Back to Journals » Clinical Ophthalmology » Volume 19
Correlating the Optical Coherence Tomography Patterns and Biomarkers of Diabetic Macular Edema with Hemoglobin Level in Diabetic Kidney Disease
Authors Gopalakrishnan N, Nagaraju SP, George NM, Bhandary SV, Kamath YS , Shetty SS, Nair SS, Kuzhuppilly NI , S S
Received 17 August 2024
Accepted for publication 23 December 2024
Published 21 January 2025 Volume 2025:19 Pages 209—215
DOI https://doi.org/10.2147/OPTH.S447826
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Nikhil Gopalakrishnan,1 Shankar Prasad Nagaraju,2 Neenu Mariam George,3 Sulatha V Bhandary,4 Yogish S Kamath,4 Sushan Shankar Shetty,4 Soumya S Nair,4 Neetha IR Kuzhuppilly,4 Shailaja S4
1Department of Retina, Narayana Nethralaya, Bangalore, Karnataka, India; 2Department of Nephrology, Kasturba Medical College Manipal, Manipal Academy of Higher Education, Manipal, Udupi, Karnataka, India; 3Aravind Eye Hospital, Coimbatore, Tamil Nadu, India; 4Department of Ophthalmology, Kasturba Medical College Manipal, Manipal Academy of Higher Education, Manipal, Udupi, Karnataka, India
Correspondence: Shailaja S; Sushan Shankar Shetty, Department of Ophthalmology, Kasturba Medical College Manipal, Manipal Academy of Higher Education, Manipal, Udupi, Karnataka, 576104, India, Tel +91 9686125861, Fax +91-820-2571934, Email [email protected]; [email protected]
Purpose: To correlate the optical coherence tomography (OCT) based morphological patterns of diabetic macular edema (DME) and prognostic biomarkers with severity of anaemia in patients with diabetic kidney disease (DKD).
Patients and Methods: Single centre, observational cross sectional study of 42 eyes of 42 patients with DME and DKD. Eyes were divided into 2 groups: Group A (Haemoglobin level above 10 g% and group B with haemoglobin less than 10 g%). The OCT pattern and biomarkers were compared between the two groups.
Results: Mean age of the patients was 56.9 ± 8.1 (range 42– 79). Out of 42 participants, 22 patients had hemoglobin level more than 10g%(group A) while 20 patients had hemoglobin less than 10g%(Group B). The most common morphological pattern seen was combination of cystoid macular edema (CME) with diffuse retinal thickening (DRT) in both groups. Disruption of the external limiting membrane (ELM) and ellipsoid zone (EZ) was found to be associated with lower hemoglobin levels (p= 0.0155 and p= 0.0154 respectively). None of the other OCT biomarkers showed correlation with hemoglobin levels.
Conclusion: CME with DRT is the most common morphological pattern of macular edema seen in DKD patients with anemia. ELM-EZ disruption can be considered as important biomarker of anaemia in patients with DKD.
Keywords: anemia, central foveal thickness, external limiting membrane, cystoid macular edema, ellipsoid zone
Introduction
Diabetes mellitus (DM) is an important public health concern, a chronic condition affecting the quality of life of millions of people worldwide and imposing significant burden on the society and health care systems across nations. The number of diabetics in the world is estimated to be 366 million by the year 2030.1 Nephropathy, peripheral neuropathy and diabetic retinopathy are microvascular complications of diabetes. Diabetic retinopathy (DR) is a leading cause of visual impairment in the working age population2 and the most common microvascular complication of chronically elevated blood sugars. Commonest cause of vision loss in patients with DR is Diabetic macular edema (DME).3
Diabetic kidney disease (DKD), until recently known as diabetic nephropathy is another microvascular complication of diabetes. DKD is a leading cause of end stage renal disease affecting about 40% of the diabetics.4 Patients with DKD are at risk of developing renal anaemia mainly due to erythropoietin deficiency.5 Reduced red blood cell survival, systemic inflammation and autonomic neuropathy all work together to make anemia very prevalent in patients with diabetes.6 Anemia is associated with increased severity of DME and risk of progression to high risk proliferative diabetic retinopathy (PDR).7
Optical Coherence tomography (OCT) is invaluable in the diagnosis, management and follow up of patients with DME. Diffuse spongiform thickening, cystoid macular edema (CME) and serous retinal detachment (SRD) are the different morphological types of DME on OCT8,9 (Figure 1). Various OCT biomarkers like disorganisation of inner retinal layers (DRIL), hyperreflective dots (HRD) and hyperreflective foci (HRF), disruption of Ellipsoid zone (EZ) and external limiting membrane (ELM) help in prognosticating DME.9
 |
Figure 1 Morphological patterns of DME. Blue star: CME, Yellow star: DRT, Red star: SRD. DRT: increased retinal thickness with reduced intraretinal reflectivity and expanded areas of lower reflectivity;8 CME: Presence of low reflective cystoid spaces separated by high reflective intervening septae;8 SRD: accumulation of the subretinal fluid seen as hyporeflective space with the high reflective outer border.8 |
Till date no study has been conducted to correlate the degree of anaemia in DKD patients with the OCT pattern of DME and OCT biomarkers. The present study was undertaken to correlate the morphological patterns of DME and OCT biomarkers of DME with the degree of anaemia in DKD patients.
Materials and Methods
This was a single centre, observational cross-sectional study conducted at a tertiary care hospital in South India. Patients with type 1 or type 2 diabetes aged 18 years or more, with co-existing diabetic kidney disease (DKD) and having centre involving DME defined as central subfield macular thickness of more than 320 microns for men and more than 305 microns for women were included in the study.10 Patients with chronic kidney disease (CKD) due to causes other than diabetes and non-diabetic macular oedema were excluded from the study. Patients with significant media opacities hindering the acquisition of good quality OCT were also excluded from the study. Patients who had undergone any intraocular surgery, laser photocoagulation or received intravitreal injections in the past 6 months were also excluded.
After Institutional Ethical Committee clearance (IEC Project No.- 708-2019) and CTRI registration (CTRI Reg. No- 2020/07/026783), the recruitment of participants was initiated. Patients attending the outpatient departments of Ophthalmology and Nephrology in our hospital, Manipal between September 2019 to December 2021 were included in the study once they were seen to meet the above-mentioned inclusion criteria. The tenets of Declaration of Helsinki were adhered to and written informed consent was obtained from study participants. Standardized data collection forms were used. All participants underwent comprehensive ophthalmologic evaluation including Snellen visual acuity, slit-lamp biomicroscopy, and Spectral Domain OCT of macula using Cirrus HD-OCT 5000 (Carl Zeiss Meditec, Dublin, CA). Values of relevant laboratory parameters done not more than one week prior to the examination including Haemoglobin, HbA1c, serum urea, creatinine and electrolytes, and proteinuria were collected. OCT features were assessed by one of the authors (SS) who is a retina specialist. If both eyes of a patient fitted the criteria for recruitment, the eye with greater central subfield thickness was selected for the study. Snellen best corrected visual acuity (BCVA) was converted to logarithm of minimum angle of resolution (log MAR) BCVA using standardised tables.
DRT was defined as increased retinal thickness with reduced intraretinal reflectivity and expanded areas of lower reflectivity.8 Presence of low reflective cystoid spaces separated by high reflective intervening septae in the macular area was categorised as CME.8 Serous retinal detachment was defined as the accumulation of subretinal fluid seen as hyporeflective space with the high reflective outer border.8
Diabetic retinopathy was graded using the ETDRS classification into mild, moderate, severe, very severe non-proliferative diabetic retinopathy and proliferative diabetic retinopathy.11
Estimated glomerular filtration rate (eGFR) was calculated using the CKD-EPI creatinine formula.12 DKD was staged based on the estimated glomerular filtration rate (eGFR).13 Anaemia was graded according to the World health organisation (WHO) guidelines as mild (10mg/dl to 13mg/dl in males and 10mg/dl to 12mg/dl in females), moderate (8mg/dl to 9.9mg/dl in males and females) severe (6mg/dl to 7.9 mg/dl in males and females) and life threatening if less than 6 mg/dl.14
Data was expressed as mean ± standard deviation. Fisher exact test, Pearson correlation analysis, Kruskal–Wallis one-way analysis of variance, linear regression and multiple logistic regression were used to measure significance of correlation between the recorded parameters. P value less than 0.05 was taken as significant. R software was used for statistical analysis.
Results
42 eyes of 42 participants were included in the study. Mean age of the patients was 56.9 ± 8.1 (range 42–79) years. Six were female patients and the remaining 36 were male patients. The mean duration of diabetes was 12.7 ± 6.7 (range 2–30) years. The median haemoglobin was found to be 10 gm/dl and was taken as a cut off value to divide the sample into two groups, group A with haemoglobin above or equal to 10 gm/dl and group B with haemoglobin below 10gm/dl. The mean HbA1c was 8.0 ± 2.5% and mean serum creatinine was 3.7 ± 2.5. The average log MAR BCVA was found to be 0.5 ± 0.36 which corresponds to Snellen visual acuity of 6/12 (Table 1).
 |
Table 1 Demographics of the Two Groups of Participants Along with the Clinical Characteristics |
The severity of diabetic retinopathy as well as kidney disease were noted to be worse in the group B than in group A, but this difference was not of statistical significance in our sample (p=0.1747).
CME in combination with DRT was the commonest morphological pattern seen in the study population. This pattern was seen in 19 eyes (45%), 11 eyes (50%) in group A and eight eyes (40%) in group B. The second common pattern seen was the combination of all three patterns ie CME, SRD and DME which was seen in seven eyes (31.8%) in group A and six eyes (30%) in group B respectively. Only DRT was seen in three eyes, all of which were in group A. SRD in combination with CME, DRT or both was seen in 17 eyes, eight in group A and nine in group B (Table 2).
 |
Table 2 Showing the Distribution of OCT Patterns of DME in Two Groups |
Mean central foveal thickness (CFT) was 439.29 microns for the entire study population with the CFT in group A being 449.18 microns and that in the group B was 429.4 microns. DRIL was present in 70% (30 out of 42) of the total sample. DRIL was seen in 16 eyes in group A (72.73%) and 14 eyes in group B (70%). Disruption of the EZ and ELM was each seen in nine eyes in group A (40.9%) and 12 eyes (60%) in group B and 50% among all participants. Hyperreflective foci were seen in five (27.7%) and three (15%) cases in group A and B respectively (19.05% of total cases). Hyperreflective dots were seen in 12 (54.5%) and 11 (55%) cases in groups A and B respectively, which was close to the overall average of 54.76%. Epiretinal membrane formation was seen in two cases in group A (9%) and six cases in group B (30%), bringing the total prevalence to 19.05% across the entire sample. Vitreomacular adhesion (VMA) and vitreomacular traction (VMT) was seen in one (4%) and two (8%) cases in group A respectively. In group B, VMA was seen in four cases (20%) and VMT was seen in five cases (11.9%) (Table 3).
 |
Table 3 Comparison Between the OCT Biomarkers of DME |
Multivariate logistic regression analysis was done for the analysis of OCT derived variables with binary results against laboratory parameters. Lower serum Haemoglobin was found to have statistically significant associations with disruption of the ELM (p= 0.0155) and EZ (p= 0.0154) (Table 4).
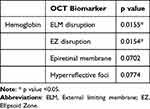 |
Table 4 Comparison Between the OCT Biomarkers with Hemoglobin Level |
Age in years was found to be significantly correlated with presence of subretinal fluid (p= 0.0363), ELM disruption (p= 0.0085) and ERM (p= 0.0144). Duration of diabetes (in years) also showed significant correlation with disruption of ELM (p= 0.03).
Serum haemoglobin (p=0.0774) as well as creatinine (p=0.0139) values were found to have statistically significant relation with presence of hyper-reflective foci.
Discussion
Anaemia resulting in reduced oxygen carrying capacity of haemoglobin leads to retinal hypoxia which in turn causes the production of angiogenic factors and inflammatory mediators. These factors are responsible for the progression of diabetic retinopathy and diabetic macular oedema. Mohan et al showed a correlation between low haemoglobin levels and hard exudate formation and occurrence of clinically significant macular oedema.15 Traveset et al demonstrated an independent association between low levels of haemoglobin and diabetic macular edema.16 On literature review we came across some studies that have looked into the correlation of OCT features of DME with CKD.17,18 But none of these studies have particularly looked at the impact of levels of haemoglobin on the morphological pattern and OCT biomarkers of DME.
Combination of CME with DRT was found to be the commonest morphological pattern seen in the present study followed by combination of all three patterns. Another multicentric study from India by Agarwal et al reported that no particular morphological pattern of macular oedema can be attributed to patients with renal involvement and DME.17 However there are no studies which have correlated the haemoglobin levels in DKD patients with the pattern on OCT. In our set of eyes, CME with DRT was found to be commonest morphological pattern in patients with all degrees of anaemia. In the study by Zhang et al albuminuria was associated with presence of sub retinal fluid (SRF) and low serum albumin level was associated with thickness of SRF. However, they did not find any relation with eGFR.19
ELM and EZ disruption were found to have a statistically significant association with lower haemoglobin levels in DKD patients. Previous studies have highlighted the importance of ELM and EZ integrity as a third blood-retinal barrier,20 and the findings in our study support the same. ELM has tight junctions which are altered by VEGF.20 EZ is indicative of the photoreceptor integrity.20 These defects are usually associated with poorer baseline visual acuity and are predictive of poorer visual prognosis following treatment. DRIL, HRD and HRF did not show any correlation with the level of haemoglobin. DKD patients who have ELM and EZ disruption on OCT should be evaluated for coexisting anaemia.
Though it was beyond the scope of the present study, it would be interesting to study the effect of anti-vascular endothelial (VEGF) drugs and intravitreal steroid injections on the DME and OCT biomarkers in DKD patients with anaemia. Future prospective studies exploring these questions can be useful to understand the role of anaemia in these set of eyes.
A small sample size is a limitation of the present study. Many eyes had to be excluded as they did not meet the inclusion criteria of minimum CFT.
Conclusion
CME with DRT is the most common morphological pattern of macular edema seen in DKD patients with anaemia. ELM-EZ disruption can be considered as the important biomarkers of anaemia in patients with DKD.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethical Statement
This research was reviewed and approved by the institutional review board of Kasturba Medical College Manipal, Manipal Academy of Higher Education (registration number ECR/146/Inst/KA/2013/RR-19). Informed consent was obtained from all participants.
Acknowledgments
The authors would like to thank the nurses and technicians of Department of Ophthalmology, Kasturba medical college and hospital, Manipal for general support and translations.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Aiello LP, Gardner TW, King GL, et al. Diabetic retinopathy. Diabetes Care. 1998;21(1):143–156. doi:10.2337/diacare.21.1.143
2. Stefánsson E, Bek T, Porta M, et al. Screening and prevention of diabetic blindness. Acta Ophthalmol Scand. 2000;78:374–385. doi:10.1034/j.1600-0420.2000.078004374.x
3. Klein R, Klein BE, Moss SE, et al. The Wisconsin epidemiologic study of diabetic retinopathy: IV. Diabetic macular edema. Ophthalmology. 1984;91(12):1464–1474. doi:10.1016/S0161-6420(84)34102-1
4. Packham DK, Alves TP, Dwyer JP, et al. Relative incidence of ESED versus cardiovascular mortality in proteinuric type 2 diabetes and nephropathy: results from the diametric database. Am J Kidney Dis. 2012;59(1):75–83. doi:10.1053/j.ajkd.2011.09.017
5. Tsai SF, Tarng DC. Anaemia in patients of diabetic kidney disease. J Chin Med Assoc. 2019;82(10):752–755. doi:10.1097/JCMA.0000000000000175
6. Thomas M, Tsalamandris C, MacIsaac R, Jerums G. Anaemia in diabetes: an emerging complication of microvascular disease. Curr Diabetes Rev. 2005;1:107–126. doi:10.2174/1573399052952587
7. Li Y, Yu Y, Brian L, et al. Anaemia and the risk of progression from non-proliferative diabetic retinopathy to vision threatening diabetic retinopathy. Eye. 2020;343:934–941. doi:10.1038/s41433-019-0617-6
8. Otani T, Kishi S, Maruyama Y. Patterns of diabetic macular edema with optical coherence tomography. Am J Ophthalmol. 1999;127(6):688–693. doi:10.1016/S0002-9394(99)00033-1
9. Das R, Spence G, Hogg RE, et al. Disorganization of inner retina and outer retinal morphology in diabetic macular edema. JAMA Ophthalmol. 2018;136(2):202–208. doi:10.1001/jamaophthalmol.2017.6256
10. Wells JA, Glassman AR, Ayala AR, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema: two-year results from a comparative effectiveness randomized clinical trial. Ophthalmology. 2016;123:1351–1359. doi:10.1016/j.ophtha.2016.02.022
11. Early Treatment Diabetic Retinopathy Study Research Group. Grading diabetic retinopathy from stereoscopic color fundus photographs-an extension of the modified airlie house classification. ETDRS report number 10. Ophthalmology. 1991;98(5):786–806. doi:10.1016/S0161-6420(13)38012-9
12. Levey AS, Steven LA. Estimating GFR using the CKD epidemiology collaboration (CKD-EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates and better risk predictions. Am J Kidney Dis. 2010;55(4):622–627. doi:10.1053/j.ajkd.2010.02.337
13. Horton WB, Barrett EJ. Microvascular dysfunction in diabetes mellitus and cardiometabolic disease. Endocr Rev. 2021;42(1):29–55. doi:10.1210/endrev/bnaa025
14. Nutritional anaemias. Report of a WHO scientific group. World Health Organ Tech Rep Ser. 1968;405:5–37.
15. Mohan VK, Nithyanandam S, Idiculla J. Microalbuminuria and low haemoglobin as risk factors for the occurrence and increasing severity of diabetic retinopathy. Ind J Ophthalmol. 2011;59:207–210.
16. Traveset A, Rubinat E, Ortega E, et al. Lower hemoglobin concentration is associated with retinal ischemia and the severity of diabetic retinopathy in type 2 diabetes. J Diabetes Res. 2016;2016:3674946. doi:10.1155/2016/3674946
17. Agarwal M, Sachdeva M, Shah S, et al. India retinal disease study group correlating the patterns of diabetic macular edema, optical coherence tomography biomarkers and grade of diabetic retinopathy with stage of renal disease. Int Ophthalmol. 2022;42(11):3333–3343. doi:10.1007/s10792-022-02332-3
18. Temkar S, Karuppaiah N, Takkar B, et al. Impact of estimated glomerular filtration rate on diabetic macular edema. Int Ophthalmol. 2018;38(3):1043–1050. doi:10.1007/s10792-017-0557-8
19. Zhang X, Hau X, Wang L, et al. Association of abnormal renal profiles with subretinal fluid in diabetic macular edema. J Ophthalmol. 2022;2022:1–5. doi:10.1155/2022/5581679
20. Nadri G, Saxena S, Stefanickova J, et al. Disorganization of retinal inner layers correlates with ellipsoid zone disruption and retinal nerve fiber layer thinning in diabetic retinopathy. J Diabetes Complications. 2019;33:550–553. doi:10.1016/j.jdiacomp.2019.05.006
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

