Back to Journals » Journal of Hepatocellular Carcinoma » Volume 12
Comparison of Computed Tomography and Ultrasound-Guided Radiofrequency Ablation for Recurrent Subdiaphragmatic Hepatocellular Carcinoma After Resection
Authors Liu HY, Hsiao CY , Hu RH, Liang PC, Wu CH
Received 1 March 2025
Accepted for publication 19 June 2025
Published 27 June 2025 Volume 2025:12 Pages 1231—1240
DOI https://doi.org/10.2147/JHC.S524399
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr David Gerber
Hao-Yun Liu,1 Chih-Yang Hsiao,2,3 Rey-Heng Hu,3 Po-Chin Liang,1 Chih-Horng Wu4– 6
1Department of Medical Imaging, National Taiwan University Hospital Hsin-Chu Branch, Hsin-Chu, Taiwan; 2Department of Traumatology, National Taiwan University Hospital, Taipei, Taiwan; 3Department of Surgery, National Taiwan University, Taipei, Taiwan; 4Department of Medical Imaging, National Taiwan University Hospital, Taipei, Taiwan; 5Hepatitis Research Center, National Taiwan University Hospital, Taipei, Taiwan; 6Center of Minimal-Invasive Interventional Radiology, National Taiwan University Hospital, Taipei, Taiwan
Correspondence: Chih-Horng Wu, Department of Medical Imaging, National Taiwan University Hospital, No. 7, Chung-Shan South Road, Taipei, 100, Taiwan, Email [email protected]
Objective: Hepatocellular carcinoma (HCC) remains one of the leading causes of cancer-related mortality worldwide. Although surgical resection and liver transplantation are considered curative, recurrence is common, especially after hepatectomy. Radiofrequency ablation (RFA) offers a minimally invasive alternative for treating recurrent HCC. However, its efficacy is influenced by tumor location and imaging guidance. This study aims to compare the outcomes of CT-guided and US-guided RFA in patients with single small recurrent HCCs located in the subdiaphragmatic region after hepatectomy.
Methods: In this retrospective single-center study, we included patients who received RFA for recurrent HCC following curative hepatectomy between 2008 and 2020. Patients were categorized into CT-guided or US-guided RFA groups. RFA was performed by experienced interventional radiologists, and follow-up imaging was conducted every 3– 6 months to assess recurrence. The primary outcome was recurrence-free survival (RFS), and the secondary outcome was overall survival (OS).
Results: In this study, 59 and 32 patients with subdiaphragmatic lesions underwent CT-guided- and US-guided RFA, respectively, for single recurrent HCC. The CT-guided group showed larger tumor size, lower recurrence rates, and significantly better RFS in Kaplan-Meier analysis compared to the US-guided group (49.5 months vs 35.7 months, p value= 0.042). Multivariate analysis confirmed a superior RFS hazard ratio (HR=0.551) for CT-guided RFA, although the overall survival showed no significant difference. Major complications were absent in both groups.
Conclusion: CT-guided RFA provides improved RFS for subdiaphragmatic recurrent HCC, highlighting its potential as a preferred technique for challenging anatomical locations. Further multicenter prospective studies are necessary to validate these findings and assess the long-term survival outcomes.
Keywords: hepatocellular carcinoma, radiofrequency ablation, recurrence, image-guiding, post-operative treatment
Introduction
Hepatocellular carcinoma (HCC) is the third most common cause of cancer-related death worldwide.1 Curative therapies for HCC include liver resection, transplantation, and ablation.2 However, the recurrence rates after hepatectomy and transplantation are approximately 70% and 15%, respectively.3 For surgeons, repeat operations for recurrent HCC are challenging due to altered anatomy, dense adhesions, and hepatic insufficiency, all of which increase the surgical complexity.4 RFA may offer a solution to overcome these issues. The treatment outcomes of percutaneous RFA have been shown to be comparable to surgical hepatectomy in both primary and recurrent HCC.5,6 Furthermore, with advancements in navigation systems and high-resolution imaging tools—such as computed tomography (CT) and ultrasound (US)—the complication rates associated with RFA have decreased, and clinical outcomes have improved in recent years.7,8
Although RFA provides a curative alternative for patients who are not candidates for surgery, its success depends heavily on tumor location and the guiding modality.9,10 CT-guided RFA allows visualization of the entire liver, including the subdiaphragmatic region and liver tip.10 US-guided RFA enables real-time monitoring of needle placement and intraprocedural bubble formation. However, CT-guided RFA is generally more costly and less accessible compared to US-guided RFA.11,12 Additionally, ultrasound windows may be limited for deeper lesions or may be obscured by air or bone, reducing visibility compared to CT. Therefore, in cases of recurrent HCC located in the subdiaphragmatic regions, tumor visibility and operator confidence under US guidance may be insufficient to ensure high-quality ablation.
A previous study demonstrated that ethiodized-oil CT-guided RFA resulted in better outcomes than US-guided RFA for primary HCCs located in the subdiaphragmatic regions.13 However, in recurrent HCCs, the hepatic segments are often altered due to previous resection. Most existing studies have only evaluated the outcomes of RFA for recurrent HCC using a single imaging modality.5,6,10,14 To our knowledge, no prior study has directly compared CT-guided and US-guided RFA for recurrent HCC after hepatectomy. The aim of this study is to compare the outcomes of CT-guided versus US-guided RFA in patients with a single small recurrent HCC located in the subdiaphragmatic region. We hypothesized that for recurrent HCCs located within 1 cm of the diaphragm, CT-guided RFA would result in better prognosis than US-guided RFA.
Methods
Patient and Data Collections
This retrospective single-center cohort study enrolled patients who underwent RFA for HCC following liver resection at National Taiwan University Hospital (NTUH) between January 2008 and December 2020. The inclusion criteria were as follows: 1) patients had received hepatectomy for primary HCC at NTUH; 2) no additional medical treatments were administered after surgery; 3) HCC recurrence was confirmed either by pathological examination or typical imaging features, and the patient received percutaneous RFA treatment; 4) the patient had regular follow-up at NTUH, including imaging studies every 3–6 months. The exclusion criteria were: 1) recurrence involving more than one HCC lesion; 2) the distance between the tumor and the diaphragm or heart was greater than 1 cm; 3) incomplete or missing medical records. The RFA procedures were primarily performed by two senior interventionalists (Po-Chin Liang, Chih-Horng Wu), both with over 15 years of consistent clinical experience in RFA. They are certified as instructors for tumor ablation by Taiwan Society of Interventional Radiology to ensure a comparable and proficient level of technical expertise throughout the study. The protocol and the request for the waiver of informed consent for retrospective collection of medical records (Department of Medical Imaging, from Jan, 2005 to Mar, 2022) have been approved by the 157th meeting of Research Ethics Committee B of the National Taiwan University Hospital (NTUH-202204096RINB) on May 27, 2022.
Clinical Variables and Follow up
In this study, the enrolled patients were divided into two groups: CT-guided and US-guided RFA. Liver function was assessed using the Albumin-Bilirubin (ALBI) score. The ALBI score was calculated based on serum albumin and total bilirubin using the following formula: (log10 [total bilirubin, μmol/L] × 0.66) + ([albumin, g/L] × −0.085). The ALBI grade was then classified as follows: Grade 1 (score < −2.60), Grade 2 (−2.60 ≦ score < −1.39), and Grade 3 (score ≧−1.39).15 Following RFA, dynamic computed tomography (CT) or magnetic resonance imaging (MRI) was performed 1–2 months after the procedure to evaluate technical efficacy. If no viable tumor was identified, follow-up imaging or alpha-fetoprotein (AFP) levels were assessed every 3–6 months to monitor for recurrence. A major complication was defined as any adverse event occurring after RFA, such as vascular injury, pneumothorax, diaphragmatic injury, or fulminant hepatic failure. The primary endpoint of the study was HCC recurrence after RFA. Recurrence-free survival (RFS) was defined as the interval between the date of RFA for recurrent HCC and the date of last follow-up or recurrence diagnosis. The secondary endpoint was patient mortality. Overall survival (OS) was defined as the interval between the date of RFA for recurrent HCC and the date of death.
CT-Guided RFA: Procedure
CT-guided RFA was performed in four sequential steps: First, tumor tagging with ethiodized oil (Lipiodol, Andre Guerbet, Aulnay-sous-Bois, France) was conducted in the angiographic suite. A catheter was positioned in either the right or left hepatic artery to deliver approximately 2–5 mL of Lipiodol, depending on tumor size. This technique enhanced tumor visualization under CT imaging. Second, the patient was then transferred to the CT room and positioned supine. Local or general anesthesia was administered based on tumor burden, expected procedure time, and anesthesiologist availability. Third, after anesthesia, a 21-gauge Chiba needle was inserted to determine the appropriate angle and depth for the RFA needle. Multiple CT scans were performed to confirm the needle tip position. Fourth, the RFA needle was then inserted parallel to the Chiba needle until the tumor was targeted. Ablation was performed until the ablation zone fully encompassed the lesion with an adequate safety margin. Following the procedure, a repeat CT scan was conducted to evaluate for potential complications.
US-guided RFA was performed in two main steps and was relatively more straightforward compared to CT-guided RFA. First, the patient was transferred to the operating room, and anesthesia was administered using the same protocol as in CT-guided RFA. Second, the RFA needle was inserted under real-time ultrasound guidance. Ultrasonographic monitoring was performed throughout the ablation process. The endpoint of RFA was determined by the complete gas bubble coverage over the viable tumor. In addition, a color Doppler map or microvascular imaging was used to assess for potential bleeding. The post-procedural follow-up protocol was identical to that used in CT-guided RFA.
Statistical Analysis
Clinical data were retrieved from patients’ medical records. All statistical analyses were performed using R software (version 4.3.1; R Foundation for Statistical Computing, Vienna, Austria). Descriptive statistics are presented as mean ± standard deviation for continuous variables and as counts for categorical variables. Differences between the two groups were assessed using the independent samples t-test for continuous variables and the chi-squared test for categorical variables. The Kaplan–Meier method was used to estimate recurrence-free survival (RFS) and overall survival (OS), and comparisons between the CT-guided and US-guided groups were made using the Log rank test. The effects of clinical factors and guidance modalities on survival were evaluated using univariable Cox proportional hazards models. Variables with a p-value < 0.5 in the univariable analysis were included in the multivariable analysis.
Results
Patients’ Characteristics
Among the study cohort, 187 and 117 patients received CT-guided and US-guided RFA, respectively, for single recurrent HCC (Figure 1). In both groups, males were predominant, accounting for approximately 75% of the patients. Most patients had liver function–related blood tests within the normal range. Over 80% of the patients were classified as ALBI grade 1, with no significant differences between the two groups. There were also no significant differences in tumor size, number of HCC recurrences, recurrence-free interval, number of deaths, or overall survival duration between the two groups (Table 1). There were no significant differences in recurrence-free survival (RFS) or overall survival (OS) between the two groups (Supplementary Figures E1 and E2).
 |
Table 1 Characteristics of Patients with Single HCC Recurrence After Hepatectomy |
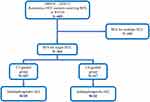 |
Figure 1 The flow chart for patients’ selection. |
The Patients’ Characteristics of Single HCC at Subdiaphragmatic Region and Less Than 1 Centimeter
Fifty-nine patients in the CT-guided RFA group and 32 patients in the US-guided RFA group had single HCCs located in the subdiaphragmatic region (Figure 1). The CT-guided RFA group had a larger mean tumor size (2.4 cm) and a lower recurrence rate (49.6%, 29/59) compared to the US-guided group (mean = 2.0 cm, p = 0.019; recurrence rate = 71.9%, 23/32, p = 0.036). More than 80% of patients in both groups had preserved liver function. No major complications occurred after RFA, and all patients were discharged within 1 to 2 days post-procedure. Compared to the US-guided group, the CT-guided RFA group showed a trend toward a longer recurrence-free period, although the difference did not reach statistical significance. No other significant demographic differences were observed between the two groups (Table 2).
 |
Table 2 Characteristics of Patients with Tumor Located Less Than 1 cm from the Diaphragm |
Survival Analysis
Survival analysis was performed to compare outcomes between patients undergoing CT-guided and US-guided RFA. The Kaplan–Meier (KM) survival curves demonstrated that the CT-guided RFA group had significantly better recurrence-free survival (RFS) than the US-guided group (Figure 2). In addition, univariate analysis showed that the CT-guided RFA group had a significantly lower hazard ratio (HR) for RFS compared to the US-guided group (p < 0.05), indicating superior outcomes. Multivariate analysis—including covariates with a univariate p-value < 0.5—also demonstrated a statistically significant advantage in RFS for the CT-guided group (Table 3). However, there was no significant difference in overall survival (OS) between the two groups (Figure 3). Furthermore, the univariate analysis results were consistent with this finding (Supplementary Table E1). These results suggest that while CT-guided RFA may improve local control, it does not translate into a significant survival benefit.
 |
Table 3 Univariate and Multivariate Analyses of Recurrence-Free Survival for Subdiaphragmatic Tumors |
 |
Figure 2 The KM curve for RFS. |
 |
Figure 3 The KM curve for OS. |
Discussion
To our knowledge, this is the first study to compare CT-guided RFA with US-guided RFA for recurrent HCC after liver resection. This study demonstrated a significant difference in the prognosis between the two groups. The results showed that for a single recurrent HCC below the diaphragm within 1 cm, CT-guided RFA provided a longer RFS than US-guided RFA.
Subdiaphragmatic HCC presents unique challenges for percutaneous RFA treatment. These difficulties stem from a suboptimal US window due to interference from the overlying lung and ribs, difficulty in needle placement, and the inability to achieve an adequate ablative margin along the hepatic capsule.16–18 In this setting, CT may offer superior image quality compared to US, allowing better needle guidance and a more accurate delineation of the ablation zone. When performing ablation with curative intent, it is crucial to ensure that the ablation zone fully encompasses the target tumor, including the circumferential ablative margin. Ideally, this margin should be at least 5 mm, with a preferred width of 1 cm, around the tumor.19–21 This approach maximizes the likelihood of complete tumor eradication and reduces the risk of recurrence.
Our study specifically focused on tumors located less than 1 cm below the diaphragm. Patients in the CT-guided RFA group exhibited superior RFS compared to those in the US-guided RFA group, suggesting a potential prognostic advantage of CT-guided RFA in managing subdiaphragmatic lesions. However, no significant differences in OS were observed between CT- and US-guided RFA groups. According to the 2022 EASL Clinical Practice Guidelines and Bai et al study,22 overall survival in HCC patients is influenced by multiple factors, including liver function status, tumor biology, and subsequent treatments. These factors may obscure the impact of the initial ablation modality on overall survival, necessitating larger studies with longer follow-up to elucidate these relationships. Several factors influence OS following treatment, making it difficult to attribute outcomes solely to the choice of therapy. Longer follow-up periods and larger sample sizes are likely necessary to detect differences in survival. Further investigations are warranted to better understand the impact of treatment modalities on the OS of patients with recurrent HCC.
For superficial recurrent HCCs, there is a minimal difference between performing RFA under CT or US guidance. Further subgroup analysis of patients without subdiaphragmatic lesions showed no significant differences in RFS or OS (Supplementary Table E2 and Figures E3, E4). This suggests that when tumors are more superficial and imaging windows are optimal, both modalities provide adequate visualization for treatment. However, for deeper-seated tumors, ultrasound may not provide a comprehensive view of the entire lesion. Furthermore, in patients with a history of hepatectomy, postoperative fibrosis of the skin and the hepatic capsular connective tissue may compromise ultrasound penetration, thereby degrading image quality. As a result, complete tumor ablation during RFA may be challenging. This could explain why RFS outcomes for subdiaphragmatic lesions treated with US-guided RFA are generally inferior to those treated with CT-guided RFA.
While conventional B-mode US has the advantages of lower cost, easier accessibility, and real-time imaging, CT offers superior resolution and deeper penetration. Although newer US-CT/MR fusion imaging combines the benefits of both modalities,23,24 it requires specialized expertise for accurate operation, limiting its availability to general clinicians.
RFA for HCC is generally regarded as a safe procedure, with a relatively low incidence of complications.25–27 In this cohort, no major complications were observed after the procedure. Minor adverse events were limited to one patient experiencing generalized weakness and another presenting with transient hypotension despite normal hematological parameters. Both patients recovered uneventfully and were discharged the following day without further sequelae. Furthermore, none of the patients in this study required repeat RFA within the first month after treatment, suggesting a high initial success rate of the ablation procedures.
This study has several limitations. First, as it was conducted at a single center, its findings may have limited generalizability owing to the specific patient demographics and clinical environments. Further multicenter studies are needed to validate these results. Second, its retrospective design poses risks of selection and information bias, which can be mitigated by prospective randomized trials. Third, the small sample size reduced the statistical power of the subgroup analyses, although statistical textbook states that a minimum of 30 samples is generally considered necessary to ensure adequate statistical power in analytical research.28 Larger-scale studies are still needed in the future to further validate these findings. Fourth, most of the patients had good liver function. However, it is unknown whether this outcome can be applied to patients with poor liver function. In addition, operator expertise may have influenced the outcomes, particularly when comparing CT- and US-guided RFA. Finally, the follow-up period may be insufficient to fully capture long-term survival outcomes, particularly OS.
Conclusion
This study highlights the potential advantages of CT-guided RFA over US-guided RFA for subdiaphragmatic recurrent HCC, particularly in terms of RFS. Although the study’s limitations underscore the need for future multicenter prospective research, these efforts will further validate the efficacy of CT-guided RFA and clarify its role in improving the long-term clinical outcomes.
Abbreviations
HCC, Hepatocellular carcinoma; RFA, Radiofrequency ablation; CT, computed tomography; US, ultrasound; ALBI, Albumin-Bilirubin; MRI, magnetic Resonance Imaging; RFS, Recurrence-free survival; OS, Overall survival.
Data Sharing Statement
Data supporting the findings of this study are available upon request from the corresponding author. The data were not publicly available due to privacy or ethical restrictions.
Ethics Approval
Written informed consent was waived for this retrospective study. All clinical information, laboratory data, and images were collected under routine workup. This study followed the Declaration of Helsinki of 1975, as revised in 2008. Institutional Review Board of National Taiwan University Hospital approval was obtained (NTUH-202204096RINB).
Acknowledgments
Hepatitis Research Center, National Taiwan University Hospital, Taipei, Taiwan. Center of Minimal-Invasive Interventional Radiology, National Taiwan University Hospital, Taipei, Taiwan.
Funding
The related work had financial support from the National Taiwan University Hospital with grant: NTUH 108-M4430; NTUH 109-M4557; NTUH 110-PC1374; NTUH 111-PC1418; NTUH 111-TUM363 and NTUH 113-A170.
Disclosure
All authors have completed the Journal of Hepatocellular Carcinoma disclosure form and declare that no support, financial or otherwise, has been received from any organization that may have an interest in the submitted work and that there are no other relationships or activities that could appear to have influenced the submitted work.
References
1. Kinsey E, Lee HM. Management of hepatocellular carcinoma in 2024: the multidisciplinary paradigm in an evolving treatment landscape. Cancers. 2024;16(3):666. doi:10.3390/cancers16030666
2. Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681–693. doi:10.1016/j.jhep.2021.11.018
3. Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American association for the study of liver diseases. Hepatology. 2018;68(2):723–750. doi:10.1002/hep.29913
4. Goh BKP, Syn N, Teo J-Y, et al. Perioperative outcomes of laparoscopic repeat liver resection for recurrent HCC: comparison with open repeat liver resection for recurrent HCC and laparoscopic resection for primary HCC. World J Surg. 2019;43(3):878–885. doi:10.1007/s00268-018-4828-y
5. Xia Y, Li J, Liu G, et al. Long-term effects of repeat hepatectomy vs percutaneous radiofrequency ablation among patients with recurrent hepatocellular carcinoma: a randomized clinical trial. JAMA Oncol. 2020;6(2):255–263. doi:10.1001/jamaoncol.2019.4477
6. Takayama T, Hasegawa K, Izumi N, et al. Surgery versus radiofrequency ablation for small hepatocellular carcinoma: a randomized controlled trial (SURF Trial). Liver Cancer. 2021;11(3):209–218. doi:10.1159/000521665
7. Bai X-M, Zhong-Hu H, Hao W, et al. An evaluation of 20-year survival of radiofrequency ablation for hepatocellular carcinoma as first-line treatment. Eur J Radiol. 2023;168:111094. doi:10.1016/j.ejrad.2023.111094
8. Deng Q, Minglian H, Fu C, et al. Radiofrequency ablation in the treatment of hepatocellular carcinoma. Int J Hyperthermia. 2022;39(1):1052–1063. doi:10.1080/02656736.2022.2059581
9. Hu G-J, Zheng Q-Y, Tsai F-G, et al. Ablative margin assessment for recurrence prediction in patients with hepatocellular carcinoma receiving radiofrequency ablation. J Formos Med Assoc. 2025:S0929–6646(25)00065–8.
10. Mitani H, Naito A, Chosa K, et al. Safety margin for CT- and US-guided radiofrequency ablation after TACE of HCC in the hepatic dome. Minim Invasive Ther Allied Technol. 2022;31(6):894–901. doi:10.1080/13645706.2021.1995436
11. Huo J, Aloia TA, Xu Y, et al. Comparative effectiveness of computed tomography- versus ultrasound-guided percutaneous radiofrequency ablation among medicare patients 65 years of age or older with hepatocellular carcinoma. Value Health. 2019;22(3):284–292. doi:10.1016/j.jval.2018.10.004
12. Luo M, Peng S, Yang G, et al. Percutaneous ablation of liver metastases from colorectal cancer: a comparison between the outcomes of ultrasound guidance and CT guidance using propensity score matching. Ultrasonography. 2023;42(1):54–64. doi:10.14366/usg.21212
13. Wu C-H, Liang P-C, Su T-H, et al. Iodized oil computed tomography versus ultrasound-guided radiofrequency ablation for early hepatocellular carcinoma. Hepatol Int. 2021;15(5):1247–1257. doi:10.1007/s12072-021-10236-0
14. Koh YH, Choi J-I, Kim HB, et al. Computed tomographic-guided radiofrequency ablation of recurrent or residual hepatocellular carcinomas around retained iodized oil after transarterial chemoembolization. Korean J Radiol. 2013;14(5):733–742. doi:10.3348/kjr.2013.14.5.733
15. Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33(6):550–558. doi:10.1200/JCO.2014.57.9151
16. Nam SY, Rhim H, Kang TW, et al. Percutaneous radiofrequency ablation for hepatic tumors abutting the diaphragm: clinical assessment of the heat-sink effect of artificial ascites. AJR Am J Roentgenol. 2010;194(2):W227–31. doi:10.2214/AJR.09.2979
17. Kang TW, Rhim H, Kim EY, et al. Percutaneous radiofrequency ablation for the hepatocellular carcinoma abutting the diaphragm: assessment of safety and therapeutic efficacy. Korean J Radiol. 2009;10(1):34–42. doi:10.3348/kjr.2009.10.1.34
18. Song KD, Lim HK, Rhim H, et al. Hepatic resection vs percutaneous radiofrequency ablation of hepatocellular carcinoma abutting right diaphragm. World J Gastrointest Oncol. 2019;11(3):227–237. doi:10.4251/wjgo.v11.i3.227
19. Wang X, Sofocleous CT, Erinjeri JP, et al. Margin size is an independent predictor of local tumor progression after ablation of colon cancer liver metastases. Cardiovasc Intervent Radiol. 2013;36(1):166–175. doi:10.1007/s00270-012-0377-1
20. Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria--a 10-year update. Radiology. 2014;273(1):241–260. doi:10.1148/radiol.14132958
21. Sarioglu AG, Wehrle CJ, Akgun E, et al. Radiofrequency ablation versus microwave ablation for colorectal liver metastases: long-term results of a retrospective cohort surgical experience. Hepatobiliary Surg Nutr. 2024;13(5):759–770. doi:10.21037/hbsn-23-677
22. Bai X-M, Cui M, Yang W, et al. The 10-year survival analysis of radiofrequency ablation for solitary hepatocellular carcinoma 5 cm or smaller: primary versus recurrent HCC. Radiology. 2021;300(2):458–469. doi:10.1148/radiol.2021200153
23. European Society of Radiology (ESR). Abdominal applications of ultrasound fusion imaging technique: liver, kidney, and pancreas. Insights Imaging. 2019;10(1):6. doi:10.1186/s13244-019-0692-z
24. Ahn SJ, Lee JM, Lee DH, et al. Real-time US-CT/MR fusion imaging for percutaneous radiofrequency ablation of hepatocellular carcinoma. J Hepatol. 2017;66(2):347–354. doi:10.1016/j.jhep.2016.09.003
25. Koda M, Murawaki Y, Hirooka Y, et al. Complications of radiofrequency ablation for hepatocellular carcinoma in a multicenter study: an analysis of 16 346 treated nodules in 13 283 patients. Hepatol Res. 2012;42(11):1058–1064. doi:10.1111/j.1872-034X.2012.01025.x
26. Lahat E, Eshkenazy R, Zendel A, et al. Complications after percutaneous ablation of liver tumors: a systematic review. Hepatobiliary Surg Nutr. 2014;3(5):317–323. doi:10.3978/j.issn.2304-3881.2014.09.07
27. Maeda M, Saeki I, Sakaida I, et al. Complications after radiofrequency ablation for hepatocellular carcinoma: a multicenter study involving 9,411 Japanese patients. Liver Cancer. 2020;9(1):50–62. doi:10.1159/000502744
28. Gay LR. Educational Research: Competencies for Analysis and Application. New York: Macmillan; 1992.
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

