Back to Journals » Clinical Ophthalmology » Volume 18
Comparison of Vessel Density and Retinal Sensitivity After Scleral Buckling and Phacovitrectomy in the Management of Macula-on Primary Rhegmatogenous Retinal Detachment
Authors Zabel P , Charytoniuk T, Zabel K, Kazmierczak K, Suwala K, Buszko K, Kaluzny JJ
Received 1 June 2024
Accepted for publication 10 October 2024
Published 6 November 2024 Volume 2024:18 Pages 3161—3170
DOI https://doi.org/10.2147/OPTH.S480833
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Przemyslaw Zabel,1– 3 Tomasz Charytoniuk,2,4 Katarzyna Zabel,1– 3 Karolina Kazmierczak,2 Karolina Suwala,1,3 Katarzyna Buszko,5 Jakub J Kaluzny1,3
1Department of Sensory Organ Studies, Collegium Medicum, Nicolaus Copernicus University, Bydgoszcz, Poland; 2Department of Ophthalmology, Collegium Medicum, Nicolaus Copernicus University, Bydgoszcz, Poland; 3Oftalmika Eye Hospital, Bydgoszcz, Poland; 4Department of Physiology, Medical University of Bialystok, Bialystok, Poland; 5Department of Biostatistics and Biomedical Systems Theory, Collegium Medicum, Nicolaus Copernicus University, Bydgoszcz, Poland
Correspondence: Przemyslaw Zabel, Department of Sensory Organ Studies, Collegium Medicum, Nicolaus Copernicus University, Bydgoszcz 85-830, Sandomierska 16, Poland, Email [email protected]
Purpose: The choice of surgical method for rhegmatogenous retinal detachment (RRD) may have a significant impact on the retina. In this study, we aimed to compare retinal function and structure after scleral buckling (SB) and phacovitrectomy (phaco-PPV) for macula-on RRD.
Patients and Methods: This cross-sectional study included patients who underwent anatomically successful repair of macula-on RRD managed with SB (n=35) and phaco-PPV (n=35) between 2019– 2023. All participants were examined within 6– 20 months of surgery to evaluate the retinal structure using spectral domain optical coherence tomography (SD-OCT) and vessel density (VD) by OCT angiography (OCTA). Best-corrected visual acuity (BCVA) and microperimetry (MP) tests were used to assess the retinal function.
Results: Analysis of the microvascular network with OCTA between eyes after surgery and healthy eyes showed a decrease in VD. Significant changes in the superficial vascular plexus (SVP) and deep vascular plexus (DVP) were observed only in eyes after SB surgery (p < 0.001 and p=0.02, respectively). Analysis of retinal function assessed by MP showed a significant decrease (p< 0.05) in retinal sensitivity after phaco-PPV (24.81± 2.25 dB) and SB (24.18± 2.14 dB) operations compared to the healthy control group (25.97 ± 1.51 dB), whereas postoperative BCVA showed no differences (p> 0.05).
Conclusion: Changes in retinal sensitivity were accompanied by impairment of the microvascular network in the eyes after SB and phaco-PPV surgeries due to macula-on RRD. Disorders were more pronounced in eyes following SB surgery, possibly secondary to mechanical stress.
Keywords: scleral buckling, phacovitrectomy, macula-on rhegmatogenous retinal detachment, microperimetry, spectral domain optical coherence tomography, optical coherence tomography angiography, microvascular network
Introduction
Retinal detachment (RD) is defined as detachment of the neurosensory retina from the underlying retinal pigment epithelium (RPE).1,2 It is commonly known that primary rhegmatogenous RD (RRD) might be successfully managed with various surgical interventions, especially scleral buckling (SB), and both pars plana vitrectomy (PPV) and combined phacoemulsification with vitrectomy (phaco-PPV).3–5 The choice of surgery method and its specific modifications vary significantly between surgeons and centers, partly due to the fact that there is still no strong certainty evidence of significant differences in both the structural and functional outcome between these major RRD management procedures. To date, the comparative efficacy of SB and PPV has been widely studied using major endpoints, including the final best-corrected visual acuity (BCVA), reattachment rates, and the occurrence of adverse effects.3 Interestingly, some recent studies have indicated that preoperative and postoperative foveal and perifoveal retinal sensitivity assessed using the microperimetry (MP) technique might also be a crucial factor in comparing the effectiveness of retinal surgeries.6,7 Moreover, the optical coherence tomography angiography (OCTA) technique has been demonstrated to be an essential comparative point for assessing both pre- and postoperative macular microcirculation and vessel density (VD) with good reproducibility and repeatability.8 Notably, a few recent studies have shown reduced retinal microcirculation even after successful anatomical repair, particularly after SB surgery.9,10 Although a number of studies have analyzed various parameters after PPV and SB due to primary macula-on RRD, none of the studies combined and correlated functional, structural, and vascular outcomes.8,11,12
Thus, the aim of this study was to compare the retinal changes in function, structure, and microvascular network assessed by MP, spectral domain OCT (SD-OCT), and OCTA between eyes after phaco-PPV and SB operations for macula-on RRD and unaffected fellow eyes. In addition, the correlations of the retinal structural changes assessed with both OCTA and SD-OCTA with the retinal sensitivity analyzed with MP and BCVA tests were determined. We hypothesized that significant disturbances in the microvascular network may occur in eyes after SB, which may result in impaired retinal function.
Materials and Methods
The Setting of the Study
This retrospective, double-center study was conducted in the Department of Ophthalmology, Nicolaus Copernicus University, Collegium Medicum, Bydgoszcz, Poland, and Oftalmika Eye Hospital, Bydgoszcz, Poland. The study protocol was approved by the Bioethical Commission of Nicolaus Copernicus University, Collegium Medicum, Bydgoszcz, Poland (KB442/2020), and was conducted according to the principles of the Declaration of Helsinki. All participants provided informed consent.
Patient Recruitment and Study Design
This study involved individuals who underwent anatomically successful surgery for primary uncomplicated macula-on RRD managed with SB and phaco-PPV between 2019–2023. Each patient included in the study was diagnosed with fresh clinical RRD in phakic eye without fibrosis, epi- or subretinal membranes, and proliferative vitreoretinopathy (PVR) during qualification for surgery. The decision to perform SB or phaco-PPV depended mainly on the presence of optical media opacity and location of the retinal break(s). SB was performed when both the lens and vitreous were clear and retinal break(s) were sufficiently visible in the far periphery to fall on the posterior one-third of the buckle. Otherwise, a phaco-PPV was performed. All the procedures were performed by the same experienced surgeon (K.K). SD-OCT examination confirmed the absence of fluid in the macular area before surgery in each individual, indicating that the neurosensory retina was not detached from the RPE in the posterior pole of the eye. The healthy control (HC) group comprised fellow eyes in which any disease had been ruled out and had not undergone any surgery.
The inclusion criteria were as follows: 1) primary unilateral RRD, 2) history indicating that the time from the first symptoms of retinal detachment to the surgical procedure was less than 7 days 3) effective management of RDD with phaco-PPV or SB surgery, 3) period from surgery to study inclusion ranging at least 6 months from surgery, and 4) normal structure of the retina in the macular area in preoperative and postoperative SD-OCT assessments, 5) axial length (AXL) between 21–27 mm.
The exclusion criteria were as follows: 1) re-detachment of the retina 2) any ophthalmic procedures other than phaco-PPV or SB for primary macula-on RRD, 3) amblyopia and preoperative anisometropia, 4) any degenerative ophthalmological conditions (eg epiretinal membrane, drusen), intra- or subretinal fluid, and glaucoma, 5) diabetes, 6) history of retinal vascular occlusion, 7) history of ocular surgeries, and 8) PVRs diagnosed pre- or postoperatively.
Within 6–20 months of the procedure, all individuals underwent precise ophthalmological examinations by the same ophthalmologist (P.Z. and T.C)., including: 1) BCVA, 2) IOP (Icare TAO1i, Finland Oy, Vantaa, Finland), 3) slit-lamp biomicroscopy with a Volk lens, 4) AXL measurement (IOL Master 500, Zeiss Humphrey, Dublin, CA, USA), 5) macular analyzer integrity assessment (MAIA) MP (Centervue, Padova, Italy), 6) SD-OCT (Heidelberg Engineering, Heidelberg, Germany), and 7) OCTA (Optovue, Inc., Fremont, CA, USA).
Surgical Procedures
The Constellation Vision System (Alcon Laboratories, Inc., Fort Worth, TX, USA) was used to perform all the combined phacoemulsification and PPV procedures. After completing phacoemulsification, the same surgeon performed the second part, ie, PPV. The vitrectomy procedure began by inserting three 23 gauge-valved cannulas in the pars plana placed 3.5 mm from the corneal limbus. Diluted triamcinolone acetonide was used to dye vitreous humor. Following core vitrectomy, detachment of the posterior hyaloid was induced if needed, and vitreous base shaving was performed. Peripheral vitrectomy and traction release were then accomplished. The retina was flattened by draining subretinal fluid from a pre-existing break or a recently created drainage retinotomy. Subsequently, fluid-air exchange was performed in these eyes prior to retinal tamponade. Once the retina was flattened, endolaser photocoagulation was performed around the retinal break(s) or areas of retinal degeneration if needed. The 20% sulfur hexafluoride (SF6) gas was applied to the vitreous chamber as an endotamponade.
The SB surgeries were performed by the same experienced surgeon. After conjunctival peritomy, rectus muscles were isolated and looped off. A combination of cryotherapy under indirect ophthalmoscopy and exoplanets (circumferential segmental silicone tire (No. 287, MIRA, MIRA Inc., Waltham, MA, USA) and silicone encircling band (No. 240)) were used to treat retinal break(s). Decisions regarding the external drainage of subretinal fluid (SRF) and the use of intraocular tamponade depended on the surgeon’s judgment as to whether the amount of SRF would affect the reattachment of the retina. 6–0 Vicryl suture (Ethicon Inc., USA) was used to suture the conjunctiva.
Images
Retinal images were recorded using the SD-OCT Spectralis to characterize the peripapillary retinal nerve fiber layer (pRNFL) and macular structure. To quantify retinal ganglion cell complex (GCC) thickness, the glaucoma mode built into the Avanti RTVue XR equipment was used.
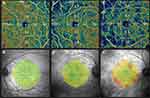
OCTA imaging was determined with an Avanti RTVue XR device with AngioVue software (version 2017.1.0.151). A 6×6 mm2 high-definition (400 × 400) angio scan pattern, centered on the fixation point, and a 4.5×4.5 mm2 centered on the ONH was carried out. The software automatically analyzed the VD in the superficial vascular plexus (SVP) and deep vascular plexus (DVP) of the scanned area of the macula. Moreover, the area of the foveal avascular zone (FAZ) was automatically analyzed. A scan covering the ONH was used to measure the VD of the radial peripapillary capillaries (RPCs) in the peripapillary area, extending between 2- and 4-mm-diameter elliptical contour lines around the disc margin. Only good technical measurements with a scan quality (SQ) index of 6 or higher were included in the analyses. Measurements with incorrect segmentation of individual vascular plexuses and those with motion artifacts in en face images (irregular vascular patterns or blurred ONH borders) were rejected.
Microperimetry
A combination of fundus imaging, scanning laser ophthalmoscopy, static perimetry, and fixation analysis might be achieved using MAIA microperimetry. The expert examination option was used to determine the average threshold (AT) of macular sensitivity using the standard macular grid pattern (37 stimuli points) built into the MAIA MP, covering the central 10° of the retina (±5° around the macula). MAIA measurements were performed without dilation of the pupil in a dim room. A 4–2 threshold strategy was used during the MAIA examinations.
Statistical Analysis
Descriptive statistics for normally distributed data are presented as mean ± standard deviation (SD). Categorical data were presented as structure percentage indexes. The normal distribution of the data was assessed using the Shapiro–Wilk test. Differences between continuous variables were evaluated using the Mann–Whitney U-test. Linear associations between continuous variables were assessed using Pearson’s correlation coefficients with significance tests. A linear mixed effects model (LMM) was used to analyze the association between VD in RPC, SVP, DVP and SQ, AXL. All statistical analyses were conducted at a significance level α=0.05. The calculations were performed using the R software (version 4.3.1) and Statistica (version 13.2).
Results
This study initially enrolled 123 eyes (122 patients) that met the inclusion criteria. Owing to poor image quality (motion artifacts, media opacities, incorrect segmentation) in the OCTA and SD-OCT examinations, two eyes from the phaco-PPV group, three eyes from the SB group, and one eye from the HC group were excluded.
Finally, analyses were carried out on the data from 117 eyes (116 patients): 35 eyes in the phaco-PPV group, 35 eyes in the SB group, and 47 eyes in the HC group. Table 1 presents the demographic and clinical characteristics of the study groups. In eyes with macula-on RRD before phaco-PPV and SB surgeries, BCVA was worse than that in healthy fellow eyes (0.15±0.82, 0.15±0.28, 0.02±0.05, respectively; p<0.05 for both groups). The groups were well-matched by age and sex distribution. The phaco-PPV and SB groups were similar in number of retinal tears (1.74±0.81 vs 1.57±0.74, p=0.429), the average duration of visual symptoms (4.72±2.31 vs 4.75±2.19 days, p=0.896) and the time elapsed between surgery and examinations (9.17±1.98 vs 9.06±2.37 months, p=0.682). In both the phaco-PPV and SB groups, retinal detachment was most common in one quadrant (48.57% vs 52.94%), followed by two quadrants (40% vs 38.24%), less often in three quadrants (8.57% vs 8.82%), while one person (2.84%) in the phaco-PPV group had retinal detachment in all four quadrants.
 |
Table 1 Baseline Clinical Characteristics of Treated and Healthy Fellow Eyes in the Study Groups |
The results, which present functional and morphological data of the studied groups, are included in Table 2. The eyes that underwent phaco-PPV and SB did not show significant differences from the control group in IOP (p=0.847, p=0.673, respectively) and AXL (p=0.684, p=0.168, respectively). Assessment of retinal function using MAIA MP showed a significant decrease of AT after both phaco-PPV and SB operations due to macula-on RRD (p=0.047, p<0.001, respectively). It was different when the analysis of retinal function was carried out using Snellen charts, because no significant differences were found in groups after phaco-PPV or SB compared to the HC group (p=0.821, p=0.324, respectively).
 |
Table 2 Comparison of Functional and Morphological Characteristics Between Study Groups |
In the macular area, assessment of the microvascular network with OCTA between the eyes after surgery for macula-on RRD and the healthy eyes showed a decrease of VD. In the eyes after SB surgery, significant changes were observed in SVP and DVP of the whole area 6×6 mm2 en face images (p<0.001 and p=0.002, respectively), the parafoveal area (p<0.001 and p=0.026, respectively) and perifoveal area (p<0.001 and p=0.001, respectively), as opposed to the foveal area (p=0.455 and p=0.115, respectively), where these changes were not statistically significant. In the phaco-PPV group, despite a decrease of VD in both vascular plexuses, these changes were insignificant (p>0.05).
The decrease in VD was also related to the RPC layer; however, significant changes were observed only in the eyes after SB (p=0.041). Moreover, comparative analysis of the retinal structure showed insignificant differences in GCC and pRNFL thicknesses in both groups: phaco-PPV (p=0.722 and p=0.825, respectively) and SB (p=0.066 and p=0.293, respectively). The structural and retinal vascular results for the study groups are shown in Table 3.
 |
Table 3 Comparison of Microvascular Network and Structural Characteristics Between Study Groups |
Table 4 presents Pearson’s correlations between AT of the retinal sensitivity and structural data in the peripapillary and macular areas. Significant Pearson’s correlations were observed only in eyes after SB between retinal AT and VD in SVP and RPC (R=0.493, p=0.003 and R=0.466, p=0.004, respectively). Postoperative BCVA did not show any correlation with structural parameters in both groups.
 |
Table 4 Pearson’s Correlation Coefficient Between Structural and Functional Parameters for Operated Eyes |
Discussion
In this retrospective study, we found an effect of RRD, even when the macula was not involved, on retinal sensitivity assessed by MP and the microvascular network evaluated using OCTA. The decrease in retinal AT was more significant in eyes undergoing SB surgery than in eyes undergoing phaco-PPV, similar to the reduction in VD. In addition, we showed statistically significant but weak association between the reduction of VD in the SVP and RPC and decreased AT of the retina in the eyes after SB surgery (Figure 1).
Numerous studies have attempted to determine the optimal surgical technique for RRD treatment. Designing a randomized clinical trial is difficult because of the many differences associated with RD. However, the general consensus based on data available in the published literature, including a systemic review, recommends SB as the primary surgery for uncomplicated RRD, in phakic eyes, especially in young patients.13–15 However, with recent significant advances in PPV surgery, many studies have shown that the two procedures are comparable in the treatment of primary RRD, especially in those without PVR.5 Currently, there is a tendency to choose primary PPV as the first surgery to treat any type of RRD despite the advantage of SB over PPV in relatively young phakic patients.16
To thoroughly remove the vitreous body and avoid the effect of lens opacity on the functional and structural results of the retina in our study, we performed a combined procedure that included phacoemulsification and PPV. Previous research has shown that anatomical and functional results are comparable to those obtained with PPV and delayed cataract surgery.17–19 In our study, we avoided delayed cataract surgery during follow-up and the effect of lens opacity on the postoperative outcomes. Furthermore, owing to the inclusion of eyes with macula-on RRD in the study group, the estimation of the implant power was burdened with a lower refractive error than when the detachment involved the macula.
This study found that eyes after phaco-PPV and SB surgeries due to macula-on RRD showed no significant differences in postoperative BCVA compared with HC eyes. In turn, a more detailed assessment of retinal function using MP showed a significant reduction in AT of the retina in both operated groups, despite the normal structure of the retina in the macula on SD-OCT examination. Previous studies confirms that BCVA assessment may be an insufficient indicator of many aspects of their visual functions since it imprecisely and subjectively determines the retinal function after it is reattached, and it is common knowledge that the most depends on the involvement of the macular area.10,19 Our findings confirm that when the macular region is uninvolved, more precise methods for evaluating the function of the macula are required because the AT of retinal sensitivity may decrease even with normal BCVA. In our previous study, we also used MAIA MP to assess retinal function; however, we compared eyes only after SB due to macula-on RRD with healthy fellow eyes. In both studies, we showed that AT of the retina is reduced in RRD with an attached macula, even after successful repair surgery, which was confirmed by previous studies using other methods of assessing retinal function.12,20,21 These findings support our hypothesis that the state of the retinal microvascular network in the macula substantially influences the maintenance of proper function. We demonstrated that eyes after phaco-PPV had a smaller decrease in both VD and retinal sensitivity than eyes following SB surgery. In 1983, Cardillo Piccolino F, during a fluorescein angiography examination, noticed reduced in microcirculation in patients with RRD, even beyond the area of detachment, which was the result of an increase in the peripheral resistances.22 Subsequent reports confirmed that reduced microcirculation occurs not only in areas with detachment, but also within the ONH and macula, even if macula-on RRD is diagnosed.21,23,24 Proven factors associated with reduced VD in eyes after RRD are endothelin-1 (ET-1) secretion and Müller cell activation. Secretion of the vasoconstrictor peptide ET-1 in SRF leads to narrowing of the microvessels and, as a result, a decrease in retinal blood flow.25,26 Müller cells also have some pathological effects on the retinal vasculature, in the form of cell hyperplasia, gliosis, as well as subretinal fibrosis, even when no other microstructural changes are observed.27–29 Although detachment occurs outside the macula, this abnormal mechanism may explain why macular blood flow is diminished in the eyes following phaco-PPV. A systematic review and meta-analysis assessing macular microcirculation changes after repair of RRD assessed with OCTA showed that significant changes in vessel density reduction occur in eyes after successful repair surgery for macula-off RRD. However, decrease VD could also be observed in some eyes after repair surgery for macula-on RRD. The results of this meta-analysis underscore the importance of RD in the macular area as a factor that has a major impact on the microvascular network and quality of vision.30
Importantly, our study showed that not only does the state of prior RRD affect VD in the macular area, even if it is not affected by detachment, but the type of repair surgery also has a more significant impact. Compared with the HC and phaco-PPV groups, eyes after SB surgery exhibited significantly worse VD parameters evaluated by OCTA. This thesis is supported by another studies that demonstrate disturbances in choroidal perfusion occurring after SB operation for RRD. D’Aloisio et al used OCTA to demonstrate that SB surgery is also associated with a significant reduction in the total perfusion of the iris. The decrease in iris perfusion is probably secondary to the tension of the ocular rectus muscles and mechanical compression of the ocular vessels by silicone exoplanets.31 Moreover, other studies evaluating choroidal perfusion indices such as choriocapillaris flow area (CFA) and choroidal vascularity index (CVI) in eyes that were successfully treated for RRD due to giant retinal tears (GRTs) have also shown significant changes. Long-term CVI and CFA were lower in eyes after RRD with GRTs than in fellow eyes. Among eyes with GRT, those with SB had significantly lower CVI and CFA, confirming the additional mechanical effect of SB on vascular perfusion.32
The strength of this study comparing retinal function and structure in eyes following phaco-PPV and SB surgery for macula-on RRD is that the macula was carefully examined with SD-OCT both before and after surgery, allowing patients with subretinal fluid in the macular region to be excluded. Undoubtedly, both AXL and the presence of lens and vitreous opacities had a significant impact on the VD results obtained from the OCTA examination. Therefore, in statistical analyses, despite the lack of significant differences in AXL and SQ in OCTA between the study groups, we used the linear mixed-effect model, which considers the impact of the difference in AXL and the quality of OCTA scans on the obtained VD results.
This study has several limitations. First, the number of eyes in the operated group was relatively small because this study was based on the macula-on RRD. Therefore, many eyes with SRF in the macular area were excluded. Therefore, we decided to enroll the patients at various follow-up periods, as specified in the inclusion criteria. Second, since we did not perform an OCTA examination prior to the procedure, we do not know the state of the microvascular network before surgery, which makes it impossible to assess changes over time. Accordingly, it is worth planning a multicenter prospective study in the future, which, based on this research, may expand our knowledge of the functional and anatomical changes in the retina over time.
In conclusion, we found that even after successful repair surgery for macula-on RRD, there is a crucial change in retinal function that can be accurately assessed using the MP. Changes in AT of retinal sensitivity were associated with loss of the microvascular network on OCTA examination. We believe that the decrease in AT sensitivity of the retina may be mediated by the reduction in VD, and that these changes are more significant in eyes following SB surgery than in eyes after phaco-PPV. SB surgery may result in reduced vascular perfusion possibly secondary to mechanical stress.
Acknowledgments
We would like to thank all study participants who kindly provided consent to participate in this study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ghazi NG, Green WR. Pathology and pathogenesis of retinal detachment. Eye. 2002;16:411–421. doi:10.1038/sj.eye.6700197
2. Mitry D, Charteris DG, Fleck BW, et al. The epidemiology of rhegmatogenous retinal detachment: geographical variation and clinical associations. Br J Ophthalmol. 2010;94:678–684. doi:10.1136/bjo.2009.157727
3. Dhoot AS, Popovic MM, Nichani PAH, et al. Pars plana vitrectomy versus scleral buckle: a comprehensive meta-analysis of 15,947 eyes. Surv Ophthalmol. 2022;67:932–949. doi:10.1016/j.survophthal.2021.12.005
4. Nossair AA, Ewais WA, Eissa SA. Chandelier-assisted scleral buckling using wide angle viewing contact lens for pseudophakic retinal detachment repair. Int J Ophthalmol. 2019;12:627–633. doi:10.18240/ijo.2019.04.17
5. Narayanan R, Tyagi M, Hussein A, et al. Scleral buckling with wide-angled endoillumination as a surgical educational tool. Retina. 2016;36:830–833. doi:10.1097/IAE.0000000000000792
6. Noda H, Kimura S, Hosokawa MM, et al. Effect of rhegmatogenous retinal detachment on preoperative and postoperative retinal sensitivities. Sci Rep. 2020;10:21497. doi:10.1038/s41598-020-78693-5
7. Molina-Martín A, Pérez-Cambrodí RJ, Piñero DP. Current clinical application of microperimetry: a review. Semin Ophthalmol. 2018;33:620–628. doi:10.1080/08820538.2017.1375125
8. Gironi M, D’Aloisio R, Verdina T, et al. Long-term macular vascular changes after primary rhegmatogenous retinal detachment surgery resolved with different tamponade or different surgical techniques. Life. 2022;12:1525. doi:10.3390/life12101525
9. Iwase T, Kobayashi M, Yamamoto K, et al. Change in choroidal blood flow and choroidal morphology due to segmental scleral buckling in eyes with rhegmatogenous retinal detachment. Sci Rep. 2017;7:5997. doi:10.1038/s41598-017-05126-1
10. Lina G, Xuemin Q, Qinmei W, Lijun S. Vision-related quality of life, metamorphopsia, and stereopsis after successful surgery for rhegmatogenous retinal detachment. Eye. 2016;30:40–45.
11. Barca F, Bacherini D, Dragotto F, et al. Oct angiography findings in Macula-on and Macula-off rhegmatogenous retinal detachment: a prospective study. J Clin Med. 2020;9:3982. doi:10.3390/jcm9123982
12. Zabel P, Zabel K, Kazmierczak K, et al. Vascular density and macular sensitivity in eyes after scleral buckling surgery for macula-on rhegmatogenous retinal detachment. PLoS One. 2023;18:e0279683. doi:10.1371/journal.pone.0279683
13. Sun Q, Sun T, Xu Y, et al. Primary vitrectomy versus scleral buckling for the treatment of rhegmatogenous retinal detachment: a meta-analysis of randomized controlled clinical trials. Curr Eye Res. 2012;37:492–499. doi:10.3109/02713683.2012.663854
14. Adelman RA, Parnes AJ, Ducournau D. Strategy for the management of uncomplicated retinal detachments. Ophthalmology. 2013;120:1804–1808. doi:10.1016/j.ophtha.2013.01.070
15. Heimann H, Bartz-Schmidt KU, Bornfeld N, et al. Scleral buckling versus primary vitrectomy in rhegmatogenous retinal detachment. A prospective randomized multicenter clinical study. Ophthalmology. 2007;114:2142–2154. doi:10.1016/j.ophtha.2007.09.013
16. Kobashi H, Takano M, Yanagita T, et al. Scleral buckling and pars plana vitrectomy for rhegmatogenous retinal detachment: an analysis of 542 eyes. Curr Eye Res. 2014;39:204–211. doi:10.3109/02713683.2013.838270
17. Kim MS, Woo SJ, Park KH. Phacovitrectomy versus lens-sparing vitrectomy for rhegmatogenous retinal detachment repair according to the surgical experience. Retina. 2021;41:1597–1604. doi:10.1097/IAE.0000000000003090
18. Tan A, Bertrand-Boiché M, Angioi-Duprez K, et al. Outcomes of combined phacoemulsification and pars plana vitrectomy for rhegmatogenous retinal detachment: a comparative study. Retina. 2021;41:68–74. doi:10.1097/IAE.0000000000002803
19. Owsley C, Sloane ME. Contrast sensitivity, acuity, and the perception of “real-world” targets. Br J Ophthalmol. 1987;71. doi:10.1136/bjo.71.10.791:791-6
20. Okamoto F, Sugiura Y, Okamoto Y, et al. Changes in contrast sensitivity after surgery for macula-on rhegmatogenous retinal detachment. Am J Ophthalmol. 2013;156:667–672. doi:10.1016/j.ajo.2013.05.017
21. Akiyama K, Fujinami K, Watanabe K, et al. Macular dysfunction in patients with macula-on rhegmatogenous retinal detachments. Br J Ophthalmol. 2019;103:404–409. doi:10.1136/bjophthalmol-2018-312153
22. Cardillo Piccolino F. Vascular changes in rhegmatogenous retinal detachment. Ophthalmologica. 1983;186:17–24. doi:10.1159/000309255
23. Iwase T, Kobayashi M, Yamamoto K, et al. Changes in blood flow on optic nerve head after vitrectomy for rhegmatogenous retinal detachment. Invest Ophthalmol Vis Sci. 2016;57:6223–6233. doi:10.1167/iovs.16-20577
24. Eshita T, Shinoda K, Kimura I, et al. Retinal blood flow in the macular area before and after scleral buckling procedures for rhegmatogenous retinal detachment without macular involvement. Jpn J Ophthalmol. 2004;48:358–363. doi:10.1007/s10384-004-0096-5
25. Polak K, Luksch A, Frank B, et al. Regulation of human retinal blood flow by endothelin-1. Exp Eye Res. 2003;76:633–640. doi:10.1016/S0014-4835(02)00312-3
26. Hiscott P, Wong D. Proliferative vitreoretinopathy. Encyclop Eye. 2010;2010:1.
27. Iandiev I, Uhlmann S, Pietsch UC, et al. Endothelin receptors in the detached retina of the pig. Neurosci Lett. 2005;384:72–75. doi:10.1016/j.neulet.2005.04.056
28. Iandiev I, Uckermann O, Pannicke T, et al. Glial cell reactivity in a porcine model of retinal detachment. Invest Ophthalmol Vis Sci. 2006;47:2161–2171. doi:10.1167/iovs.05-0595
29. Anderson DH, Guerin CJ, Erickson PA, et al. Morphological recovery in the reattached retina. Invest Ophthalmol Vis Sci. 1986;27:168–183.
30. Chen X, Li W, Jin X, et al. Macular microcirculation changes after repair of rhegmatogenous retinal detachment assessed with optical coherence tomography angiography: a systematic review and meta-analysis. Front Physiol. 2022;13(995353). doi:10.3389/fphys.2022.995353
31. D’aloisio R, Viggiano P, Borrelli E, et al. Changes in iris perfusion following scleral buckle surgery for rhegmatogenous retinal detachment: an anterior segment optical coherence tomography angiography (as-octa) study. J Clin Med. 2020;9:1231. doi:10.3390/jcm9041231
32. Quiroz-Reyes MA, Quiroz-Gonzalez EA, Quiroz-Gonzalez MA, et al. Postoperative choroidal vascular biomarkers in eyes with rhegmatogenous retinal detachment-related giant retinal tears. Int J Retina Vitreous. 2023;9(1):45. doi:10.1186/s40942-023-00482-9
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.