Back to Journals » ClinicoEconomics and Outcomes Research » Volume 17
Cost-Effectiveness Analysis of Ofatumumab versus Teriflunomide for Relapsing-Remitting Multiple Sclerosis: A 10-Year Markov Model
Authors Almalki ZS , Alshammari MM, Almazrou SH , Alqahtani OAA , Alkhayat MR, Alnemari SF, Mukhemair HS, Alkredeas SM, Alsuhibani AA , Asiri BY, Alalawi TN, Alahmari AK, Alotaibi FO
Received 30 October 2024
Accepted for publication 4 March 2025
Published 20 March 2025 Volume 2025:17 Pages 217—232
DOI https://doi.org/10.2147/CEOR.S503842
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Xing Lin Feng
Ziyad Saeed Almalki,1 Mashael Mafleh Alshammari,2 Saja H Almazrou,2 Ohud Abd Alhadi Alqahtani,3 Maryam Riyadh Alkhayat,3 Shahad Fahad Alnemari,3 Haya Showky Mukhemair,3 Sara Mohamaad Alkredeas,3 Abdulrahman A Alsuhibani,4 Bushra Yousif Asiri,3 Tala Nouraldin Alalawi,3 Abdullah K Alahmari,1 Fahad Obaid Alotaibi5
1Department of Clinical Pharmacy, College of Pharmacy, Prince Sattam bin Abdulaziz University, Al-Kharj, Riyadh, Saudi Arabia; 2Department of Clinical Pharmacy, College of Pharmacy, King Saud University, Riyadh, Saudi Arabia; 3Collage of Pharmacy, Almaarefa University, Riyadh, Saudi Arabia; 4Department of Pharmacy Practice, College of Pharmacy, Qassim University, Qassim, Saudi Arabia; 5Forensic Medical Services Center, Ministry of Health, Riyadh, Saudi Arabia
Correspondence: Ziyad Saeed Almalki, Department of Clinical Pharmacy, College of Pharmacy, Prince Sattam bin Abdulaziz University, Al-Kharj, Riyadh, Saudi Arabia, Tel +966-11 588 7315, Email [email protected]
Background and Objectives: Ofatumumab, a fully human anti-CD20 monoclonal antibody, is a promising disease-modifying therapy (DMT) for relapsing-remitting multiple sclerosis (RRMS). This study investigates its cost-effectiveness compared to teriflunomide from the perspective of Saudi healthcare payers. This comparison is crucial for informing treatment strategies and resource allocation in Saudi Arabia, where RRMS poses a significant healthcare burden and access to newer DMTs is evolving.
Patients and Methods: A Markov model was constructed to evaluate the long-term cost-effectiveness of ofatumumab compared to teriflunomide for treating RRMS in Saudi Arabia. This model simulates disease progression over 10 years, a timeframe chosen for its clinical relevance and consistency with similar studies. To reflect the Saudi patient population, the model uses a hypothetical cohort with characteristics mirroring those in the ASCLEPIOS I/II clinical trials. The model incorporates transition probabilities between disease states, primarily derived from the British Columbia MS (BCMS) database and further refined using data from the ASCLEPIOS trials. To ensure relevance to the Saudi context, local data sources were utilized, including drug costs from the Saudi Food and Drug Authority (SFDA) and health state costs from published local studies. Clinical expert input was incorporated to validate model assumptions.The primary outcome measure was the incremental cost per quality-adjusted life-year (QALY) gained. Sensitivity analyses were conducted to assess the robustness of the model findings.
Results: Compared to teriflunomide, ofatumumab yielded incremental cost-effectiveness ratios (ICERs) of $46,188 per QALY over the 10-year period. Ofatumumab demonstrated a greater impact on reducing disability progression, particularly in the early stages of the disease. At a willingness-to-pay (WTP) threshold of $99,120 per QALY, ofatumumab demonstrated a 99.14% probability of cost-effectiveness in probabilistic sensitivity analyses.
Conclusion: This cost-effectiveness analysis demonstrates that ofatumumab is a cost-effective treatment for RRMS in Saudi Arabia, with an ICER below the WTP. Policymakers should consider including ofatumumab in national formularies and prioritize its use in early-stage RRMS to maximize patient benefit and cost-effectiveness.
Keywords: cost-effectiveness analysis, ofatumumab, teriflunomide, relapsing-remitting multiple sclerosis, expanded disability status scale, ASCLEPIOS clinical trials, Saudi Arabia, disease-modifying therapy
Introduction
Multiple sclerosis (MS) is a significant global health challenge that affects an estimated 2.8 million individuals worldwide.1 A 2020 meta-analysis revealed that the yearly occurrence of MS increased by 2.3% between 1985 and 2018.2 MS is a chronic and unpredictable neuroinflammatory disease that leads to irreversible physical and neurological impairments. Relapsing-remitting multiple sclerosis (RRMS), the most prevalent form of MS is characterized by sporadic relapses or a deterioration of neurological symptoms. Patients with RRMS have intermittent relapses that are followed by periods of complete or partial recovery.3
The projected prevalence rate of MS among the member nations of the Arabian Gulf Cooperation Council (GCC) exceeds 30 cases per 100,000 individuals. A registry-based study in Saudi Arabia indicated a concerning rise in MS cases, with a prevalence of 40.40 per 100,000 individuals across 20 hospitals.4 Fifty percent of MS Caregivers report moderate to severe burden.5 This increase not only affects individuals and caregivers but also places significant pressure on global healthcare systems. In 2020, the average annual expenditure per MS patient in Saudi Arabia was reported to be $15,582. However, this figure did not cover indirect costs.6 According to a systematic review of the costs of illness studies in Europe, increased disease severity is associated with higher costs. Patients with mild disability incur annual costs of $25,677, while those with severe disability face costs averaging $64,567 per year. This severe disability group accounts for nearly 49% of the total disease-related expenses.7
The treatment landscape of RRMS has evolved significantly, with a growing number of disease-modifying therapies (DMTs) offering diverse options. Among these, teriflunomide, an oral immunomodulatory agent, has proven valuable in reducing relapse rates and slowing disability progression, as demonstrated in clinical trials.8 While not always a first-line therapy, its oral administration and manageable safety profile make it suitable for certain individuals.9 However, the emergence of newer DMTs like ofatumumab, a fully human anti-CD20 monoclonal antibody, necessitates careful evaluation of their comparative effectiveness and cost-effectiveness.10 Ofatumumab has shown significant efficacy in clinical trials, notably reducing annualized relapse rates and delaying confirmed disability progression compared with other DMTs, including teriflunomide.11,12 The introduction of any new therapy, especially one with superior efficacy, necessitates a thorough cost-effectiveness evaluation, considering the range of available DMTs with varying efficacy, safety, and cost profiles. This is crucial when comparing new therapies to established treatments like teriflunomide, enabling informed decision-making by clinicians and payers.
While previous studies, such as those conducted in Canada and Spain, have demonstrated the cost-effectiveness of ofatumumab compared to other DMTs for RRMS, cost-effectiveness analyses are inherently context-specific.12–15 Factors such as the healthcare system structure, drug pricing, and local epidemiology can significantly influence the results.16 Therefore, this study aimed to evaluate the cost-effectiveness of ofatumumab compared to teriflunomide for the treatment of RRMS from the perspective of Saudi healthcare payers. Specifically, the objectives were to: 1) estimate the incremental cost-effectiveness ratio (ICER) of ofatumumab compared to teriflunomide; 2) assess the probability of ofatumumab being cost-effective at a range of willingness-to-pay (WTP) thresholds relevant to the Saudi context; and 3) identify the key drivers of cost-effectiveness through sensitivity analyses. This analysis addresses a significant gap in local cost-effectiveness data for RRMS treatments in Saudi Arabia, providing crucial information for informed decision-making regarding the adoption and reimbursement of these therapies. The findings will inform policymakers, clinicians, and stakeholders, ultimately contributing to improved management of RRMS in Saudi Arabia.
Materials and Methods
Study Population and Interventions
The patient cohort investigated in this cost-effectiveness analysis was a hypothetical cohort designed to reflect the baseline characteristics of patients enrolled in the ASCLEPIOS I and II clinical trials. These two Phase III, double-blind, double-dummy trials compared subcutaneous ofatumumab (20 mg every 4 weeks after loading doses of 20 mg on days 1, 7, and 14) with oral teriflunomide (14 mg daily) for a duration of up to 30 months in patients with RRMS. The primary outcomes were the annualized relapse rate and the time to confirmed disability progression. ASCLEPIOS I enrolled 927 patients, with 463 assigned to ofatumumab and 464 to teriflunomide. ASCLEPIOS II enrolled 882 patients, with 441 assigned to ofatumumab and 441 to teriflunomide, for a combined total of 1809 patients (904 ofatumumab, 905 teriflunomide). The hypothetical cohort size was determined based on the sample sizes of the ASCLEPIOS trials, ensuring that the modeled patient population was representative of the clinical trial participants. This approach allowed for the alignment of the baseline characteristics and outcome measures, thereby enhancing the accuracy of the cost-effectiveness analysis. Table 1 indicates the baseline characteristics of the modeled patient cohort, which were consistent with those reported in the ASCLEPIOS trials. Additional details regarding the ASCLEPIOS trials have been previously published.17
 |
Table 1 Baseline Characteristics of the Modeled Cohort, Mirroring the ASCLEPIOS Trial Population. |
Markov Model Structure
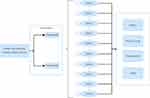
For the cost-effectiveness analysis, we constructed a Markov model to simulate the progression of MS and ran it over a time horizon of 10 years from a Saudi payer perspective. This model aimed to capture the clinical pathways and health outcomes of a hypothetical cohort of individuals diagnosed with RRMS. Patients with RRMS who were actively ill were allocated to the baseline Expanded Disability Status Scale (EDSS) state distributions at the time of model entry, according to a pooled analysis of the ASCLEPIOS trials.17 The patients in the model transitioned from EDSS states 0 (normal neurological examination) to 9 and 10 (death state). In every cycle, patients had the option to either remain in their present EDSS state or progress to a higher or lower EDSS state (ie, improve or deteriorate, respectively) (Figure 1). The natural history transition probabilities between the EDSS states were obtained from the BCMS database, an internationally recognized reference used in multiple cost-effectiveness studies (Table 2).18 Mortality was modeled in accordance with the subsequent discussion, as EDSS 10 (death) was excluded from the EDSS scale. The current study’s extrapolation of disability progression is consistent with numerous published cost-effectiveness analyses and health technology assessment submissions.18–21 During treatment, patients may encounter several outcomes such as relapse, adverse events, discontinuation of therapy, or death. Relapses were classified by severity status derived from a prospective cohort of individuals with RRMS, categorizing them as mild, moderate, or severe.22
 |
Table 2 EDSS Transition Rates |
Model Inputs
The cost-effectiveness model employed a comprehensive approach, integrating clinical inputs, such as transition probabilities between EDSSs, relapse rates, mortality rates, and treatment-specific factors, including comparative efficacy, adverse events, and discontinuation probabilities. To ensure the accuracy and relevance of our cost-effectiveness analysis within the Saudi Arabian healthcare system, we incorporated several key economic factors. This included direct medical costs associated with each treatment strategy, such as drug acquisition, administration, and monitoring, as well as health state utilities. Furthermore, we integrated Saudi-specific data, including direct medical costs and health state costs derived from local studies. Clinical experts provided valuable input to validate the model, particularly in estimating adverse event costs, ensuring the model accurately reflects the economic and clinical realities of managing RRMS in Saudi Arabia.
EDSS Health States
In alignment with established methods for the economic modeling of MS, this study derived transition probabilities by integrating efficacy data into the natural history of disability progression. This approach allows for a more realistic projection of the disease course under different treatment scenarios. Specifically, the model incorporated treatment effects on disability progression by utilizing the hazard ratio for time to 6-month confirmed disability progression (6-month CDP) and the relative risk for relapse rate, which were sourced from Hauser et al.17 For patients undergoing treatment, natural history data were modified using a treatment effect estimated by Samjoo et al.23 The efficacy of a variety of disease-modifying therapies on a 6-month CDP was compared to that of a placebo in their network meta-analysis (Table 3). Ofatumumab and teriflunomide’s annual discontinuation probabilities were obtained from Hauser et al, Inshasi et al, and Mauskopf et al Studies.17,24,25
 |
Table 3 Model Inputs: Treatment Efficacy, Discontinuation Rate, and Adverse Events |
Relapse Rate
Due to data limitations, a uniform relapse rate was assumed across all treatments and EDSS scores, using the Annual Natural History Relapse Rate by EDSS Health State (Table 4).26 While relapse rates may vary across EDSS stages, the available data does not allow for accurate modeling of this relationship. To avoid introducing bias, we opted for the more conservative assumption of uniform relapse rates, consistent with other cost-effectiveness models in similar contexts. A sensitivity analysis was performed to explore the potential impact of this assumption. Based on published data on relapse severity, three relapse levels were incorporated into the model: severe (requiring hospitalization, 21%), moderate (requiring an emergency department visit, 46%), and mild (requiring outpatient management only, 33%).22
 |
Table 4 Annual Natural History Relapse Rate and Mortality Multiplier by EDSS Health State |
Adverse Events and Mortality
Serious adverse events were defined according to the criteria used in the ASCLEPIOS trials, with serious infection identified as the most frequently reported serious adverse events. All-cause mortality rates, stratified by age and gender, were derived from national life tables.27 The standardized mortality ratio by EDSS state was modeled using data from Sadovnick et al and Pokorski, applying a mortality multiplier to both populations (Table 4). 28,29
Model Assumptions
The clinical and economic outcomes of treatment were evaluated by applying treatment effects to the model without any treatment switching. To clearly assess the potential benefits of each treatment, our model assumes that patients fully adhered to their prescribed treatment regimens. This assumption mirrors the controlled environment of the ASCLEPIOS trials, allowing us to compare the cost-effectiveness of ofatumumab and teriflunomide under ideal conditions. While real-world adherence may vary, potentially affecting the generalizability of these results, it’s important to remember that the ASCLEPIOS trials themselves were designed to evaluate treatment efficacy under conditions of high adherence. In accordance with the ASCLEPIOS trials, patients who discontinued ofatumumab/teriflunomide treatment either when they reached an EDSS of 7 or greater or during all-cause discontinuation. Based on clinician input, a ten-year time horizon was selected, assuming that treatment was typically discontinued after ten years. Clinical expert input was crucial for validating model assumptions, particularly regarding adverse event costs. We conducted semi-structured interviews with seven experienced neurologists in Saudi Arabia, using a consensus approach to incorporate their cost estimates. These estimates were validated by comparison with published data and through sensitivity analyses.
Costs
As this study adopted a payer perspective, only direct medical costs were included in the analysis (Table 5). All costs were transformed from Saudi Arabian Riyal (SAR) to 2024 US dollars (USD, $) using the Saudi consumer price index to adjust for inflation and a SAR/USD conversion factor of 0.266.30 The prescription cost, drug monitoring, and adverse event management costs constituted the majority of the total cost. It was presumed that these expenses would accrue during the time that patients were actively engaged in therapy. Treatment costs were based on wholesale acquisition cost (WAC) drug costs obtained from the Saudi Food and Drug Authority (SFDA) online drug database. This database provides a comprehensive list of approved medications in Saudi Arabia, including their WAC prices.31 Annual monitoring costs were applied for each cycle, which included liver profile tests, complete blood counts, and neurologist visits, based on cost data published in Bohlega et al, which provides detailed cost breakdowns for these procedures in the Saudi context.32 Each EDSS health state was designated annualized direct medical costs in accordance with previous local studies. Specifically, we utilized cost data from Alsaqa’aby et al to estimate the direct medical costs associated with each EDSS health state. This study provided a comprehensive analysis of resource utilization and costs for MS patients in Saudi Arabia.6,33 Relapses experienced were weighted by their severity, as discussed above, and the corresponding management costs by severity level obtained from the literature were applied. The costs associated with relapse management were primarily based on data from Alsaqa’aby et al6,33 In order to quantify the cost of severe adverse event management, the most common types of severe adverse events (severe infections) and their associated treatment costs were identified. The adverse event cost was based on expert opinion.
 |
Table 5 Model Inputs: Costs |
Utilities
Utility values represent the preference-weighted measure of health-related quality of life, usually expressed as quality-adjusted life-years (QALYs). Each Markov model’s health state has an associated utility value, generally derived from the published literature (Table 6). 32–34
 |
Table 6 Model Inputs: Patient Quality-of-Life Utilities and Disutilities |
Statistical Analysis
Transition probabilities were estimated by integrating natural history data from the BCMS database with treatment effects from the ASCLEPIOS trials. Hazard ratios for 6-month CDP and relative risks for relapse rates were used to adjust the natural history probabilities. These probabilities were then converted to annual rates to align with the model cycle length.
Equation 1 was employed to convert the probabilities to yearly rates (event per patient per year) in order to account for the annual model cycle length,
(Equation 1)
where r = rate; t = time in years; p = probability of an event occurring during time t.
These annual rates were then converted to annual probabilities using Equation 2,
(Equation 2)
where r = one-year rate; t = time in years; p = probability of an event occurring during time t.37–39
The incremental cost per QALY was used as a metric for incremental cost-effectiveness. In order to determine if treatments were cost-effective, they were evaluated using the World Health Organization’s 2001 guidelines and a WTP threshold of SAR 99,120 (almost three times the gross domestic product per capita, $33,040) per QALY gained.40
Sensitivity Analyses
Sensitivity assessments were performed using both deterministic and probabilistic methods. For each parameter, we ran a one-way deterministic sensitivity analysis. A probabilistic sensitivity analysis was conducted to address the uncertainty surrounding parameter estimates. Five thousand iterations were used in the simulation. This number of iterations was deemed sufficient to achieve convergence in the results, as demonstrated by visually inspecting trace plots and confirming the stabilization of the mean and standard deviation of key outcomes (ICER, cost, and QALYs). Probabilistic sensitivity analyses make use of the parameters and distributions shown in Tables 2–4. We used TreeAge Pro 2024, developed by TreeAge Software, Inc. (Williamstown, MA, USA), to perform all simulations.
Results
Table 7 presents the results of the cost-effectiveness analysis. Over a 10-year time horizon, the total direct cost was $150,826 for the ofatumumab group and $121,463 for the teriflunomide group. Ofatumumab was more effective than teriflunomide, resulting in an incremental gain of 0.64 QALYs. Specifically, the QALYs were calculated at 7.78 for the ofatumumab group and 7.14 for the teriflunomide group. From a Saudi payer perspective, ofatumumab was found to be cost-effective, with an ICER of $46,188 per QALY. This ICER is particularly relevant within the Saudi context, as it falls well below the commonly cited WTP threshold of $99,120 per QALY, which is approximately three times the Saudi Arabian GDP per capita.
 |
Table 7 Results of the Cost-Effectiveness Analysis |
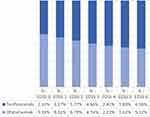
Figure 2 illustrates the distribution of the EDSS levels for both treatment groups at the end of the 10-year time horizon. Ofatumumab demonstrated a consistently higher proportion of patients with EDSS levels 0–3 than teriflunomide, suggesting a potential benefit in slowing disease progression. Notably, the magnitude of these differences, particularly the 2.15% higher proportion at EDSS 1 with ofatumumab, reflects the specific natural history data and treatment effects applied within the Saudi model.
Deterministic one-way sensitivity analyses (Table 8) confirmed the robustness of the base-case results across a range of parameter values. However, the results were most sensitive to variations in the cost of both treatments. This highlights the importance of accurate drug pricing and resource allocation within the Saudi healthcare system.
 |
Table 8 Results of Deterministic One-Way Sensitivity Analyses |
The probabilistic sensitivity analysis (Figure 3) showed that ofatumumab was cost-effective in 99.14% of the simulated iterations, given the Saudi WTP threshold. This high probability of cost-effectiveness reinforces the potential value of ofatumumab for Saudi payers. The cost-effectiveness acceptability curves (Figure 4) further illustrate this point, demonstrating the increasing likelihood of ofatumumab being the preferred strategy as the WTP threshold rises.
 |
Figure 3 Incremental cost-effectiveness scatter plot. |
 |
Figure 4 Cost-Effectiveness Acceptability Curve. |
Discussion
The Saudi healthcare system is committed to providing comprehensive and accessible treatment for individuals with MS. Universal healthcare coverage ensures all citizens have access to necessary medical services, including a range of DMTs and specialized care. However, the cost of treatment, particularly for newer DMTs, remains a concern. While the SFDA has approved medications like ofatumumab and teriflunomide, their reimbursement may be subject to specific criteria or require co-payments, potentially limiting access for some patients. This study investigates the cost-effectiveness of ofatumumab compared to teriflunomide from the perspective of Saudi healthcare payers. This economic evaluation is crucial for informing treatment strategies and resource allocation in Saudi Arabia, where relapsing-remitting MS (RRMS) constitutes a significant healthcare burden and access to newer DMTs is evolving. This study provides the first cost-effectiveness analysis of ofatumumab compared to teriflunomide specifically tailored to the Saudi Arabian healthcare context. While cost-effectiveness analyses of ofatumumab in other settings exist, their findings may not be directly applicable to Saudi Arabia due to variations in drug pricing, resource allocation, and standard clinical practices. This study addresses this gap by utilizing local cost data, incorporating clinical expert opinion specific to the Saudi context, and modeling a hypothetical cohort representative of the Saudi RRMS population.This cost-effectiveness analysis examined the economic implications of utilizing ofatumumab compared with teriflunomide for treating RRMS from a Saudi payer perspective. Our findings indicate that while ofatumumab is associated with higher direct costs over a 10-year time horizon than teriflunomide, it also yields an incremental gain of 0.64 QALYs, which is a cost-effective treatment option.
Our cost-effectiveness analysis indicates that ofatumumab is a promising treatment option for RRMS in Saudi Arabia. This aligns with previous research in other settings,13–16 though it’s important to acknowledge that cost-effectiveness can vary depending on healthcare system structures, drug pricing, and local epidemiology. The clinical benefits of ofatumumab, along with its favorable ICER, support its inclusion in reimbursement policies. Further research, including analyses of treatment sequences, is needed to determine its optimal place within the RRMS treatment landscape. However, treatment decisions should always be individualized based on patient characteristics, preferences, and potential side effects.
This finding regarding the distribution of EDSS levels after 10 years provides further support for the long-term benefits of ofatumumab, although it comes at a higher initial cost. The fact that a consistently higher proportion of patients on ofatumumab had lower EDSS levels (0–3) compared to teriflunomide suggests that the higher efficacy of ofatumumab likely translates to a delay in disability accumulation for a significant portion of patients. The observation that the largest difference in proportions was observed at EDSS 1 further suggests that ofatumumab might be particularly effective in the earlier stages of RRMS, potentially delaying the onset of significant disability. The observation that ofatumumab is associated with a more favorable distribution of EDSS levels after 10 years, with more patients remaining in the lower disability categories than teriflunomide, aligns with previous research on high-efficacy DMTs in several ways.41 Freeman et al found that patients treated with high-efficacy DMTs, especially B-cell targeting therapies like ofatumumab, experienced slower disability progression compared to those on less effective treatments.42 Hauser et al found that ofatumumab effectively reduced disability progression in patients with RRMS.17
We employed deterministic one-way sensitivity analyses to assess the robustness of our findings by examining the impact of varying individual model parameters. Of note, ofatumumab remained the dominant strategy (more effective and less costly) across all tested parameter ranges, indicating that our base-case conclusion—that ofatumumab is more cost-effective than teriflunomide—is robust. However, the analysis revealed notable sensitivity to variations in the costs of both treatments. This suggests that the relative cost-effectiveness of ofatumumab is particularly dependent on its price compared to teriflunomide, which could highlight the need for ongoing monitoring of drug prices and careful consideration of pricing strategies to ensure the long-term affordability and accessibility of ofatumumab in RRMS patients.
Our probabilistic sensitivity analysis strongly suggests that ofatumumab, although more expensive than teriflunomide, is highly likely to be a cost-effective treatment for RRMS in the Saudi Arabian context. This conclusion stems from the finding that 99.14% of the simulated scenarios were positioned in the more effective, albeit costlier, quadrant. Furthermore, the cost-effectiveness acceptability curve demonstrated a 99.14% probability of ofatumumab achieving cost-effectiveness at a WTP threshold of SAR 99,120 per QALY gained.
These findings are significant considering the intricacies of the Saudi Arabian healthcare landscape. First, Saudi Vision 2030 emphasizes both enhanced healthcare quality and efficiency. Our findings suggest that ofatumumab aligns with this vision by potentially improving patient outcomes while remaining economically justifiable. This is particularly relevant given the rising prevalence of non-communicable diseases, such as RRMS, which pose a growing burden on the healthcare system. Second, as a key player in pharmaceutical management, the National Unified Procurement Company can leverage its findings to inform drug procurement, formulary development, and reimbursement policies. The robust cost-effectiveness evidence presented strengthens the inclusion of ofatumumab in national and institutional formularies.
Limitations and Future Directions
The present cost-effectiveness analysis, which was strengthened by its reliance on head-to-head clinical trial data, is subject to several limitations inherent to this methodological approach. First, our analysis focused on direct medical costs from a payer perspective, but it did not capture the full economic burden of RRMS.43 Future research should incorporate indirect costs, such as productivity losses and informal caregiving costs, to provide a more comprehensive societal perspective on the economic impact of MS and its treatment. Second, the study relies on average cost data sourced from the published literature. However, this approach may not accurately reflect the actual costs incurred in specific clinical settings. Variations in resource utilization, treatment patterns, and local pricing agreements can significantly influence actual costs.44 Future research should endeavor to collect primary cost data specific to the Saudi Arabian healthcare context to enhance the accuracy and relevance of the findings. Third, it is important to acknowledge that our study population, based on the ASCLEPIOS trials, may not fully represent the real-world distribution of EDSS scores in MS cohorts. The significant difference in proportions observed at EDSS 1 may be more applicable to patients with lower EDSS scores. Further research is needed to evaluate the cost-effectiveness of ofatumumab in broader MS populations with a wider range of EDSS scores, including those with higher levels of disability. Fourth, the model assumes a cost constancy over a defined time horizon. However, healthcare costs, particularly pharmaceutical prices, fluctuate due to inflation, market dynamics, and policy changes.45 While this limitation was partially addressed through sensitivity analyses in the present study, future research should explore more dynamic modeling approaches to capture the real-world variability of healthcare costs better. Fifth, the study utilized utility values derived from published literature, which may not fully encapsulate the preferences and health-related quality of life experienced by the Saudi Arabian population with MS. While sensitivity analyses have explored the impact of varying utilities, future research should prioritize the elicitation of utility values directly from the target population to enhance the cultural sensitivity and generalizability of the findings. Finally, this analysis employed a 10-year time horizon, which is clinically relevant as it captures a significant period of disease progression and treatment impact in RRMS. This timeframe also aligns with common practice in cost-effectiveness analyses of MS treatments, enabling comparisons with existing literature. While acknowledging that long-term cost-effectiveness may differ, this 10-year horizon balances relevance with feasibility, minimizing the uncertainty associated with extrapolating data over extended periods. Our model can be adapted for longer durations by incorporating updated natural history data, extrapolating transition probabilities (with careful consideration of potential changes in treatment patterns and disease progression), and addressing uncertainty through sensitivity analyses. Future research should explore longer time horizons to provide a more comprehensive assessment of the long-term value of different treatment strategies in the Saudi Arabian context.
Conclusion
These findings have important implications for healthcare policy in Saudi Arabia. Given its demonstrated cost-effectiveness, policymakers should consider including ofatumumab in national and institutional formularies for RRMS and align reimbursement policies to ensure patient access. The National Unified Procurement Company and other relevant authorities should leverage these findings in price negotiations with pharmaceutical companies to ensure the long-term affordability and accessibility of ofatumumab for RRMS patients. Furthermore, as our analysis suggests that ofatumumab may be particularly effective in the earlier stages of RRMS, policymakers could consider prioritizing access for patients with early-stage disease to potentially delay the onset of significant disability. Continuous monitoring and evaluation of real-world treatment patterns, costs, and patient outcomes are essential to assess the long-term impact of ofatumumab on the healthcare system and to inform future policy adjustments. This comprehensive approach, integrating clinical evidence with economic considerations, can contribute to a more robust and sustainable healthcare system in Saudi Arabia, particularly in the context of rising healthcare costs and the increasing prevalence of chronic diseases like RRMS.
Acknowledgments
This study is supported via funding from Prince Sattam bin Abdulaziz University project number (PSAU/2024/R/1446).
Funding
This study is supported via funding from Prince Sattam bin Abdulaziz University project number (PSAU/2024/R/1446).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Walton C, King R, Rechtman L, et al. Rising prevalence of multiple sclerosis worldwide: insights from THE Atlas of MS, third edition. Mult Scler. 2020;26(14):1816. doi:10.1177/1352458520970841
2. Etemadifar M, Nikanpour Y, Neshatfar A, Mansourian M, Fitzgerald S. Incidence and prevalence of multiple sclerosis in persian gulf area: a systematic review and meta-analysis. Mult Scler Relat Disord. 2020;40:101959. doi:10.1016/J.MSARD.2020.101959
3. Tafti D, Ehsan M, Xixis KL. Multiple Sclerosis. StatPearls.
4. Aljumah M, Bunyan R, Al Otaibi H, et al. Rising prevalence of multiple sclerosis in Saudi Arabia, a descriptive study. BMC Neurol. 2020;20(1):1–7. doi:10.1186/S12883-020-1629-3/TABLES/3
5. Algahtani H, Shirah B, Bayazeed A, et al. Assessment of the burden of multiple sclerosis patients’ caregivers in Saudi Arabia. Cureus. 2020;12(1). doi:10.7759/cureus.6658.
6. Economic burden of multiple sclerosis in Saudi Arabia – 13TH COLLEGE OF PHARMACY RESEARCH DAY. Available from:. https://coprd-ksu.com/economic-burden-of-multiple-sclerosis-in-saudi-arabia/.
7. Paz-Zulueta M, Parás-Bravo P, Cantarero-Prieto D, Blázquez-Fernández C, Oterino-Durán A. A literature review of cost-of-illness studies on the economic burden of multiple sclerosis. Mult Scler Relat Disord. 2020;43:102162. doi:10.1016/j.msard.2020.102162
8. Miller AE. Teriflunomide for the treatment of relapsing–remitting multiple sclerosis. Expert Rev Clin Immunol. 2015;11(2):181–194. doi:10.1586/1744666X.2015.993611
9. O’Connor P, Comi G, Freedman MS, et al. Long-term safety and efficacy of teriflunomide: nine-year follow-up of the randomized TEMSO study. Neurology. 2016;86(10):920. doi:10.1212/WNL.0000000000002441
10. Hauser SL, Kappos L, Bar-Or A, et al. The development of ofatumumab, a fully human anti-CD20 Monoclonal antibody for practical use in relapsing multiple sclerosis treatment. Neurol Ther. 2023;12(5):1491–1515. doi:10.1007/S40120-023-00518-0
11. Samjoo IA, Worthington E, Drudge C, et al. Comparison of ofatumumab and other disease-modifying therapies for relapsing multiple sclerosis: a network meta-analysis. J Comp Eff Res. 2020;9(18):1255–1274. doi:10.2217/cer-2020-0122
12. Gärtner J, Hauser SL, Bar-Or A, et al. Efficacy and safety of ofatumumab in recently diagnosed, treatment-naive patients with multiple sclerosis: results from ASCLEPIOS I andII. Mult Scler. 2022;28(10):1562. doi:10.1177/13524585221078825
13. Baharnoori M, Bhan V, Clift F, et al. Cost-effectiveness analysis of ofatumumab for the treatment of relapsing-remitting multiple sclerosis in Canada. PharmacoEconomics Open. 2022;6(6):859. doi:10.1007/S41669-022-00363-1
14. Bhan V, Clift F, Baharnoori M, et al. Cost–consequence analysis of ofatumumab for the treatment of relapsing-remitting multiple sclerosis in Canada. J Comp Eff Res. 2023;12(9). doi:10.57264/CER-2022-0175/SUPPL_FILE/SUPPLEMENTARY
15. Vudumula U, Patidar M, Gudala K, Karpf E, Adlard N. Evaluating the impact of early vs delayed ofatumumab initiation and estimating the long-term outcomes of ofatumumab vs teriflunomide in relapsing multiple sclerosis patients in Spain. J Med Econ. 2023;26(1):11–18. doi:10.1080/13696998.2022.2151270
16. Hutubessy R, Chisholm D, Tan-Torres Edejer T, et al. Generalized cost-effectiveness analysis for national-level priority-setting in the health sector. Cost Eff Resour Alloc. 2003;1:8. doi:10.1186/1478-7547-1-8
17. Hauser SL, Bar-Or A, Cohen JA, et al. Ofatumumab versus Teriflunomide in Multiple Sclerosis. N Engl J Med. 2020;383(6):546–557. doi:10.1056/NEJMOA1917246
18. Palace J, Bregenzer T, Tremlett H, et al. UK multiple sclerosis risk-sharing scheme: a new natural history dataset and an improved Markov model. BMJ Open. 2014;4(1):e004073. doi:10.1136/BMJOPEN-2013-004073
19. Taheri S, Sahraian MA, Yousefi N. Cost-effectiveness of alemtuzumab and natalizumab for relapsing-remitting multiple sclerosis treatment in Iran: decision analysis based on an indirect comparison. J Med Econ. 2019;22(1):71–84. doi:10.1080/13696998.2018.1543189
20. Versteegh MM, Huygens SA, Wokke BWH, Smolders J. Effectiveness and cost-effectiveness of 360 disease-modifying treatment escalation sequences in multiple sclerosis. Value Health. 2022;25(6):984–991. doi:10.1016/J.JVAL.2021.11.1363
21. CADTH COMMON DRUG REVIEW Pharmacoeconomic Review Report.
22. Goldberg LD, Edwards NC, Fincher C, Doan QV, Al-Sabbagh A, Meletiche DM. Comparing the cost-effectiveness of disease-modifying drugs for the first-line treatment of relapsing-remitting multiple sclerosis. J Manag Care Pharm. 2009;15(7):543–555. doi:10.18553/JMCP.2009.15.7.543
23. Samjoo IA, Drudge C, Walsh S, et al. Comparative efficacy of therapies for relapsing multiple sclerosis: a systematic review and network meta-analysis. J Comp Eff Res. 2023;12(7). doi:10.57264/CER-2023-0016
24. Inshasi JS, Almadani A, Al Fahad S, et al. High-efficacy therapies for relapsing-remitting multiple sclerosis: implications for adherence. An expert opinion from the United Arab Emirates. Neurodegener Dis Manag. 2020;10(4):257–266. doi:10.2217/NMT-2020-0016
25. Mauskopf JA, Paul JE, Grant DM, Stergachis A. The role of cost-consequence analysis in healthcare decision-making. Pharmacoeconomics. 1998;13(3):277–288. doi:10.2165/00019053-199813030-00002
26. Weinshenker BG. Natural history of multiple sclerosis. Ann Neurol. 1994;36:S6–S11. doi:10.1002/ANA.410360704
27. Life tables: life tables by country Saudi Arabia. Available from:. https://apps.who.int/gho/data/view.searo.61440?lang=en.
28. Sadovnick AD, Ebers GC, Wilson RW, Paty DW. Life expectancy in patients attending multiple sclerosis clinics. Neurology. 1992;42(5):991–994. doi:10.1212/WNL.42.5.991
29. Pokorski RJ. Long-term survival experience of patients with multiple sclerosis. J Insur Med. 1997;29(2):101–106.
30. Consumer Price Index | General Authority for Statistics. Available from:. https://www.stats.gov.sa/en/394.
31. drugs list | Saudi Food and Drug Authority. Available from:. https://www.sfda.gov.sa/en/drugs-list?TradeName=&ScientificName=Risankizumab&Agent=&ManufacturerName=&RegNo=&pg=1.
32. Bohlega S, Elboghdady A, Al-Johani A, et al. Economic evaluation of cladribine tablets in patients with high disease activity-relapsing-remitting multiple sclerosis in the Kingdom of Saudi Arabia. Value Heal Reg Issues. 2021;25:189–195. doi:10.1016/J.VHRI.2021.03.007
33. Alsaqa’aby MF, Vaidya V, Khreis N, Al Khairallah T, Al-Jedai AH. Cost-effectiveness of oral agents in relapsing-remitting multiple sclerosis compared to interferon-based therapy in Saudi Arabia. Ann Saudi Med. 2017;37(6):433–443. doi:10.5144/0256-4947.2017.433
34. Guertin JR, Feeny D, Tarride JE. Age- and sex-specific Canadian utility norms, based on the 2013-2014 Canadian Community Health Survey. CMAJ. 2018;190(6):E155–E161. doi:10.1503/CMAJ.170317
35. Tappenden P, McCabe C, Chilcott J, et al. Cost-effectiveness of disease-modifying therapies in the management of multiple sclerosis for the Medicare population. Value Health. 2009;12(5):657–665. doi:10.1111/J.1524-4733.2008.00485.X
36. Prosser LA, Kuntz KM, Bar-Or A, Weinstein MC. Cost-effectiveness of interferon beta-1a, interferon beta-1b, and glatiramer acetate in newly diagnosed non-primary progressive multiple sclerosis. Value Health. 2004;7(5):554–568. doi:10.1111/J.1524-4733.2004.75007.X
37. Edlin R, McCabe C, Hulme C, Hall P, Wright J. Cost effectiveness modelling for health technology assessment. cost eff model heal technol assess. doi:10.1007/978-3-319-15744-3
38. Decision modelling for health economic evaluation - Andrew Briggs, Mark Sculpher, Karl Claxton - Oxford University Press. Available from:. https://global.oup.com/academic/product/decision-modelling-for-health-economic-evaluation-9780198526629?cc=sa&lang=en&.
39. Fleurence RL, Hollenbeak CS. Rates and probabilities in economic modelling: transformation, translation and appropriate application. Pharmacoeconomics. 2007;25(1):3–6. doi:10.2165/00019053-200725010-00002
40. Robinson LA, Hammitt JK, Chang AY, Resch S. Understanding and improving the one and three times GDP per capita cost-effectiveness thresholds. Health Policy Plan. 2017;32(1):141–145. doi:10.1093/HEAPOL/CZW096
41. Mouallif S, Kim T, Adlard N, et al. Cost effectiveness of ofatumumab in comparison with other disease modifying therapies and best supportive care for the treatment of relapsing-remitting multiple sclerosis in Canada (P1-1.Virtual). Neurology. 2022;98(18_supplement). doi:10.1212/WNL.98.18_SUPPLEMENT.3753
42. Freeman L, Longbrake EE, Coyle PK, Hendin B, Vollmer T. High-efficacy therapies for treatment-naïve individuals with relapsing–remitting multiple sclerosis. CNS Drugs. 2022;36(12):1285. doi:10.1007/S40263-022-00965-7
43. Sarhan AA, El-Sharkawy KA, Mahmoudy AM, Hashim NA. Burden of multiple sclerosis: impact on the patient, family and society. Mult Scler Relat Disord. 2022;63. doi:10.1016/J.MSARD.2022.103864
44. Shankaran V, Chennupati S, Sanchez H, et al. Clinical characteristics, treatment patterns, and healthcare costs and utilization for hepatocellular carcinoma (HCC) patients treated at a large referral center in Washington State 2007-2018. J Hepatocell Carcinoma. 2021;8:1597–1606. doi:10.2147/JHC.S328274
45. Movahed MS, Rezapour A, Vahedi S, et al. The impact of inflation and its uncertainty on pharmaceutical prices: evidence from Iran. Iran J Pharm Res IJPR. 2021;20(3):94–101. doi:10.22037/IJPR.2020.114071.14646
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.