Back to Journals » Drug Design, Development and Therapy » Volume 19
Comparison of Remimazolam versus Dexmedetomidine on Hemodynamics in Older Patients Under Lower Extremity Orthopedic Surgery with Spinal Anesthesia: A Randomized Controlled Trial
Authors Wang D , Liu Z, Zhang W, Li S, Chen Y, Jiang C, Su N, Liu T, Li X, Bi C
Received 20 November 2024
Accepted for publication 30 June 2025
Published 14 July 2025 Volume 2025:19 Pages 6037—6046
DOI https://doi.org/10.2147/DDDT.S504371
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Prof. Dr. Georgios Panos
Di Wang,1 Zhi Liu,2 Wenhui Zhang,1 Siru Li,1 Yutao Chen,1 Chenxin Jiang,1 Naying Su,1 Tianxin Liu,1 Xingguo Li,1 Congjie Bi1
1Department of Anesthesiology, Central Hospital of Dalian University of Technology (Dalian Municipal Central Hospital), Dalian, Liaoning, People’s Republic of China; 2Intensive Care Unit, The Second Affiliated Hospital of Dalian Medical University, Dalian, Liaoning, People’s Republic of China
Correspondence: Xingguo Li, Department of Anesthesiology, Central Hospital of Dalian University of Technology (Dalian Municipal Central Hospital), Dalian, Liaoning, People’s Republic of China, Tel +86 13940878435, Email [email protected] ) Congjie Bi, Department of Anesthesiology, Central Hospital of Dalian University of Technology (Dalian Municipal Central Hospital), Dalian, Liaoning, People’s Republic of China, Tel +86 19969312108, Email [email protected] )
Purpose: To compare the effects of remimazolam and dexmedetomidine on the hemodynamics in elderly patients undergoing orthopedic surgery under spinal anesthesia.
Methods: This study evaluated 126 patients aged ≥ 60 years undergoing lower-extremity orthopedic surgery under spinal anesthesia, randomizing them into remimazolam and dexmedetomidine groups. The primary outcome was the incidence of hemodynamic fluctuations, such as hypotension and bradycardia. The secondary endpoints included the cumulative dose vasoactive medication and the incidence of hypertension, tachycardia, postoperative nausea and vomiting (PONV), postoperative delirium (POD), and hypoxemia. Continuous hemodynamic variables including mean arterial pressure (MAP) and heart rate (HR) were recorded at baseline, every 5 min for the first 20 min after intravenous infusion of sedatives, every 10 min thereafter, up to one hour, at the end of the surgery, and in the post-anesthesia care unit (PACU).
Results: Compared to dexmedetomidine group, patients in the remimazolam group demonstrated significantly higher MAP at three specific time points (60 minutes after baseline, at the end of surgery, and in the PACU) and higher HR at all time points after T3 (15 minutes after baseline). The remimazolam group also reduced norepinephrine and atropine interventions. There were no statistically significant differences in other adverse events between the two groups.
Conclusion: Remimazolam demonstrated superior hemodynamic stability and fewer adverse cardiovascular events than dexmedetomidine, along with reduced requirements for vasoactive medications, making it an alternative to intraoperative sedation in older patients undergoing lower limb surgery under spinal anesthesia.
Keywords: benzodiazepines, hemodynamics, older adults, orthopedic surgery, dexmedetomidine, remimazolam
Introduction
Spinal anesthesia combined with intraoperative sedation is widely used as an important anesthesia method for lower-limb fracture surgery in older patients.1 Compared to general anesthesia, spinal anesthesia is a cost-effective, physiologically less invasive alternative that promotes patient recovery, enhances comfort, and reduces postoperative complications.2,3 During spinal anesthesia, sedatives are commonly administered to alleviate anxiety and tension in patients. Midazolam, propofol, and dexmedetomidine are the most commonly used medications for sedation. However, some studies have indicated that the use of sedatives may lead to intraoperative hypotension.4 Hypotension can precipitate renal or myocardial injury; potentially prolonging intensive care unit stay and increasing the risk of perioperative complications and mortality.5 This concern is particularly among older patients, whose physiological decline results in altered tolerance and responsiveness to anesthetic drugs compared with younger patients, intraoperative hypotension warrants heightened vigilance.
Remimazolam is a novel, ultra-short-acting γ-aminobutyric acid type A (GABA-A) receptor agonist belonging to the benzodiazepine class of drugs.6–8 Notably, remimazolam is characterized by a consistent context-sensitive and rapid elimination half-life.9 Furthermore, it exerts minimal respiratory and circulatory depressive effects, virtually eliminating injection pain, and administration of the specific antagonist flumazenil can achieve selective reversal of the sedative effects of remimazolam.10–12 However, the hemodynamic effects of remimazolam remain inconclusive, particularly in older patients undergoing lower-extremity orthopedic surgery under spinal anesthesia.
This study aimed to compare the hemodynamics of remimazolam and dexmedetomidine in older patients undergoing lower-limb orthopedic surgery under spinal anesthesia. By evaluating the efficacy and safety of these sedative agents, we aimed to provide evidence-based guidance to clinicians in selecting the most appropriate adjunctive medication, thereby enhancing patient comfort and perioperative outcomes.
Materials and Methods
Study Design
This single-blind, randomized controlled trial was approved by the Ethics Committee of the Central Hospital of Dalian University of Technology (Dalian Municipal Central Hospital) (Liaoning, China; 2024–039-01; April 2024) and was registered prior to patient enrollment at https://www.chictr.org.cn (ChiCTR2400083380; principal investigator: Di Wang; registered on April 22, 2024). All experimental participants or their authorized representatives signed an informed consent form on the document outlining the details of the study. This study was conducted at the Central Hospital of Dalian University of Technology (Dalian Municipal Central Hospital) from April 2024 to September 2024.
Participants, Study Design, and Randomization
The study population comprised older patients aged ≥60 years who were scheduled to undergo lower limb orthopedic surgery involving spinal anesthesia. We included patients who met the American Society of Anesthesiologists physical status (ASA) I–III. Exclusion criteria included deviation from the designated anesthetic plan, such as patients intending to undergo general anesthesia, or those presenting with contraindications to spinal anesthesia. Additional exclusion criteria included chronic use of opioids or benzodiazepines (defined as use exceeding three months). Furthermore, patients with severe heart failure, characterized by a left ventricular ejection fraction (EF) < 30%, were excluded because pre-existing conditions could potentially impact hemodynamics. Communication impediments such as severe hearing disorders or diminished communicative abilities were also excluded from the study.
This study was a randomized parallel-group trial with equal group sizes. Randomization sequences were generated using SPSS version 25.0 to ensure a 1:1 allocation ratio. Anesthesiologists were responsible for executing randomization and preparing individual opaque sealed envelopes containing computer-generated group assignments for each participant. On the day of surgery, prior to entering the operating room, patients were randomly assigned to either remimazolam or dexmedetomidine group. The patients were blinded to the group allocation and the medications administered.
Preoperative Baseline Characteristics and Comorbidities
The baseline characteristics and comorbidities of each patient were recorded before surgery. This mainly included weight, sex, age, blood pressure, heart rate, ASA physical status classification, and relevant medical comorbidities.
Intervention and Control
No patients received pre-anesthetic medication. Upon arrival in the operating room, venous access was established and 500 mL of crystalloid (Ringer lactate) was administered at a rate 5–7 mL·kg−1·h−1. Standard monitoring included electrocardiography, percutaneous arterial oxygen saturation monitoring, and invasive arterial pressure monitoring were implemented. Supplemental oxygen was delivered via face mask at a flow rate of 2–3 L/min under medical supervision. After positioning the patient appropriately, a midline approach was utilized to insert a Quincke needle with the bevel oriented cephalad into the L2-L3 or L3-L4 interspace. Upon confirmation of cerebrospinal fluid return, 2–2.5 mL of 0.5% hyperbaric bupivacaine was administered intrathecally. Patients in whom the neuraxial anesthesia level extends above the T8 level were excluded.
After confirming the appropriate neuraxial anesthesia level, remimazolam or dexmedetomidine was intravenously administered for intraoperative sedation until the end of surgery. Given the original development of the Bispectral Index (BIS) for propofol and the weaker correlation between the BIS and depth of sedation with remimazolam and dexmedetomidine, as demonstrated in studies,13–16 we opted to solely utilize the Modified Observer’s Assessment of Alertness/Sedation (MOAA/S) scale for assessing sedation depth in this study.
In the dexmedetomidine group, an intravenous infusion of dexmedetomidine was initiated at a loading dose of 0.5 µg· kg −1 over the first 10 minutes, followed by a maintenance infusion rate ranging from 0.2 to 0.6 µg·kg−1·h−1. In the remimazolam group, the loading dose was 0.1 mg·kg−1 for the remimazolam group for the first 10 min, followed by a maintenance rate of 0.1 to 0.3 mg·kg−1·h−1. Adjustments to the dosage were made to maintain a moderate level of sedation (MOAA/S score of 2–3).
The baseline point (T0) was defined as the initiation of loading sedation. Mean Arterial Pressure (MAP) and Heart Rate (HR) were recorded at 5, 10, 15, 20, 30, 40, 50, and 60 min after baseline (T1-T8), at the end of surgery (T9), and in the post-anesthesia care unit (PACU) (T10). Hypotension was defined as systolic blood pressure < 70% of baseline or < 90 mmHg, received intravenous norepinephrine for treatment. Intraoperative bradycardia was identified when the patient’s HR < 55 beats·min−1. Considering the advanced age of the patients, the anesthesiologist administered intravenous atropine (0.5 mg) only when the HR < 40 beats·min−1. If hypertension (systolic arterial pressure > 180 mm Hg or diastolic arterial pressure > 110 mm Hg) occurred, 0.5–1 mg of nicardipine hydrochloride was administered intravenously. In the event of tachycardia, defined as a heart rate exceeding 110 beats·min−1, patients were administered 5–10 mg of esmolol intravenously. For cases of respiratory depression or hypoxia, indicated by SpO2 < 90%, patients received jaw-thrust assistance. If additional intraoperative blood loss occurred, an estimated equivalent volume of colloid solution (Hydroxyethyl Starch 130/0.4 in Sodium Chloride Injection) was administered to maintain hemodynamic stability.
After the surgical procedure, patients underwent a femoral nerve block, lumbar plexus block, or sciatic nerve block using 20–30 mL of ropivacaine 0.375% for postoperative analgesia. Subsequently, all patients were observed in the PACU for 20 minutes.
Outcome Measures
The primary endpoint was to determine whether there was a significant difference in the occurrence of hemodynamic fluctuations, specifically hypotension and bradycardia, between groups.
Secondary endpoints included the total doses of norepinephrine, atropine and the incidence of hypertension, tachycardia, postoperative nausea and vomiting (PONV), postoperative delirium (POD), and hypoxemia requiring treatment. Additionally, we analyzed continuous hemodynamic variables, including MAP and heart rate. Measurements were recorded at baseline, every 5 minutes during the first 20 minutes after sedative infusion, every 10 minutes thereafter up to 60 minutes, at the end of surgery, and in the PACU.
Sample Size
This randomized controlled trial compared remimazolam with dexmedetomidine. The primary outcome used for sample size estimation was the incidence of intraoperative hypotension. Based on our pre-test results, the incidence of intraoperative hypotension was 31% in the remimazolam group and 56% in the dexmedetomidine group. With a two-sided α of 0.05 and power of 80% (β = 0.20), a sample size of 60 patients per group was calculated. Allowing for a 10% dropout rate, at least 66 patients were required in each group, resulting in a total sample size of 132 patients.
Statistical Analysis
Quantitative data were assessed for normality using the Shapiro–Wilk test. Normally distributed continuous variables were analyzed using the independent samples t-test and reported as mean ± standard deviation (SD). Non-normally distributed variables were summarized as median (interquartile range, IQR: 25th–75th percentile) and compared using the Mann–Whitney U-test. Categorical variables were described using frequencies (percentages) and analyzed with chi-square tests or Fisher’s exact tests. Repeated-measures ANOVA was used to assess the differences in variables measured multiple times, conducting both between- and within-group statistical comparisons. Mauchly's sphericity test was performed. If the sphericity assumption was violated, the modified statistic tests as Greenhouse-Geisser and Huynh-Feldt were used. If there was a significant interaction with time and group, post hoc Bonferroni corrections were used to correct the type I error. Results of missing data were excluded from the analysis. All statistical analyses were performed using SPSS version 25.0 (IBM, Armonk, NY, USA), with two-tailed P-values < 0.05 considered statistically significant.
Results
Our clinical trial entailed screening of 144 patients to assess their eligibility. Among these individuals, eight were deemed ineligible for participation and four met the exclusion criteria. The remaining 132 patients were randomly allocated in a 1:1 ratio to receive either remimazolam or dexmedetomidine After excluding 3 patients in remimazolam group and 3 patients in dexmedetomidine group, due to sedation failure or surgery cancellation. A total of 126 patients were included in the intention-to-treat analysis. We recruited participants until the target was reached and recruitment was stopped (Figure 1).
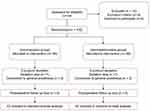 |
Figure 1 Trials diagram. |
Patient characteristics and procedural data in both groups are presented in Table 1, and no statistically significant differences were observed between the two groups.
 |
Table 1 Patient Characteristics and Procedural Data |
For the primary outcome, the remimazolam group had a significantly reduced frequency of hypotension (22.4% vs 52.4%, p <0.001) and bradycardia (9.5% vs 61.9%, p <0.001) compared to the control group. Additionally, the secondary endpoint, which included the application of atropine (p = 0.012) and the total administered dose of norepinephrine (p=0.001), was significantly lower in the remimazolam group.
The incidence of hypertension (41.3% vs 41.3%, p = 1.000), tachycardia (6.3% vs 3.2%, p = 0.403), or hypoxemia (6.3% vs 3.2%, p = 0.409) was comparable between the two groups. The incidences of PONV (19.4% vs 28.4%, p = 0.156) and POD (6.3% vs 3.2%, p = 0.51) did not differ significantly between the two groups (Table 2).
 |
Table 2 Primary and Secondary Endpoint |
Analysis of hemodynamic variables using linear mixed-effects models showed that the between - group effect on MAP not statistically significant (p=0.092, Figure 2A), while that on HR was significant (p=0.001, Figure 2B). In the dexmedetomidine group, MAP was decreased at all time points except at 5 min compared to baseline (p < 0.05 for all time points). In contrast, in the remimazolam group, MAP decreased at 10 min time points (all time points p < 0.05) but returned to the baseline level by T9 and T10 (p >0.05).
The serially assessed HR showed significant differences at from 15 minutes after the initiation of sedation onward (p < 0.01, for all time). In contrast, MAP showed significant differences only 60 minutes after dosing (p=0.041), at the end of the procedure (p < 0.001), and in the PACU (p<0.001). At all other time points, HR (baseline p = 0.971, 5 min p = 0.370, 10 min p = 0.059) and MAP (baseline p=0.601; 5 min p=0.486; 10 min p=0.729; 15 min p=0.699; 20 min p=0.836; 30 min p=0.834; 40 min p=0.354; 50 min p=0.180) did not show any significant intergroup difference.
Discussion
Our findings indicate that older patients undergoing spinal anesthesia for lower-extremity orthopedic procedures in the remimazolam group exhibited a significantly lower incidence of hypotension and bradycardia, along with reduced requirements for norepinephrine and atropine, than those in the dexmedetomidine group. These results suggested that remimazolam may provide superior hemodynamic stability during spinal anesthesia in orthopedic procedures.
In our study, remimazolam demonstrated significant hemodynamic advantages over dexmedetomidine, manifesting as a lower incidence of hypotension and bradycardia, alongside a reduced total requirement for norepinephrine and atropine. Importantly, the comparable MAP values observed at multiple time points must be interpreted in the context of substantially higher vasopressor requirements in the dexmedetomidine group; this pharmacological compensation potentially masked underlying hemodynamic differences, further underscoring the stability advantage of remimazolam. Compared to dexmedetomidine, remimazolam was associated with a higher HR from T3 to T10 and significantly higher MAP from T8 to T10. The dexmedetomidine group exhibited persistently lower MAP and HR, extending into the PACU, likely attributable to its longer half-life. This prolonged effect, coupled with the greater need for vasopressors to maintain perfusion, poses potential risks for older patients with limited cardiovascular reserve. Conversely, the faster offset of remimazolam facilitates a swifter return to baseline hemodynamics, reducing complication risks and reliance on pharmacological support, positioning it as a potentially safer option for procedural sedation in vulnerable populations.
Many studies have showed that intraoperative use of remimazolam to maintain sedation was associated with more stable hemodynamic parameters in general anesthesia. In a randomized controlled trial, the researchers assigned 60 patients undergoing hip replacement surgery receiving general anesthesia to receive either remimazolam (initiated with a loading dose of 0.2–0.4 mg·kg−1, followed by a maintenance infusion of 0.3–0.5 mg·kg−1·h−1) or propofol (starting with a loading dose of 1.5–2 mg·kg−1, then maintained with 4–8 mg·kg−1·h−1).17 The study revealed that patients in the remimazolam group experienced reduced respiratory and circulatory suppression, attenuated stress responses, and lower rates of cognitive dysfunction than those in the propofol group did. Similarly, patients undergoing general anesthesia for laparoscopic radical resection for gastric cancer, the intraoperative administration of remimazolam (with a loading dose of 0.2mg·kg−1, followed by a maintenance dose of 0.3–0.5 mg·kg−1·h−1) was linked to more stable hemodynamic parameters and a lower prevalence of early POCD compared to dexmedetomidine.18 In our study, for intraoperative sedation during spinal anesthesia, remimazolam (with a loading dose of 0.1 mg·kg−1, and a maintenance dose of 0.1–0.3 mg·kg−1·h−1) exhibited more stable perioperative hemodynamics than dexmedetomidine (with a loading dose of 0.5 µg·kg−1, and a maintenance dose of 0.2–0.6 µg·kg−1·h−1). The loading doses and maintenance rates of dexmedetomidine and remimazolam used in our study were aligned with the sedative dosing regimens utilized in other studies and were appropriate for older patients.19,20
While our results underscore the hemodynamic advantages of remimazolam, particularly in reducing hypotension incidence compared to dexmedetomidine, some studies report comparable hypotension rates between the two drugs. These discrepancies likely stem from significant methodological heterogeneity across trials. Notably, studies employing higher maintenance doses—often necessary for deeper sedation in complex procedures—observed attenuated hemodynamic divergence. For instance, one study involving continuous intravenous infusion during regional anesthesia found no difference in hypotension between remimazolam (loading: 6 mg·kg−1·h−1 for 10 min, maintenance: 1 mg·kg−1·h−1) and dexmedetomidine (loading: 6 μg·kg⁻¹·h⁻¹ for 10 min, maintenance: 1 μg·kg−1·h−1).21 Similarly, in patients undergoing fiberoptic bronchoscopy, remimazolam (loading: 6 mg·kg⁻¹·h⁻¹ for 10 min, maintenance: 1–2 mg·kg−1·h−1) did not significantly reduce hypotension incidence compared to dexmedetomidine (loading: 0.5 μg·kg⁻¹ for 10 min, maintenance: 0.2–0.7 μg·kg−1·h−1).22 In contrast, our weight-adjusted titration protocol utilized significantly lower maintenance doses (remimazolam: 0.1–0.3 mg·kg−1·h−1; dexmedetomidine: 0.2–0.6 μg·kg−1·h−1) targeting moderate sedation (MOAA/S 2–3), consistent with guideline-recommended dosing for older adults. Crucially, equipotent sedation was confirmed by identical MOAA/S targets, suggesting that observed hemodynamic differences are attributable to pharmacological profiles rather than sedation depth disparities. Furthermore, procedural and population differences are critical factors. Studies involving younger cohorts or general anesthesia settings may obscure age-related pharmacodynamic vulnerabilities. Our cohort’s advanced age (median 71 years) and standardized spinal anesthesia (sensory block lower than T8) heightened sensitivity to hemodynamic perturbations,23 thereby amplifying the observed hemodynamic advantages of remimazolam in our setting.
Hypotension is a prevalent adverse event in orthopedic surgery, and its incidence is further increased by spinal anesthesia.24,25 The vasodilation of arteries and veins due to sympathetic blockage along with paradoxical activation of cardioinhibitory receptors is a primary cause of spinal anesthesia-induced hypotension.26 Hypotension in older patients is a significant predictor of organ damage, cardiovascular condition, and postoperative cognitive decline. This condition not only increases the risk of 30-day mortality, but also correlates with acute kidney injury (AKI) and major adverse cardiac events, such as myocardial injury or infarction. Additionally, hypotension can extend hospital stays and increase healthcare costs, placing a substantial burden on both patients and the healthcare system.27–29
The divergent hemodynamic outcomes fundamentally stem from distinct pharmacological mechanisms, rather than disparities in sedative potency or dosing. Dexmedetomidine, an α2-adrenoceptor agonist, centrally inhibits sympathetic outflow while potentiating vagal activity—inherently predisposing to dose-dependent hypotension and bradycardia, which could lead to an increased risk of hemodynamic instability.24,30 Conversely, remimazolam’s ultra-short-acting GABA-A receptor modulation, characterized by rapid esterase metabolism and context-independent half-life, minimizes cardiovascular depression while reducing drug accumulation.9,31,32 By strictly adhering to guideline-recommended geriatric dosing and achieving equivalent MOAA/S-targeted sedation depth (scores of 2–3) between groups, our methodology ensured that sedation depth and pharmacological exposure were comparable.33,34 Despite this rigorous control, dexmedetomidine was associated with a significantly higher incidence of hypotension (52.4% vs 22.2%) and bradycardia (61.9% vs 9.5%) than remimazolam. This finding confirms that the divergent hemodynamic profiles are directly attributable to dexmedetomidine’s intrinsic pharmacological properties,30 rather than pharmacological overexposure or inadequate sedation. Additionally, standardized 10-minute infusions were implemented to preclude rapid hemodynamic fluctuations potentially arising from differential onset kinetics between remimazolam and dexmedetomidine. Notably, studies reporting non-significant hemodynamic differences21,22 were conducted in distinct clinical contexts—involving procedures requiring deeper sedation (eg, fiberoptic bronchoscopy22) or complex analgesic regimens—that necessitated higher dosing. Such regimens may obscure intrinsic pharmacologic differences, whereas our optimized protocol in spinal anesthesia clearly demonstrates the inherent hemodynamic advantage of remimazolam.
In our study, remimazolam not only effectively maintained the hemodynamic stability of the patient, but also demonstrated non-inferiority to dexmedetomidine in terms of POD, respiratory depression, and PONV. These effects are associated with the capacity of remimazolam to modulate microglial activity in a beneficial manner similar to dexmedetomidine, and its impact on the GABA-BDZ receptor complex, which reduces the activity of dopaminergic neurons and the release of serotonin like midazolam.35–37 This finding is corroborated by a wealth of literature highlighting the multifaceted benefits of remimazolam in various surgical settings.38–41
However, our study has some limitations. First, it was conducted at a single center with a limited sample size. Second, the study only enrolled patients classified as ASA I–III, which necessitates further investigation of the effect of remimazolam on hemodynamic stability in high-risk populations. Future multi-center trials should validate these findings in broader populations, including those with significant cardiopulmonary comorbidities. Additionally, dose-response studies could optimize remimazolam dosing for specific surgical contexts.
Conclusion
Our findings show that remimazolam is a safe and effective alternative to dexmedetomidine for sedation in patients undergoing lower-extremity orthopedic surgery with spinal anesthesia own to its lower incidence of hypotension and bradycardia. In this context, remimazolam is a promising alternative to dexmedetomidine in minimizing the risk of cardiac-related adverse events.
Data Sharing Statement
The datasets for this study are available from the corresponding author (email: [email protected]) upon reasonable request.
Ethical Approval Statement
This study was approved by the Ethics Committee of the Central Hospital of Dalian University of Technology (Dalian Municipal Central Hospital) (Liaoning, China; 2024-039-01; April 2024). Informed consent was obtained from the study participants prior to the commencement of the study.
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Acknowledgments
We would like to acknowledge the hard and dedicated work of all the staff who implemented the intervention and evaluation components of the study.
Funding
This study did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
The authors have no relevant financial or non-financial interests to disclose.
References
1. Nabatame M, Takeuchi M, Takeda C, Kawakami K. Association between sedation during spinal anesthesia and mortality in older patients undergoing Hip fracture surgery: a nationwide retrospective group study in Japan. J Clin Anesth. 2024;92:111322. doi:10.1016/j.jclinane.2023.111322
2. Turcotte JJ, Stone AH, Gilmor RJ, Formica JW, King PJ. The effect of neuraxial anesthesia on postoperative outcomes in total joint arthroplasty with rapid recovery protocols. J Arthroplasty. 2020;35(4):950–954. doi:10.1016/j.arth.2019.11.037
3. Kelly ME, Turcotte JJ, Aja JM, MacDonald JH, King PJ. General vs neuraxial anesthesia in direct anterior approach total hip arthroplasty: effect on length of stay and early pain control. J Arthroplasty. 2021;36(3):1013–1017. doi:10.1016/j.arth.2020.09.050
4. Arain SR, Ebert TJ. The efficacy, side effects, and recovery characteristics of dexmedetomidine versus propofol when used for intraoperative sedation. Anesthesia Analg. 2002;95(2):461–466. doi:10.1097/00000539-200208000-00042
5. Wesselink EM, Kappen TH, Torn HM, Slooter AJC, van Klei WA. Intraoperative hypotension and the risk of postoperative adverse outcomes: a systematic review. Br J Anaesth. 2018;121(4):706–721. doi:10.1016/j.bja.2018.04.036
6. Masui K. Remimazolam besilate, a benzodiazepine, has been approved for general anesthesia!! J Anesth. 2020;34:479–482. doi:10.1007/s00540-020-02755-1
7. Chang Y, Huang Y-T, Chi K-Y, Huang Y-T. Remimazolam versus propofol for procedural sedation: a meta-analysis of randomized controlled trials. PeerJ. 2023;11:e15495. doi:10.7717/peerj.15495
8. Kilpatrick GJ, McIntyre MS, Cox RF, et al. Tilbrook GS: CNS 7056: a novel ultra-short-acting Benzodiazepine. Anesthesiology. 2007;107:60–66. doi:10.1097/01.anes.0000267503.85085.c0
9. Sheng X-Y, Liang Y, Yang X-Y, et al. Safety, pharmacokinetic and pharmacodynamic properties of single ascending dose and continuous infusion of remimazolam besylate in healthy Chinese volunteers. Eur J Clin Pharmacol. 2020;76:383–391. doi:10.1007/s00228-019-02800-3
10. Schüttler J, Eisenried A, Lerch M, Fechner J, Jeleazcov C, Ihmsen H. Pharmacokinetics and pharmacodynamics of remimazolam (CNS 7056) after continuous infusion in healthy male volunteers: part I. Pharmacokinetics Clin Pharmacodynamics Anesthesiol. 2020;132:636–651.
11. Araki H, Inoue S. Switching to remimazolam followed by flumazenil may be a promising combination for deep extubation. JA Clin Rep. 2023;9:52. doi:10.1186/s40981-023-00643-7
12. Chen X, Sang N, Song K, et al. Psychomotor recovery following remimazolam-induced sedation and the effectiveness of flumazenil as an antidote. Clin Ther. 2020;42:614–624. doi:10.1016/j.clinthera.2020.02.006
13. Schnider TW, Minto CF, Egan TD, Filipovic M. Relationship between propofol target concentrations, bispectral index, and patient covariates during Anesthesia. Anesthesia Analg. 2021;132(3):735–742. doi:10.1213/ANE.0000000000005125
14. Yang C, Jiao J, Nie Y, Shao W, Zhang H, Huang S. Comparison of the bispectral indices of patients receiving remimazolam and propofol for general anesthesia: a randomized crossover trial. Anaesth Crit Care Pain Med. 2024;43(3):101377. doi:10.1016/j.accpm.2024.101377
15. Xi C, Sun S, Pan C, Ji F, Cui X, Li T. Different effects of propofol and dexmedetomidine sedation on electroencephalogram patterns: wakefulness, moderate sedation, deep sedation and recovery. PLoS One. 2018;13(6):e0199120. doi:10.1371/journal.pone.0199120
16. Kasuya Y, Govinda R, Rauch S, Mascha EJ, Sessler DI, Turan A. The correlation between bispectral index and observational sedation scale in volunteers sedated with dexmedetomidine and propofol. Anesthesia Analg. 2009;109(6):1811–1815. doi:10.1213/ANE.0b013e3181c04e58
17. Zhang J, Wang X, Zhang Q, Wang Z, Zhu S. Application effects of remimazolam and propofol on elderly patients undergoing Hip replacement. BMC Anesthesiol. 2022;22:118. doi:10.1186/s12871-022-01641-5
18. Liao YQ, Min J, Wu ZX, Hu Z. Comparison of the effects of remimazolam and dexmedetomidine on early postoperative cognitive function in elderly patients with gastric cancer. Front Aging Neurosci. 2023;15:1123089. doi:10.3389/fnagi.2023.1123089
19. Zhu S, Liu Y, Wang X, et al. Different sedation strategies in older patients receiving spinal anesthesia for hip surgery on postoperative delirium: a randomized clinical trial. Drug Des Devel Ther. 2023;17:3845. doi:10.2147/DDDT.S439543
20. Zhao T-Y-M, Chen D, Sun H, et al. Moderate sedation with single-dose remimazolam tosilate in elderly male patients undergoing transurethral resection of the prostate with spinal anesthesia: a prospective, single-arm, single-centre clinical trial. BMC Anesthesiol. doi:10.1186/s12871-022-01788-1
21. Hong S-W, Park J-Y, Rhee K-Y, Kim S-H. Comparison emergence of sedation, using dexmedetomidine and remimazolam, in spinal anaesthesia—Double blinded randomized controlled trial. Int J Med Sci. 2024;21(8):1552. doi:10.7150/ijms.95736
22. Xu H, Wang L, Zhu W, Ren C, Liu G, Liu Y. Comparison of the safety and efficacy of remimazolam besylate versus dexmedetomidine for patients undergoing fiberoptic bronchoscopy: a prospective, randomized controlled trial. Drug Des Devel Ther. 2024;18:2317. doi:10.2147/DDDT.S460949
23. Jansen PAF, Brouwers JRBJ. 2012 clinical pharmacology in old persons. Scientifica. 2012;2012:723678. doi:10.6064/2012/723678
24. Carpenter RL, Caplan RA, Brown DL, Stephenson C, Wu R. Incidence and risk factors for side effects of spinal anesthesia. Anesthesiology. 1992;76(6):906–916. doi:10.1097/00000542-199206000-00006
25. Hartmann B, Junger A, Klasen J, et al. The incidence and risk factors for hypotension after spinal anesthesia induction: an analysis with automated data collection. Anesthesia Analg. 2002;94(6):1521–1529. doi:10.1097/00000539-200206000-00027
26. Salinas FV, Sueda LA, Liu SS. Physiology of spinal anaesthesia and practical suggestions for successful spinal anaesthesia. Best Pract Res Clin Anaesth. 2003;17(3):289–303. doi:10.1016/s1521-6896(02)00114-3
27. Cai J, Tang M, Wu H, et al. Association of intraoperative hypotension and severe postoperative complications during non-cardiac surgery in adult patients: a systematic review and meta-analysis. Heliyon. 2023;9(5):e15997. doi:10.1016/j.heliyon.2023.e15997
28. Gregory A, Stapelfeldt WH, Khanna AK, et al. intraoperative hypotension is associated with adverse clinical outcomes after noncardiac surgery. Anesthesia Analg. 2021;132(6):1654–1665. doi:10.1213/ANE.0000000000005250
29. Khanna AK, Shaw AD, Stapelfeldt WH, et al. Postoperative hypotension and adverse clinical outcomes in patients without intraoperative hypotension, after noncardiac surgery. Anesthesia Analg. 2021;132(5):1410–1420. doi:10.1213/ANE.0000000000005374
30. Bloor BC, Ward DS, Belleville JP, Maze M. Effects of intravenous dexmedetomidine in humans. II. Hemodynamic changes Anesthesiology. 1992;77(6):1134–1142. doi:10.1097/00000542-199212000-00014
31. Yoshikawa Y, Oura S, Kanda M, et al. Comparison of the negative effect of remimazolam and propofol on cardiac contractility: analysis of a randomised parallel-group trial and a preclinical ex vivo study. Clin Exp Pharmacol Physiol. 2024;51(3):e13840. doi:10.1111/1440-1681.13840
32. Zhang H, Li H, Zhao S, Bao F. Remimazolam in general anesthesia: a comprehensive review of its applications and clinical efficacy. Drug Des Devel Ther. 2024;18:3487. doi:10.2147/DDDT.S474854
33. Expert Group on Clinical Application Guidelines for Remimazolam Besylate. (2023). clinical practice guidelines for remimazolam besylate. Chinese. J Anesthesiol. 43(8):904–911.
34. Expert Group on Dexmedetomidine Clinical Application. (2018). Expert. Consensus on the clinical application of dexmedetomidine (2018). J Clin Anesthesiol. 34(8):820–823.
35. Di Florio T. The use of midazolam for persistent postoperative nausea and vomiting. Anaesth Intensive Care. 1992;20(3):383–386. doi:10.1177/0310057X9202000324
36. Wen T, Wen J, Yao C. Remimazolam inhibits postoperative cognitive impairment after cardiopulmonary bypass by alleviating neuroinflammation and promoting microglia M2 polarization. Brain Res. 2024;1838:148975. doi:10.1016/j.brainres.2024.148975
37. Zhou Z, Yang Y, Wei Y, Xie Y. Remimazolam attenuates LPS-derived cognitive dysfunction via subdiaphragmatic vagus nerve target α7nAChR-Mediated Nrf2/HO-1 signal pathway. Neurochem Res. 2024;49:1306–1321. doi:10.1007/s11064-024-04115-x
38. Liu X, Ding B, Shi F, et al. The efficacy and safety of remimazolam tosilate versus etomidate-propofol in elderly outpatients undergoing colonoscopy: a prospective, randomized, single-blind, non-inferiority trial. Drug Des Devel Ther. 2021;15:4675–4685. doi:10.2147/DDDT.S339535
39. Yoo YM, Park JH, Lee KH, Yi AH, Kim TK. The incidences of nausea and vomiting after general anesthesia with remimazolam versus sevoflurane: a prospective randomized controlled trial. Korean J Anesthesiol. 2024;77(4):441–449. doi:10.4097/kja.23939
40. Zhou B, Li S, Luo A, Zheng H. The efficacy and safety of remimazolam tosilate compared with propofol for endoscopic retrograde cholangiopancreatography under monitored anesthesia care: a single-center randomized controlled clinical trial. Heliyon. 2024;10(19):e38349. doi:10.1016/j.heliyon.2024.e38349
41. Fujimoto D, Obata N, Mizobuchi S. Effectiveness of remimazolam in preventing postoperative delirium in elderly patients with proximal femoral fractures. J Anesth. 2024;38(4):475–482. doi:10.1007/s00540-024-03339-z
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Effects of Remimazolam on Intraoperative Frontal Alpha Band Power Spectrum Density and Postoperative Cognitive Function in Older Adults Undergoing Lower Extremity Fractures Surgeries: A Randomized Controlled Trial
Wu H, Tian S, Ma H, Zhou W, Feng S, Meng L, Ou J, Xu F, Zhang Z
Clinical Interventions in Aging 2024, 19:2195-2205
Published Date: 31 December 2024
Effects of Dexmedetomidine as an Adjuvant in Preoperative Ultrasound-Guided Internal Branch of Superior Laryngeal Nerve Block on Postoperative Sore Throat and Hemodynamics in Patients With Double-Lumen Endotracheal Intubation: A Randomized Controlled Trial
Chen Z, Zhang L, Lu G, Zhang Y, Zhao D, Zhao S, Zhang H, Jin Y, Zhao X, Jin Y
Journal of Pain Research 2025, 18:229-241
Published Date: 17 January 2025
Impact of Dexmedetomidine on Hospital and Intensive Care Unit Stay Duration in Adult Traumatic Brain Injury Patients: A Systematic Review
Alaifan T, Sakhakhni A, Khojah A, Alraddadi EA, Alkhaibary A, Alqahtani AM
Drug, Healthcare and Patient Safety 2025, 17:157-171
Published Date: 11 July 2025


