAR platform has not yet been reported, limiting our conclusions regarding spot spacing.
All KLEx platforms show a gradual improvement in visual acuity over time, as demonstrated by the existing literature found in our review. One discrepancy that is important to note is the difference in visual outcomes reported in two studies using CLEAR at 6 months and 1 year, respectively. At 6 months, Leccisotti et al reported that only 55% of eyes using CLEAR achieved a UDVA of 20/20 and 20% of eyes lost one Snellen line of CDVA, whereas Bteich et al found that 100% of eyes reached 20/20 at one year and only 5% of eyes lost one Snellen line of CDVA. We believe that this may be due to the differing mean ages reported in these studies. The mean age in Leccisotti’s 6-month CLEAR study was 40 years old, while the mean age in Bteich’s 1-year CLEAR study was 26 years old. One study evaluated the relationship between age and postoperative outcomes in 53 patients (102 eyes) who underwent SMILE and found that patients aged 40 and older had worse visual and refractive outcomes compared to their younger counterparts. This is thought to be due to the biomechanical response of the human corneal stroma to lenticule extraction changes, as corneal stiffness increases with age and may alter the refractive response to the SMILE procedure.39 It may also be attributed to the greater amplitude and range of accommodation in younger patients compared to older patients.40 Together, these findings highlight the potential influence of age on visual outcomes following lenticule extraction, as observed in our literature review.
The correction of cylinder has continued to improve over time. While KLEx platforms provide predictable and effective astigmatism corrections, studies indicate a tendency for undercorrection.2,4,8,17,21,23,24 Unlike LASIK, which employs a well-defined cyclotorsion registration mechanism, early KLEx platforms, particularly SMILE, lacked this feature. These platforms instead relied on manual cyclotorsion control. A LASIK study comparing groups with and without cyclotorsion control showed a statistically significant improvement in postoperative sphere and cylinder corrections in the cyclotorsion group. This benefit likely stems from its ability to compensate for inherent eye movement and body position, ensuring precise alignment.41 Interestingly, despite only allowing manual cyclotorsion compensation, SMILE outperformed SILK, CLEAR, and SMILE Pro in astigmatism correction, even though these platforms incorporate automated cyclotorsion compensation. This may be attributed to SMILE’s widespread use, particularly in the United States, and its well-established nomograms for refining spherical and cylindrical corrections.
Although we discovered that CLEAR and SILK had the highest percentage of eyes reaching 20/20 UDVA at 1 year and 6 months, respectively, all platforms performed quite well. Some may argue that the superior visual outcomes with CLEAR at 1 year and SILK at 6 months could be due to the smaller sample sizes and the narrower range of preoperative myopic correction in these studies. Since these results are not directly compared to one another in a single study, definitive conclusions regarding the superiority of one platform over another cannot be drawn. In our review, we identify subtle differences in the postoperative visual outcomes among the KLEx platforms. However, we believe these differences are not clinically significant, as each platform has demonstrated comparable safety, efficacy, and stability across the reported time intervals.
The induction of higher order aberrations (HOAs) is a concern in any corneal refractive surgery, as it can affect visual quality, leading to symptoms such as glare, halos, and reduced contrast sensitivity.42 While all KLEx platforms induced some postoperative HOAs, particularly spherical aberration and vertical coma, this may be attributed to centration mechanisms. Increased vertical coma following LASIK and SMILE has been reported in previous studies, attributed to the structural changes from applanation to the corneal vertex.42–44 The mechanism of centration may explain these findings. Yoon et al suggested that the minimal HOA induction in their SmartSight study resulted from centration on the corneal vertex, in comparison to other SmartSight studies with more drastic increases.7 The substantial rise in horizontal coma observed with CLEAR could be linked to manual corneal markings made using a non-coaxial microscope.8 SILK demonstrated HOA stability by 1 week, with minimal long-term impact aside from vertical coma, though its findings may be limited by a small sample size.22 The persistent vertical coma in KLEx procedures suggests ongoing challenges with centration, indicating that these platforms have not yet fully optimized their centration techniques.
The current study is primarily limited by the lack of existing research on each KLEx platform and inconsistencies in follow-up times. Specifically, there is limited long-term data on SILK and SMILE Pro. This scarcity is partly due to the more recent CE approval of SmartSight, CLEAR, and SILK, as well as the lack of FDA approval for these systems, which restricts the available research on these platforms. Furthermore, SMILE Pro, having been approved in the United States only recently, has less available data compared to its predecessor, SMILE. We acknowledge the selection of the five SMILE articles as a potential limitation of our analysis, as it may introduce bias. However, these articles were selected based on their large sample sizes, methodology, and relevance to the present study, which provides a fair comparative analysis, despite the disproportionate volume of SMILE literature.
Additionally, most studies reported in this review focused on visual outcomes without assessing patient-reported outcomes such as dry eye and visual disturbances. Other important parameters, like corneal biomechanics and epithelial mapping, were not evaluated. Since these platforms are relatively new technologies, standard visual outcome analysis has been the primary focus, while other important metrics have not yet been assessed. Longer follow-ups could shed light on the stability, safety, and efficacy of these newer platforms. Future studies could integrate both objective measures (e.g., visual outcomes, corneal biomechanics, epithelial mapping) and subjective measures (e.g., patient-reported outcomes) to further distinguish the strengths and limitations of one platform over another.
Conclusions
Five KLEx platforms currently exist, each with overlapping features and distinct characteristics. Over time, optimal laser settings will be identified to enhance lenticule removal, improve visual recovery, and refine astigmatic correction through more efficient nomograms, centration, and cyclotorsion mechanisms. Further analysis and comparisons will help determine the most effective cap thickness, lenticule size, and diameter. Despite their differences, all five platforms demonstrate excellent safety and efficacy within available follow-up periods. Lastly, while “KLEx” is widely accepted as the umbrella term, the continued use of manufacturer-specific names—such as “SMILE”, “CLEAR”, and “SILK”—creates inconsistencies. Standardizing terminology under “KLEx” would streamline research and clinical discussions, reducing confusion among both scientists and patients.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ganesh S, Brar S, Arra R. Refractive lenticule extraction small incision lenticule extraction: a new refractive surgery paradigm. Indian J Ophthalmol. 2018;66(1):10–19. doi:10.4103/ijo.IJO_761_17
2. Pradhan KR, Arba-Mosquera S. Three-month outcomes of myopic astigmatism correction with small incision guided human cornea treatment. J Refract Surg. 2021;37(5):304–311. doi:10.3928/1081597X-20210210-02
3. Izquieredo LJ, Sossa D, Ben-Shaul O, Henriquez M. Corneal lenticule extraction assisted by a low-energy femtosecond laser. J Cataract Refract Surg. 2020;46(46):1217–1221. doi:10.1097/j.jcrs.0000000000000236
4. Sachdev M, Shetty R, Khamar P, et al, Safety and Effectiveness of Smooth Incision Lenticular Keratomileusis (SILKTM) using the ELITA(TM) Femtosecond laser system for correction of myopic and astigmatic refractive errors. Clin Ophthalmol. 2023;17:3761–3773. doi:10.2147/OPTH.S432459
5. Dupps WJ, Randleman JB, Kohnen T, Srinivasan S, Werner L. Scientific nomenclature for keratorefractive lenticule extraction (KLEx) procedures: a joint editorial statement. J Cataract Refract Surg. 2023;49(11):1085. doi:10.1097/j.jcrs.0000000000001328
6. Moshirfar M, Tuttle JJ, Stoakes IM, Bundogji N, Hoopes PC. SMILE, CLEAR, SILK: it’s time for a common term. J Refract Surg. 2023;39(8):575. doi:10.3928/1081597X-20230711-01
7. Yoon H, Magnago T, Yeom DJ. Three-month clinical outcomes to correct myopia or myopic astigmatism using a femtosecond laser for lenticule creation with automated centration and cyclotorsion compensation. J Refract Surg. 2024;40(1). doi:10.3928/1081597X-20231212-03
8. Bteich Y, Assaf JF, Gendy JE, Awwad ST. Keratorefractive lenticule extraction using the ziemer FEMTO LDV Z8 platform (CLEAR): one-year results. J Refract Surg. 2024;40(11). doi:10.3928/1081597X-20241016-01
9. Fuest M, Mehta JS. Advances in refractive corneal lenticule extraction. Taiwan J Ophthalmol. 2021;11(2):113–121. doi:10.4103/tjo.tjo_12_21
10. United States Food and Drug Administration. VisuMax femtosecond laser summary of safety and effectiveness data (P150040/S003). Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf15/p150040s003b.pdf.
11. Chuckpaiwong V, Chansue E, Lekhanont K, Tanehsakdi M, Jongkhajornpong P, Nonpassopon M. 12-month outcomes of small incision lenticule extraction with proper head positioning but no reference marking for the correction of astigmatism. J Refract Surg. 2023;39(10):683–692. doi:10.3928/1081597X-20230824-01
12. Reinstein DZ, Carp GI, Archer TJ, Vida RS, Yammouni R. Large population outcomes of small incision lenticule extraction in young myopic patients. J Refract Surg. 2022;38(8):488–496. doi:10.3928/1081597X-20220623-01
13. Li L, Schallhorn JM, Ma J, Cui T, Wang Y. Energy setting and visual outcomes in SMILE: a retrospective cohort study. J Refract Surg. 2018;34(1):11–16. doi:10.3928/1081597X-20171115-01
14. Kamiya K, Takahashi M, Nakamura T, Kojima T, Toda I, Kariya M. A multicenter study on early outcomes of small-incision lenticule extraction for myopia. Sci Rep. 2019;9(1):4067. doi:10.1038/s41598-019-40805-1
15. Pradhan KR, Arba Mosquera S. Comparing high and low energy outcomes on day one for smartsight myopic-astigmatism treatments with the SCHWIND ATOS: a retrospective case series. BMC Ophthalmol. 2023;23(1):328. doi:10.1186/s12886-023-03076-z
16. Gabric I, Bohac M, Gabric K, Arba Mosquera S. First European results of a new refractive lenticular extraction procedure—smartsight by SCHWIND eye-tech-solutions. Eye. 2023;37(18):3768–3775. doi:10.1038/s41433-023-02601-0
17. Pradhan KR, Arba Mosquera S. Twelve-month outcomes of a new refractive lenticular extraction procedure. J Optom. 2023;16(1):30–41. doi:10.1016/j.optom.2021.11.001
18. Pradhan KR, Arba Mosquera S. SmartSight correction of compound myopic astigmatism treatments with preoperative astigmatism > 1.00 diopter using the SCHWIND ATOS: a retrospective case series. J Refract Surg. 2024;40(5). doi:10.3928/1081597X-20240415-02
19. Pradhan KR, Arba Mosquera S. Keratorefractive lenticule extraction: early postoperative day 1 outcomes in 1350 consecutive procedures. Cornea. 2025. doi:10.1097/ICO.0000000000003813
20. Leccisotti A, Fields SV, De Bartolo G. Refractive corneal lenticule extraction with the CLEAR femtosecond laser application. Cornea. 2023;42(10):1247–1256. doi:10.1097/ICO.0000000000003123
21. Leccisotti A, Fields SV, De Bartolo G. Change in stromal thickness and anterior curvature after refractive corneal lenticule extraction with the CLEAR application. J Refract Surg. 2022;38(12):797–804. doi:10.3928/1081597X-20221107-01
22. Chen L, Khamar P, Wang Y, Fu H, Shetty R. Evaluation of higher-order aberrations after the Smooth Incision Lenticular Keratomileusis (SILKTM) procedure using the ELITATM femtosecond platform for correction of myopic and astigmatic refractive errors. Clin Ophthalmol. 2024;18:2155–2166. doi:10.2147/OPTH.S466932
23. Reinstein DZ, Archer TJ, Potter JG, Gupta R, Rainer W. Refractive and visual outcomes of SMILE for compound myopic astigmatism with the VISUMAX 800. J Refract Surg. 2023;39(5):294–301. doi:10.3928/1081597X-20230301-02
24. Sekundo W, Chang JSM, Ganesh S, Hjortdal J, Wiltfang R. Keratorefractive lenticule extraction for myopia and myopic astigmatism with the VISUMAX 800: 6-month outcomes of a prospective multi-center post-market clinical follow-up study. J Refract Surg. 2025;41(3). doi:10.3928/1081597X-20250204-03
25. Cung HS, Tran LHT, Tran TN. Three-month outcomes of SMILE pro with the VISUMAX 800 for myopic astigmatism in a large population. Clin Ophthalmol Auckl NZ. 2025;19:417–425. doi:10.2147/OPTH.S502915
26. Ganesh S, Brar S, Swamy DT. Comparison of clinical outcomes and patient satisfaction following SMILE performed with the visumax 800 in one eye and visumax 500 in the contralateral eye. J Refract Surg Thorofare NJ 1995. 2025;41(1):e14–e21. doi:10.3928/1081597X-20241113-02
27. Varman A, Balakumar D, Balakumar D. Comparison of visual and refractive outcomes of keratorefractive lenticule extraction for compound myopic astigmatism between visumax and VISUMAX 800. Clin Ophthalmol Auckl NZ. 2024;18:3557–3566. doi:10.2147/OPTH.S492552
28. Lee CY, Lian LB, Chen HC, et al. The outcomes of first-generation (visumax 500) and second-generation (Visumax 800) keratorefractive lenticule extraction surgeries for astigmatism. Sci Rep. 2024;14(1):22224. doi:10.1038/s41598-024-73303-0
29. Lee CY, Lian IB, Chen HC, et al. The efficiency, predictability, and safety of first-generation (Visumax 500) and second-generation (Visumax 800) keratorefractive lenticule extraction surgeries: real-world experiences. Life Basel Switz. 2024;14(7):804. doi:10.3390/life14070804
30. Yoo TK, Kim D, Kim JS, et al. Comparison of early visual outcomes after SMILE using VISUMAX 800 and VISUMAX 500 for myopia: a retrospective matched case-control study. Sci Rep. 2024;14(1):11989. doi:10.1038/s41598-024-62354-y
31. Saad A, Klabe K, Kirca M, Kretz FAT, Auffarth G, Breyer DRH. Refractive outcomes of small lenticule extraction (SMILE) pro® with a 2 MHz femtosecond laser. Int Ophthalmol. 2024;44(1):52. doi:10.1007/s10792-024-02915-2
32. Bohač M, Gabrić I, Gabrić K, Jagić M, Arba Mosquera S. Predictability of the achieved lenticule thickness in keratorefractive lenticule extraction for myopia correction. J Refract Surg. 2023;39(11):728–735. doi:10.3928/1081597X-20230925-02
33. SCHWIND. SCHWIND ATOS with smartsight: state-of-the-art femtosecond laser technology- safe procedure-fascinating benefits. SCHWIND.
34. Ziemer Ophthalmology. CLEAR Lenticule Extraction Redefined: The New Lenticule Application by Ziemer Available for the FEMTO Z8. Ziemer Ophthalmology; 2022.
35. New vision clinics. SILK: next-generation keyhole laser vision correction technology. Available from: https://www.newvisionclinics.com.au/silk-next-generation-keyhole-laser-vision-correction-technology/#:~:text=The%20lenticule%20is%20removed%20through,next%2Dday%20results%20and%20recovery.
36. ZEISS Meditec AG. Take the lead. feel the speed. ZEISS VISUMAX 800. Zeiss Meditec AG.
37. Donate D, Thaëron R. Lower energy levels improve visual recovery in small incision lenticule extraction (SMILE). J Refract Surg. 2016;32(9):636–642. doi:10.3928/1081597X-20160602-01
38. Wei S, Wang Y, Wu D, Zu P, Zhang H, Su X. Ultrastructural changes and corneal wound healing after SMILE and PRK procedures. Curr Eye Res. 2016;41(10):1316–1325. doi:10.3109/02713683.2015.1114653
39. Primavera L, Canto-Cerdan M, Alio JL, Alio Del Barrio JL. Influence of age on small incision lenticule extraction outcomes. Br J Ophthalmol. 2022;106(3):341–348. doi:10.1136/bjophthalmol-2020-316865
40. Luger MH, Ewering T, Arba-Mosquera S. Influence of patient age on high myopic correction in corneal laser refractive surgery. J Cataract Refract Surg. 2013;39(2):204–210. doi:10.1016/j.jcrs.2012.07.032
41. Ivarsen A, Hjortdal J. Correction of myopic astigmatism with small incision lenticule extraction. J Refract Surg. 2014;30(4):240–247. doi:10.3928/1081597X-20140320-02
42. Pajic B, Cvejic Z, Mijatovic Z, Indjin D, Mueller J. Excimer laser surgery: biometrical iris eye recognition with cyclorotational control eye tracker system. Sensors. 2017;17(6):1211. doi:10.3390/s17061211
43. Moshirfar M, Omidvarnia S, Christensen MT, et al. Comparative analysis of corneal higher-order aberrations after laser-assisted in situ keratomileusis, photorefractive keratectomy, and small incision lenticule extraction with correlations to change in myopic Q-value and spherical equivalent with and without astigmatism. J Clin Med. 2024;13(7):1906. doi:10.3390/jcm13071906
44. Chen X, Wang Y, Zhang J, Yang S, Li X, Zhang L. Comparison of ocular higher-order aberrations after SMILE and wavefront-guided femtosecond LASIK for myopia. BMC Ophthalmol. 2017;17(1):42. doi:10.1186/s12886-017-0431-5
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.