Back to Journals » Journal of Inflammation Research » Volume 17
Crescents as Independent Risk Factor in the Progression of Primary Membranous Nephropathy
Authors Li SM, Yang LW, Huang ZQ, Ma JY, Wu JH, Liu HF, Xu YZ, Luo MN
Received 25 September 2024
Accepted for publication 28 November 2024
Published 10 December 2024 Volume 2024:17 Pages 10871—10885
DOI https://doi.org/10.2147/JIR.S497939
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tara Strutt
Shang-Mei Li,1,* La-Wei Yang,2,* Zhi-Qing Huang,1 Jia-Ying Ma,1 Jiao-Hua Wu,1 Hua-Feng Liu,1,2 Yong-Zhi Xu,1,2 Mian-Na Luo1,2
1Department of Nephrology, Affiliated Hospital of Guangdong Medical University, Zhanjiang, Guangdong Province, 524001, People’s Republic of China; 2Guangdong Provincial Key Laboratory of Autophagy and Major Chronic Non-Communicable Diseases, Affiliated Hospital of Guangdong Medical University, Zhanjiang, Guangdong Province, 524001, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Mian-Na Luo; Yong-Zhi Xu, Department of Nephrology, Affiliated Hospital of Guangdong Medical University, No. 57, Renmin Avenue South, Xiashan District, Zhanjiang, Guangdong Province, 524001, People’s Republic of China, Tel +86 13763015687, Email [email protected]; [email protected]
Objective: The role of crescent formation in primary membranous nephropathy (PMN) and its potential impact on prognosis remain an area of ongoing investigation. This study stratifies patients with PMN into two cohorts: one with crescents and one without. It then compares these groups to investigate the influence of crescents on the prognosis of PMN.
Methods: In this retrospective analysis, we included patients who had a confirmed diagnosis of PMN and exhibited crescents upon renal biopsy. The study population was sourced from the medical records at the Affiliated Hospital of Guangdong Medical University in Zhanjiang, China, spanning from January 2017 to June 2023. To enable a comparative analysis of clinical, pathological, and prognostic features, a control group was established, comprising 106 patients diagnosed with PMN who did not have crescent formation. These controls were randomly selected from the same time frame. Regular follow-up of the patients continued in the outpatient setting for at least six months.
Results: A total of 53 patients with PMN and crescent formation were included in this study, while 106 individuals without crescents served as a randomly selected control group. Patients with PMN and crescents exhibited higher systolic blood pressure (P = 0.015), 24-hour proteinuria (P = 0.006), serum creatinine (P = 0.029) levels, and lower glomerular filtration rate (P = 0.002), compared to those without crescents. Histological examination revealed a higher proportion of focal segmental sclerosis (P < 0.001), spherical sclerosis (P < 0.001), arteriosclerosis (P = 0.02), and interstitial fibrosis with tubular necrosis (P = 0.002) in patients with PMN and crescent formation. Immunofluorescence staining demonstrated a weaker IgG4 fluorescence intensity in patients with PMN and crescent formation. At the end of the follow-up period, patients with PMN and crescents had a lower remission rate (P = 0.022), poorer renal function (P = 0.007), and lower albumin (P = 0.039) levels. Kaplan-Meier (KM) analysis identified proteinuria and crescent formation as independent risk factors for adverse outcomes in patients with PMN (P < 0.001 and P < 0.05). Immunohistochemistry staining revealed positive expression of CD68 and CD20 in the renal interstitium of patients with PMN, regardless of the presence of crescents.
Conclusion: Crescent formation is associated with a risk of adverse outcomes in patients with PMN. Patients with crescents exhibit severe clinical and pathological features and have poorer prognoses.
Keywords: crescents, primary membranous nephropathy, risk factor
Introduction
Primary membranous nephropathy (MN) is a prevalent glomerular disease in adults, particularly in China, with a rising incidence rate.1 In PMN patients who continue to have nephrotic syndrome, kidney failure develops in 40% - 50% over a period of 10 years.2 These patients are also at an increased risk of life-threatening thromboembolic and cardiovascular events.3 Serum creatinine levels and proteinuria are well-established biomarkers that have long been used to gauge the risk of progressive kidney disease, which is associated with heightened cardiovascular morbidity and mortality. These conditions can ultimately culminate in kidney failure.4 Characterized by significant proteinuria, MN’s etiology and pathogenesis remain elusive. While it can be secondary to autoimmune diseases, chronic infections, tumors, or drug exposure, its primary form is defined by deposition of immune complexes along the epithelial side of the glomerular capillary loop leading to diffuse basement membrane thickening. Notably, MN is associated with intrinsic glomerular cell proliferation or local inflammatory responses.5 The combination of membranous glomerular lesions with endothelial proliferation, loop necrosis, and crescent formation is often associated with secondary MN conditions like glomerular basement membrane disease (GBM) nephritis,6–10 vasculitis,11–13 and IgA nephropathy.14 However, our clinical experience along with observations of certain researchers has revealed a small subset of MN cases presenting with crescents. Despite extensive screening, these cases have shown no evidence of secondary factors or other underlying glomerular diseases.15 This has led to a need to better understand the role of crescents in PMN, their impact on prognosis, and the most effective treatment strategies.
Crescent formation is a well-established pathological feature of crescentic glomerulonephritis and is also observed in both primary and secondary kidney diseases, including IgA nephropathy and lupus nephritis.16–18 In recent years, rare crescentic glomerular diseases such as diabetic nephropathy have received increased attention, indicating crescent formation as an independent risk factor.19 Notably, crescent formation has also been observed in patients with PMN, with studies suggesting a poor prognosis.20 However, the majority of research on this topic has relied on case reports and small scale retrospective analysis.
We conducted a retrospective study of patients with PMN and crescent formation during hospitalization to investigate their clinical and pathological characteristics and their prognostic impact. After discharge, these patients were followed up regularly for at least six months in outpatient clinics. Through this study, we aim to remind clinicians of the need for aggressive management in PMN patients with crescents.
Methods
Data Collection
Patients with confirmed PMN diagnosis and presence of crescents as determined by renal biopsy, were included in this retrospective study. These patients were identified from medical records at the Affiliated Hospital of Guangdong Medical University (Zhanjiang, China) between January 2017 and June 2023. The following criteria were applied for patient inclusion: (1) patients who underwent renal biopsy; (2) pathological diagnosis: the biopsy confirmed a diagnosis of primary membranous nephropathy; (3) crescent formation was observed in the renal biopsy; (4) patients were regularly followed up in the outpatient clinic for at least 6 months or longer after discharge. All participants in the study were aged 18 years or older.
Exclusion criteria were as follows: (1) absence of autoimmune disease; (2) absence of a history of viral hepatitis; (3) absence of thyroid or other tumors; (4) absence of exposure to organic solvents or heavy metals; (5) absence of anti-GBM nephritis, systemic vasculitis, or IgA nephropathy. A control group consisting of 106 patients with PMN without crescent formation was randomly selected from the same time period to facilitate comparison of clinical, pathological, and prognostic characteristics.
All patients provided informed consent for their clinical data to be utilized in this study. The study was conducted in accordance with the principles outlined in Declaration of Helsinki and was approved by the institutional review board of the Affiliated Hospital of Guangdong Medical University (Approval Number: PJKT2022-028; Acceptance Number: KT2022-028-01).
Therapeutic Intervention
In line with previous studies, immunosuppressive regimens were tailored to individual patients based on histological lesions, proteinuria levels, and informed consent regarding potential side effects and medication costs.21,22 Patients were closely monitored in the outpatient department and necessary medication adjustments were made throughout the follow-up process. Patients with active lesions including cellular crescents and interstitial inflammatory cell infiltration received a combination therapy of prednisone plus cyclophosphamide (P+CTX), tripterygium wilfordii Hook F (TwHF), azathioprineor (AZA), or mycophenolate mofetil (MMF). Specific dosages and usage of these medications adhered to established guidelines.23
Efficacy Assessment
Treatment efficacy was evaluated using the 2012 Kidney Disease: Improving Global Outcomes (KDIGO) guidelines which defined complete remission as follows: (1) 24-h urine protein < 0.3 g, serum albumin > 35 g/L, and normal serum creatinine; (2) partial remission was characterized by 24-h urine protein quantification < 3.5 g and > 50% decrease, improved serum albumin levels, and stable creatinine level; and (3) lack of remission was indicated if these criteria were not met.
Follow-Up and Endpoints
Patients were followed up regularly from the time of renal biopsy until the occurrence of one of the following endpoint events: end stage renal disease (ESRD) [the glomerular filtration rate (GFR) < 15 mL/min/1.73 m2 or initiation of renal replacement therapy], death, or loss to follow-up. The study concluded on December 31, 2023, regardless of other endpoint events.
The primary composite outcome of interest was renal survival, defined as the time elapsed from renal biopsy to the occurrence of ESRD or death, aligning with previous studies.24 The chronic kidney disease epidemiology collaboration (CKD-EPI) formula was employed to estimate GFR.25
Renal Biopsies and Pathological Analysis
All patients underwent ultrasound guided renal biopsies. The biopsy specimens were subjected to the following histological and immunohistochemical stains: hematoxylin-eosin (HE) staining, periodic acid-Schiff metheramine (PASM) staining, Masson staining, immunofluorescent staining for IgA, IgM, IgG, C1q, fibrin, and complement C3. Additionally, immunofluorescent staining for IgG1, IgG2, IgG3, and IgG4 were also performed.
The deposition of immune complexes was semi-quantitatively graded by two renal pathologists blinded to the clinical information.23 Glomerular MN lesions were classified into four stages according to the Ehrenreich and Churg classification criteria.26 Tubular atrophy and interstitial fibrosis were graded semi-quantitatively from 1 to 3, where grade 1 represents an absence of tubular or interstitial lesions; grade 2 implies 5% to 25% of interstitial tissues are affected; grade 3 implies 25% to 50% of interstitial tissues are affected.
Immunochemistry
Renal biopsies were selected for immunohistochemical analysis to identify inflammatory cells and proliferative cells. The following markers were used: CD68 and CD20 (both from Dako). The experimental procedures followed our previously published methods.22
Statistical Analysis
Statistical analysis was conducted utilizing SPSS 26.0 software. Continuous variables were expressed as mean ± standard deviation (S.D). if normal distribution, as median (interquartile range) if non-normal distribution. Categorical variables were expressed as counts and percentages. Comparisons were based on the Wilcoxon rank-sum test, t-test, or chi-square (χ2) test. A P value < 0.05 was considered as a statistically significant difference. The Kaplan-Meier (KM) method was employed to calculate renal survival rates and the Log rank test was used to compare survival curves.
Results
Flow Chart of Patient Inclusion
As shown in Figure 1, a total of 597 patients diagnosed with PMN were hospitalized at the Affiliated Hospital of Guangdong Medical University between January 2017 and June 2023. Of these, a 106 were excluded based on the exclusion criteria, while a total of 491 patients were considered eligible for the study based on the inclusion criteria. Among these, 53 individuals (approximately 10.80%, 53/491) had crescent formation and were enrolled for the study. We randomly selected 106 patients without crescents from the remaining 438 patients with PMN for the control group. All patients were monitored for at least 6 months following their initial evaluation, until the conclusion of the study period (December 31, 2023) or until they reached an endpoint event.
 |
Figure 1 Flowchart of study participants. |
General Clinical Data
As shown in Table 1, there were no significant differences in age or gender between patients with and without crescents (P > 0.05). The age range was approximately between 50 and 55 years. Over half of the patients presented with nephrotic syndrome clinically. Patients with crescents exhibited significantly higher systolic blood pressure (P = 0.015) than those without crescents, while diastolic blood pressure remained comparable. Hemoglobin levels were similar between the two groups. Patients with crescents had a significantly higher 24-hour urinary protein levels [5.6 (IQR, 3.1–7.3) g/d vs 4.2 (IQR, 2.1–6.6) g/d, P < 0.01] than those without crescents. Biochemical results of patients with crescents demonstrated significantly higher serum creatinine levels [(77.0 (IQR, 56.5–93.5) µmol/L vs 62.0 (IQR, 48.7–80.0) µmol/L, P < 0.05], while GFR was significantly lower than in the non-crescentic nephropathy group [(94.4 (IQR, 70.7–108.6) mL/min/1.73m2 vs 108.7 (IQR, 94.9–121.3) mL/min/1.73m2, P < 0.01], both of which have statistically significant differences. No statistically significant differences were observed in the serum albumin, blood uric acid, triglycerides, or cholesterol levels. Therefore, patients with PMN and crescent formation exhibited severe clinical features, including high blood pressure and impaired renal function compared to those without crescents.
 |
Table 1 Basline Clinical Features of Patients with Biopsy-Proven PMN, Stratified by Crescents |
Pathological Data
As shown in Figure 2A, approximately 84.2% of patients with PMN and crescents have a ratio of less than 25% between the number of crescents and the number of glomeruli. Only 15.8% patients had a higher prevalence of crescents. Further analysis of the characteristics of crescents revealed that cellular crescents accounted for 34.0%, cellular fibrotic crescents accounted for 32.1%, and a combination of both types accounted for 7.5% (Figure 2B).
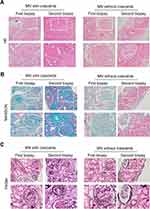
As shown in Table 2 and Figure 2C, comparatively, patients with PMN and crescents exhibited a higher prevalence of spherical sclerosis, focal segmental sclerosis, arteriosclerosis, interstitial fibrosis, and tubular atrophy in pathology (P < 0.05). There were no significant differences in mesangial cell proliferation or interstitial inflammatory cells infiltration. In terms of fluorescence staining, a weaker IgG4 fluorescence intensity was observed in patients with PMN and crescents compared to those without crescents (P < 0.01). Other fluorescence staining indicators such as IgG, IgA, IgM, C3, C1q, fiber, IgG1, IgG2, and IgG3 showed no significant statistical differences (Figure 3 and Table 2).
 |
Table 2 Comparative Analysis of the Pathological Characteristics of the Two Groups of Patients |
Follow-Up Outcomes
As shown in Table 3, at the conclusion of follow-up, an increase in serum creatinine level [77.0 (56.5–93.5) µmol/L vs 84.0 (69.0–103.5) µmol/L, P = 0.094] was observed in patients with crescents, while GFR levels decreased [94.4 (70.7–108.6) mL/min/1.73m2 vs 79.6 (59.9–101.1) mL/min/1.73m2, P < 0.01] at the initial visit. However the differences were not statistically significant. Notably, both groups showed a significant increase in serum albumin levels [23.8 (20.7–27.9) g/L vs 38 (26.3–43.2) g/L in patients with PMN and crescents, 26.0 (22.1–33) g/L vs 41.2 (36.0–45.7) g/L in patients with PMN and without crescents]. Both groups experienced a significant decrease in 24-hour urinary protein levels [5.6 (3.1–7.3) g/day vs 0.92 (0.13–4.1) g/day in patients with PMN and crescents, 4.2 (2.1–6.6) g/day vs 0.4 (0.1–1.3) g/day in patients with PMN and without crescents] compared to the initial diagnosis. The above differences were statistically significant.
 |
Table 3 Renal Outcomes of Patients of PMN with and without Crescents |
Notably, as shown in Figure 4, three patients underwent repeated renal biopsies during follow-up. One of them initially presented with crescents, while the other two had PMN without crescents. Repeated renal biopsies revealed chronic pathological changes in the patient with PMS and crescents, including glomerular segmental sclerosis, cellular crescent fibrosis, tubular atrophy, and interstitial inflammatory cell infiltration. Notably, a large accumulation of red blood cells was observed in the capillary lumen of all cases regardless of crescent presence.
As shown in Figure 5A, the KM curves demonstrated significantly worse renal survival in patients with PMN and crescents compared to those without crescents (P < 0.05). In addition, the renal survival rates were associated with proteinuria levels and serum albumin levels (<30 g/L) (Figure 5B, P < 0.001 and Figure 5D, P = 0.04). However, no significant differences in renal survival rates were observed between patients with severe interstitial fibrosis and tubular atrophy (IFTA) and the control group (Figure 5C).
Immunohistochemical Results of Inflammatory Cells
CD68 and CD20 were performed to observe the expression of inflammatory cells in patients with PMN and crescents. The results revealed that there were CD20+ (Figure 6A) and CD68+ (Figure 6B) cell infiltration in patients with PMN, both with and without crescents.
 |
Figure 6 Immunohistochemical staining of CD68 (A) and CD20 (B) (magnification, ×400). Note: All staining specimens in each group are from the same patient and the same glomerulus. |
Discussion
MN is a prevalent primary nephrotic syndrome frequently affecting middle-aged and elderly individuals, with a peak onset between 40 and 60 years of age. Consistent with these findings, the age range was approximately from 50 to 55 years. However, recent studies have highlighted a concerning trend towards onset of MN in younger individuals.27–29 International scholars have indicated that 30 to 40% of patients with nephrotic syndrome exhibit PMN, second only to focal segmental glomerulosclerosis (FSGS). Chinese data corroborates this, suggesting that 40% of individuals with nephrotic syndrome who are aged 45 years and older have MN.27 In this study over half of the patients with PMN exhibited nephrotic syndrome, regardless of crescent formation. This finding aligns with clinical practice, where many patients present with normal renal function at disease onset. While the majority of patients with PMN in this study had preserved renal function, those with crescents demonstrated a trend towards worse renal outcomes compared to those without crescents. Previous reports have indicated that 4–8% of patients with PMN may progress to renal insufficiency or even end-stage renal disease.5 The presence of crescents in renal pathology has been implicated as a potential risk factor for these adverse outcomes.30
As established in the study, hypertension, proteinuria, and elevated serum creatinine at the time of renal biopsy are associated with poor prognosis in patients with PMN.31 The findings of this study demonstrate that patients with PMN and crescents exhibited higher systolic blood pressure, proteinuria, and serum creatinine levels compared to those without crescents (Table 1). These indicators suggest a poorer prognosis for patients with PMN and crescents. Furthermore, the follow-up data revealed lower urinary protein remission rates and poorer renal function in patients with crescents (Table 3). The KM analysis also supported the view that patients with PMN and crescents have a poor prognosis, with statistically significant differences compared to those without crescents. These findings underscore the importance of heightened attention for patients with crescents as their prognosis may be more challenging. Standardized diagnosis, tailored treatment approaches, and extended follow-up education are crucial to optimize care for these patients. A limitation of this study was the relatively short follow-up duration for patients with crescents. This may be attributed to poorer treatment outcomes leading to patient disengagement or loss of confidence during the follow-up process. Despite this, both groups of patients demonstrated improvements in renal function characterized by decreased proteinuria and increased albumin levels after active treatment.
Analysis of pathological data at our center revealed that only 26.4% of patients with crescents exhibited fibrotic crescents suggesting a higher prevalence of cellular crescents. Furthermore, 84.2% of patients had a crescents proportion of less than 25%, which aligned with previous studies.32 These findings emphasize the importance of early intervention and treatment for patients with PMN with crescents to prevent the progression of cellular crescents to fibrosis or spherical sclerosis, which can lead to irreversible kidney damage. Repeated renal biopsies at our center demonstrated chronic lesions in patients with crescents, including glomerular segmental sclerosis, cellular crescent fibrosis, tubular atrophy, and interstitial inflammatory cell infiltration. These pathological features have been identified as risk factors for adverse renal outcomes.33 Repeated renal biopsy images revealed a notable accumulation of red blood cells in the glomerular capillary lumen in both patient groups. This observation underscores the importance of considering hypercoagulability in patients with PMN to mitigate the risk of thrombosis. Immunofluorescence analysis demonstrated a relatively weaker IgG4 fluorescence intensity in patients with PMN and crescents compared to those without crescents and the difference was statistically significant. However, other immunofluorescence markers did not exhibit significant variations between the two groups. These findings align with those reported by Japanese scholars.34
While the majority of immunofluorescence markers did not show significant differences between the two groups, our study revealed a higher expression of IgG2 in the crescent group compared to the non-crescent group. This finding suggests that the pathogenesis of patients with PMN may differ between these two groups, as IgG2 and IgG4 represent distinct immune pathways. Previous research has indicated that T cells undergo functional changes leading to increased production of Th2 cytokines which stimulate B cells to secrete IgG4.35 This pattern of immune response is characteristic of idiopathic membranous nephropathy.36
In particular, the pathogenesis of PMN is still being explored. How are crescents associated with PMN formed? The etiology is still unknown. Kanahara et al37 hypothesized that the deposition of in situ immune complexes in the basement membrane of PMN may result in the subsequent lysis of these immune complexes by lysosomes, which could lead to further damage to the glomerular basement membrane and eventually result in the destruction of the renal sacs and the formation of crescents. Some researchers11 believed that the appearance of these two pathological types in the same patient was not intrinsically linked, but rather just a coincidence. Current treatment options for PMN include steroids/cyclophosphamide, calcium protein inhibitors, and B cell depletion.5,38 However, the optimal treatment approach for PMN with crescents remains unclear. Immunohistochemistry was employed in this study to identify inflammatory cells revealing the expression of CD68 and CD20 positive cells in both groups. This suggests the involvement of these markers in PMN pathogenesis. The observation of CD68 and CD20 expression provides a rationale for exploring drugs targeting these pathways as potential therapeutic options to delay the progression of PMN with crescents.
Extensive research has implicated cell mediated immune mechanisms in the development of PMN, with macrophages playing a pivotal role.39,40 A previous study demonstrated a strong correlation between the degree of CD68+ interstitial macrophages infiltration during initial renal biopsy and outcome of PMN.39 Research within China has further revealed a negative correlation between CD68 expression and serum albumin levels, as well as a positive correlation with serum C3 and severity of glomerulosclerosis.41 In our study, we observed positive CD68 expression in patients with PMN regardless of crescent formation, aligning with results from existing studies. These findings suggest that the degree of interstitial macrophage infiltration in the tubules may be a crucial determinant of PMN outcomes.39,42 In recent years, the anti-CD20 monoclonal antibody rituximab has emerged as an alternative treatment option for PMN and other immune-mediated glomerular diseases.43,44 Notably, rituximab has been considered a first-line therapy for moderate and high-risk PMN.45 Our study observed CD20 expression in patients with PMN, both with and without crescents, suggesting that underlying mechanisms of PMN development may be similar in both groups. Despite the positive effects on albumin and urinary protein levels, follow-up data revealed a concerning trend of decreasing renal function in both groups of patients with PMN (Table 3). This highlights the need for ongoing efforts to develop more effective treatment strategies.
It is important to acknowledge the limitations present within this study. Firstly, the sample size was modest: the research was confined to a single-center retrospective analysis with a limited timeframe, which may introduce potential biases. Secondly, while PLAR2 is known to influence both diagnostic outcomes and prognosis, this paper concentrated solely on the role of crescents in the progression of PMN and did not incorporate PLAR2 into its analysis. Additionally, other relevant factors, including the duration of the disease and the presence of comorbidities in patients, such as diabetes, have not been taken into account in this study.
Conclusion
Patients with PMN and crescent formation exhibit severe clinical manifestations, including higher blood pressure, urinary protein excretion, and poorer renal function. Pathological examination frequently reveals increased glomerulosclerosis, renal tubulointerstitial fibrosis, and small vessel necrosis. These factors contribute to poorer therapeutic response and a worse prognosis. Crescent formation is a risk factor for the progression of PMN and necessitates active intervention. CD68 and CD20, which are involved in the occurrence and development of PMN, represent potential therapeutic targets for monoclonal antibody therapies that may delay disease progression. Further research is warranted to incorporate a broader spectrum of clinical indicators, pathological markers, and biomarkers, including PLAR2, CD68, and CD20, to gain a more holistic understanding of the critical factors that significantly influence the progression of PMN.
Abbreviations
PMN, primary membranous nephropathy; GBM, glomerular basement membrane disease; P+CTX, prednisone plus cyclophosphamide, TwHF, tripterygium wilfordii Hook F; AZA, azathioprineor, MMF, mycophenolate mofetil; KDIGO, Kidney Disease: Improving Global Outcomes; ESRD, end stage renal disease; GFR, the glomerular filtration rate; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; HE, Hematoxylin-eosin staining; PASM, Periodic acid-Schiff metheramine; IQR, inter-quartile range; IFTA, interstitial fibrosis and tubular atrophy; FSGS, focal segmental glomerulosclerosis; KM, Kaplan-Meier.
Data Sharing Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Approval and Consent to Participate
The study was approved by the ethical review committee of The Affiliated Hospital of Guangdong Medical University (Approval Number: PJKT2022-028; Acceptance Number: KT2022-028-01). Furthermore, the necessary details of the project were explained clearly to the patients and written informed consent was obtained in hospital. All participants were free to decide whether or not to participate or to withdraw at any time and for any reason without further penalty either personal or professional or affecting their future medical care.
Acknowledgments
The authors acknowledge the staff of the Institute of renal diseases, Affiliated Hospital of Guangdong Medical University.
Funding
This work was supported by the Zhanjiang City Program for tackling key problems in science and technology (No. 2021A05053), the Guangdong Medical Research Fund Project (A2023344), the National Health Commission Medical Science and Technology Development Research Center - General Research Program on Post-Marketing Clinical Studies of Innovative Drugs (WKZX2024CX401205), and the Department of Nephrology, National Clinical Key Specialty Construction Program (2023).
Disclosure
The authors declare that they have no conflict of interest regarding this work.
References
1. Tang L, Yao J, Kong X. et al. Increasing prevalence of membranous nephropathy in patients with primary glomerular diseases: a cross-sectional study in China. Nephrology. 2017;22(2):168–173. PMID: 26854278. doi:10.1111/nep.12739.
2. Troyanov S, Wall CA, Miller JA, Scholey JW, Cattran DC, Toronto Glomerulonephritis Registry Group. Idiopathic membranous nephropathy: definition and relevance of a partial remission. Kidney Int. 2004;66(3):1199–1205. PMID: 15327418. doi:10.1111/j.1523-1755.2004.00873.x
3. Fervenza FC, Sethi S, Specks U. Idiopathic membranous nephropathy: diagnosis and treatment. Clin J Am Soc Nephrol. 2008;3(3):905–919. PMID: 18235148. doi:10.2215/CJN.04321007.
4. Ronco P, Beck L, Debiec H, et al. Membranous nephropathy. Nat Rev Dis Primers. 2021;7(1):69. PMID: 34593809. doi:10.1038/s41572-021-00303-z.
5. Couser WG. Primary Membranous Nephropathy. Clin J Am Soc Nephrol. 2017;12(6):983–997. Erratum in: Clin J Am Soc Nephrol. 2017 Sep 7;12(9):1528. doi: 10.2215/CJN.07190717. PMID: 28550082; PMCID: PMC5460716.. doi:10.2215/CJN.11761116.
6. McAdoo SP, Pusey CD. Anti-Glomerular Basement Membrane Disease. Clin J Am Soc Nephrol. 2017;12(7):1162–1172. PMID: 28515156; PMCID: PMC5498345. doi:10.2215/CJN.01380217.
7. Basford AW, Lewis J, Dwyer JP, Fogo AB. Membranous nephropathy with crescents. J Am Soc Nephrol. 2011;22(10):1804–1808. PMID: 21903992. doi:10.1681/ASN.2010090923.
8. Kurki P, Helve T, von Bonsdorff M, et al. Transformation of membranous glomerulonephritis into crescentic glomerulonephritis with glomerular basement membrane antibodies. Serial determinations of anti-GBM before the transformation. Nephron. 1984;38(2):134–137. PMID: 6236378. doi:10.1159/000183294.
9. Moorthy AV, Zimmerman SW, Burkholder PM, Harrington AR. Association of crescentic glomerulonephritis with membranous glomerulonephropathy: a report of three cases. Clin Nephrol. 1976;6(1):319–325. PMID: 782751.
10. Thitiarchakul S, Lal SM, Luger A, Ross G. Goodpasture’s syndrome superimposed on membranous nephropathy. A case report. Int J Artif Organs. 1995;18(12):763–765. PMID: 8964642. doi:10.1177/039139889501801203
11. Nasr SH, Said SM, Valeri AM, et al. Membranous glomerulonephritis with ANCA-associated necrotizing and crescentic glomerulonephritis. Clin J Am Soc Nephrol. 2009;4(2):299–308. PMID: 19158367; PMCID: PMC2637583. doi:10.2215/CJN.04060808.
12. Morizane R, Konishi K, Hashiguchi A, et al. MPO-ANCA associated crescentic glomerulonephritis with numerous immune complexes: case report. BMC Nephrol. 2012;13(1):32. doi:10.1186/1471-2369-13-32 PMID: 22656245; PMCID: PMC3470990.
13. Shimada M, Fujita T, Nakamura N, et al. A case of myeloperoxidase anti-neutrophil cytoplasmic antibody (MPO-ANCA)-associated glomerulonephritis and concurrent membranous nephropathy. BMC Nephrol. 2013;14(1):73. doi:10.1186/1471-2369-14-73 PMID: 23537120; PMCID: PMC3616833.
14. Jennette JC, Newman WJ, Diaz-Buxo JA. Overlapping IgA and membranous nephropathy. Am J Clin Pathol. 1987;88(1):74–78. PMID: 3300266. doi:10.1093/ajcp/88.1.74.
15. Rodriguez EF, Nasr SH, Larsen CP, Sethi S, Fidler ME, Cornell LD. Membranous nephropathy with crescents: a series of 19 cases. Am J Kidney Dis. 2014;64(1):66–73. PMID: 24709471. doi:10.1053/j.ajkd.2014.02.018.
16. Wong MN, Tharaux PL, Grahammer F, Puelles VG. Parietal epithelial cell dysfunction in crescentic glomerulonephritis. Cell Tissue Res. 2021;385(2):345–354. PMID: 34453566; PMCID: PMC8523405. doi:10.1007/s00441-021-03513-9.
17. Trimarchi H, Barratt J, Cattran DC, et al. IgAN Classification Working Group of the International IgA Nephropathy Network and the Renal Pathology Society; Conference Participants. Oxford Classification of IgA nephropathy 2016: an update from the IgA Nephropathy Classification Working Group. Kidney Int. 2017;91(5):1014–1021. PMID: 28341274. doi:10.1016/j.kint.2017.02.003
18. Bajema IM, Wilhelmus S, Alpers CE, et al. Revision of the International Society of Nephrology/Renal Pathology Society classification for lupus nephritis: clarification of definitions, and modified National Institutes of Health activity and chronicity indices. Kidney Int. 2018;93(4):789–796. PMID: 29459092. doi:10.1016/j.kint.2017.11.023.
19. Sun L, Duan T, Zhao Q, et al. Crescents, an Independent Risk Factor for the Progression of Type 2 Diabetic Kidney Disease. J Clin Endocrinol Metab. 2022;107(10):2758–2768. PMID: 35914281. doi:10.1210/clinem/dgac416.
20. Pan Y, Liu L, Chen W, Yang H, Zhang J, Wang Y. Clinicopathological features and prognosis of primary membranous nephropathy in combination with crescent. Int Urol Nephrol. 2023;55(6):1523–1530. PMID: 36622536; PMCID: PMC10185626. doi:10.1007/s11255-022-03457-1.
21. Luo MN, Pan Q, Ye T, et al. Efficacy and safety of sequential immunosuppressive treatment for severe IgA nephropathy: a retrospective study. Front Pharmacol. 2023;14:1093442. PMID: 36998610; PMCID: PMC10043386. doi:10.3389/fphar.2023.1093442.
22. Luo MN, Yin Y, Li S, et al. Podocytes are likely the therapeutic target of IgA nephropathy with isolated hematuria: evidence from repeat renal biopsy. Front Pharmacol. 2023;14:1148553. PMID: 37089927; PMCID: PMC10119397. doi:10.3389/fphar.2023.1148553.
23. Luo MN, Yao CW, Xu BH, et al. Continuation of immunosuppressive treatment may be necessary in IgA nephropathy patients with remission of proteinuria: evaluation by repeat renal biopsy. Exp Ther Med. 2014;7(3):553–559. PMID: 24520244; PMCID: PMC3919854. doi:10.3892/etm.2013.1467.
24. Jiang S, Yu T, Zhang Z, et al. Prognostic nomogram and score to predict renal survival of patients with biopsy-proven diabetic nephropathy. Diabet Res Clin Pract. 2019;155:107809. PMID: 31401150. doi:10.1016/j.diabres.2019.107809.
25. Levey AS, Stevens LA, Schmid CH, et al. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. Erratum in: Ann Intern Med. 2011 Sep 20;155(6):408. PMID: 19414839; PMCID: PMC2763564.. doi:10.7326/0003-4819-150-9-200905050-00006.
26. Churg J, Ehrenreich T. Membranous nephropathy. Perspect Nephrol Hypertens. 1973;1(Pt 1):443–448. PMID: 4536436.
27. Zhou FD, Shen HY, Chen M, et al. The renal histopathological spectrum of patients with nephrotic syndrome: an analysis of 1523 patients in a single Chinese centre. Nephrol Dial Transplant. 2011;26(12):3993–3997. PMID: 21515637. doi:10.1093/ndt/gfr166.
28. Xie J, Chen N. Primary glomerulonephritis in mainland China: an overview. Contrib Nephrol. 2013;181:1–11. PMID: 23689562. doi:10.1159/000348642.
29. Zhu P, Zhou FD, Wang SX, Zhao MH, Wang HY. Increasing frequency of idiopathic membranous nephropathy in primary glomerular disease: a 10-year renal biopsy study from a single Chinese nephrology centre. Nephrology. 2015;20(8):560–566. PMID: 26086701. doi:10.1111/nep.12542.
30. Nayak SG, Satish R. Crescentic transformation in primary membranous glomerulopathy: association with anti-GBM antibody. Saudi J Kidney Dis Transpl. 2007;18(4):599–602. PMID: 17951950.
31. Wang R, Wang M, Xia Z, et al. Long-term renal survival and related risk factors for primary membranous nephropathy in Chinese children: a retrospective analysis of 217 cases. J Nephrol. 2021;34(2):589–596. PMID: 32770523. doi:10.1007/s40620-020-00816-y.
32. Wang J, Zhu P, Cui Z, et al. Clinical Features and Outcomes in Patients With Membranous Nephropathy and Crescent Formation. Medicine. 2015;94(50):e2294. PMID: 26683965; PMCID: PMC5058937. doi:10.1097/MD.0000000000002294.
33. Sun J, Li M, Zhu Q, et al. Glomerulosclerosis is a prognostic risk factor in patients with membranous nephropathy and non-nephrotic proteinuria. Ren Fail. 2023;45(1):2188088. PMID: 36967636; PMCID: PMC10044162. doi:10.1080/0886022X.2023.2188088.
34. Saito M, Komatsuda A, Sato R, et al. Clinicopathological and long-term prognostic features of membranous nephropathy with crescents: a Japanese single-center experience. Clin Exp Nephrol. 2018;22(2):365–376. PMID: 28852884. doi:10.1007/s10157-017-1465-y.
35. Kuroki A, Iyoda M, Shibata T, Sugisaki T. Th2 cytokines increase and stimulate B cells to produce IgG4 in idiopathic membranous nephropathy. Kidney Int. 2005;68(1):302–310. PMID: 15954921. doi:10.1111/j.1523-1755.2005.00415.x.
36. Imai H, Hamai K, Komatsuda A, Ohtani H, Miura AB. IgG subclasses in patients with membranoproliferative glomerulonephritis, membranous nephropathy, and lupus nephritis. Kidney Int. 1997;51(1):270–276. PMID: 8995742. doi:10.1038/ki.1997.32.
37. Kanahara K, Yorioka N, Nakamura C, et al. Myeloperoxidase-antineutrophil cytoplasmic antibody-associated glomerulonephritis with membranous nephropathy in remission. Intern Med. 1997;36(11):841–846. PMID: 9392363. doi:10.2169/internalmedicine.36.841.
38. Zhang S, Huang J, Dong J, et al. Efficacy and safety of rituximab for primary membranous nephropathy with different clinical presentations: a retrospective study. Front Immunol. 2023;14:1156470. PMID: 37187749; PMCID: PMC10175677. doi:10.3389/fimmu.2023.1156470.
39. Yoshimoto K, Wada T, Furuichi K, Sakai N, Iwata Y, Yokoyama H. CD68 and MCP-1/CCR2 expression of initial biopsies reflect the outcomes of membranous nephropathy. Nephron Clin Pract. 2004;98(1):c25–34. PMID: 15361701. doi:10.1159/000079924.
40. Ferrario F, Castiglione A, Colasanti G, Barbiano Di Belgioioso G, Bertoli S, D’Amico G. The detection of monocytes in human glomerulonephritis. Kidney Int. 1985;28(3):513–519. PMID: 4068484. doi:10.1038/ki.1985.158.
41. Hu W, Li G, Lin J, et al. M2 Macrophage Subpopulations in Glomeruli Are Associated With the Deposition of IgG Subclasses and Complements in Primary Membranous Nephropathy. Front Med Lausanne. 2021;8:657232. PMID: 34095170; PMCID: PMC8175664. doi:10.3389/fmed.2021.657232.
42. Wu Q, Jinde K, Nishina M, et al. Analysis of prognostic predictors in idiopathic membranous nephropathy. Am J Kidney Dis. 2001;37(2):380–387. PMID: 11157381. doi:10.1053/ajkd.2001.21319.
43. Deng L, Xu G. Update on the Application of Monoclonal Antibody Therapy in Primary Membranous Nephropathy. Drugs. 2023;83(6):507–530. PMID: 37017915. doi:10.1007/s40265-023-01855-y.
44. Rojas-Rivera JE, Ortiz Arduán A. Primary membranous nephropathy in the era of autoantibodies and biological therapies. Med Clin. 2021;157(3):121–129. PMID: 33832765. doi:10.1016/j.medcli.2021.02.010.
45. Gao S, Cui Z, Wang X, et al. Rituximab Therapy for Primary Membranous Nephropathy in a Chinese Cohort. Front Med Lausanne. 2021;8:663680. PMID: 34095173; PMCID: PMC8172988. doi:10.3389/fmed.2021.663680.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.