Back to Journals » Journal of Inflammation Research » Volume 18
Cucurbitacin IIa Alleviates Colitis via Promoting the Release of Host-Derived Extracellular Vesicles Encapsulating microRNA-30b-5p
Authors Zhao Y, Jiang B , Zuo S
Received 14 November 2024
Accepted for publication 24 January 2025
Published 31 January 2025 Volume 2025:18 Pages 1447—1458
DOI https://doi.org/10.2147/JIR.S500722
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Yinyin Zhao,1 Binyuan Jiang,2 Shengnan Zuo3
1Ningbo Institute of Innovation for Combined Medicine and Engineering (NIIME), The Affiliated LiHuiLi Hospital of Ningbo University, Ningbo, People’s Republic of China; 2Medical Research Center of the Affiliated Changsha Central Hospital of Hengyang Medical School, University of South China, Changsha, People’s Republic of China; 3Clinical Laboratory Department, Hunan Guangxiu Hospital, Changsha, People’s Republic of China
Correspondence: Binyuan Jiang; Shengnan Zuo, Email [email protected]; [email protected]
Purpose: Cucurbitacins have demonstrated anti-inflammatory effects and show promise for inflammatory bowel diseases. However, the underlying mechanisms by which cucurbitacins affect colitis remain largely unknown.
Methods: In this study, we investigated the impact of cucurbitacin IIa on dextran sulfate sodium (DSS)-induced colitis in rats and the alterations in intestinal extracellular vesicles (EVs). EVs were isolated and characterized, followed by analysis of the small RNAs and proteins encapsulated within them using small RNA sequencing and proteomics, respectively.
Results: Our results revealed that cucurbitacin IIa alleviated colitis symptoms in DSS-treated rats, along with changes in the morphology and composition of intestinal EVs. Notably, EVs from cucurbitacin IIa-treated rats also mitigated colitis symptoms in DSS-treated rats. Further analysis showed that cucurbitacin IIa modified the protein profiles and microRNA composition of EVs extracted from the feces of colitis rats. Specifically, microRNA-30b-5p, significantly increased by cucurbitacin IIa, was found to alleviate colitis symptoms in DSS-treated rats. In conclusion, cucurbitacin IIa appears to alleviate colitis by promoting the release of microRNA-30b-5p from host-derived extracellular vesicles.
Conclusion: These findings enhance our understanding of cucurbitacin IIa’s effects on intestinal health and offer potential new therapeutic targets for inflammatory bowel disease treatment.
Keywords: colitis, cucurbitacins, extracellular vesicles, inflammation, microRNA
Graphical Abstract:

Introduction
Cucurbitacins, a diverse group of tetracyclic triterpenoids derived from Cucurbitaceae plants, are recognized for their wide-ranging biological activities, including anti-inflammatory, anti-cancer, anti-bacterial, and anti-viral properties.1 Among these, cucurbitacin IIa, particularly extracted from the root of Hemsleya amabilis, has a long history of use in treating gastroenteritis.2 Recent findings suggest that Xuedan, a dry root tuber from the Hemsleya genus rich in cucurbitacin IIa, shows promise in ameliorating ulcerative colitis,3 indicating potential benefits for inflammation-related intestinal diseases. Despite these promising developments, the exact mechanisms through which cucurbitacin IIa mitigates intestinal inflammation remain unclear. Further research is needed to elucidate how cucurbitacin IIa interacts with the molecular pathways involved in inflammatory responses within the gastrointestinal tract.
Inflammatory bowel disease (IBD) is a chronic condition characterized by widespread inflammation in the intestinal tract, with its prevalence rising rapidly worldwide.4,5 Despite extensive research aimed at understanding its mechanisms and developing effective treatments, the exact causes of IBD remain largely unclear. Recently, extracellular vesicles (EVs), which play a key role in intercellular communication, have garnered growing interest.6 Studies have investigated the roles of EVs from various sources in IBD pathogenesis and their potential therapeutic applications.7 Notably, microRNAs encapsulated within EVs are considered key mediators in the interaction between diet and the host.8 Previous research has shown that EVs derived from bovine colostrum, along with the encapsulated microRNA bta-let-7a-5p, can ameliorate dextran sulfate sodium (DSS)-induced colitis.9 However, it remains unknown whether dietary anti-inflammatory substances can modify the composition of EVs and their encapsulated microRNAs, potentially leading to IBD alleviation.
In this study, we first investigated the effects of cucurbitacin IIa on colitis symptoms and inflammation using a DSS-induced colitis rat model. We then analyzed the changes in morphology, protein profiles, and microRNA composition of EVs extracted from the feces of rats treated with cucurbitacin IIa. Additionally, we explored the impact of these EVs, particularly their encapsulated microRNAs, on colitis symptoms and the inflammatory response in the colitis rats.
Materials and Methods
Animals
Male Sprague Dawley rats, weighing approximately 180 g and aged six weeks, were obtained from SLAC Laboratory Animal (Changsha, China). The rats were individually housed in a pathogen-free environment at 22 ± 2°C with a 12-hour light/dark cycle. After a one-week acclimation period, the rats were assigned to different treatment groups across three experiments. The experimental design is detailed in the Methods section. Throughout the experiments, the rats had ad libitum access to food and water. All animal procedures were conducted according to the guidelines of Hunan Province on the review of Welfare and Ethics of Laboratory Animals and approved by the Protocol Management and Review Committee of Hunan Guangxiu Hospital.
DSS-Induced Colitis
The rats were administered 3.0% (w/v) DSS (molecular weight 36–50 kDa; MP Biomedicals, Shanghai, China) dissolved in their drinking water for 7 days to induce ulcerative colitis. Body weight measurements were taken throughout the experiments.
Cucurbitacin IIa Treatment
Rats were assigned to one of four treatment groups (n=6/group): control (CON) rats, model rats (DSS-treated), cucurbitacin IIa-treated rats (CUC), and rats treated with both DSS- and cucurbitacin IIa- (CUD). Cucurbitacin IIa (Aladdin, Shanghai, China) was administered orally at a dosage of 50 mg/kg body weight daily for 14 days, with DSS treatment occurring during the final 7 days. At the end of the experiment, all rats were anesthetized and sacrificed for sample collection.
Histological Analyses of Colonic Ulceration and Goblet Cell
Approximately 1.5 cm segments of colonic tissue from the anterior region of the rats were collected and gently rinsed with cold PBS. The samples were then fixed in 4% paraformaldehyde solution. After fixation, the tissues were dehydrated through a series of increasing concentrations of alcohol, embedded in melted paraffin, and sectioned into 7-μm thick slices. The sections were initially stained with hematoxylin and then with eosin to observe histomorphology. Additionally, periodic acid-Schiff (PAS) staining was performed to visualize goblet cells secreting neutral mucus.
RT-qPCR Analysis
Total RNA was extracted from colon samples using TRIzol reagent (Sigma, Shanghai, China). cDNA was then synthesized by reverse transcription with the First-Strand cDNA Synthesis Kit (Invitrogen, Shanghai, China). Real-time PCR was performed using SYBR Green Mix, along with specific primers, template, and RNase-free H₂O. The relative expression of target genes was quantified using the comparative ΔΔCt method. Primer sequences are provided in Supplementary Table 1.
Isolation and Identification of EVs
Fecal samples were dissolved in PBS and sequentially centrifuged at 10 g, 40 g, and 500 g for 5 minutes each to collect the supernatant. The supernatant was then filtered through a 0.22-μm syringe filter following centrifugation at 10,000 g for 1 h. The filtrates were ultra-centrifuged at 100,000 g for 2 h, and fractions between 10% and 40% OptiPrep solution were collected. EVs were isolated by centrifugation at 150,000 g for 2 h using a Beckman ultracentrifuge (Beckman Coulter, Fullerton, USA). The isolated EVs were examined and analyzed by transmission electron microscopy (TEM). Briefly, EVs were placed on a copper grid and fixed with glutaraldehyde, then stained with uranyl acetate and methylcellulose. After air drying, the EVs were visualized using TEM. The size and concentration of the EVs were analyzed by nanoparticle tracking analysis (NTA). Additionally, the protein content was measured using a BCA assay kit (Solarbio, Shanghai, China), and the expression of EV protein markers was assessed using the WES Simple Western system. Antibodies against CD63 (Boster, Cat#a01080-2, RRID: AB_3662077), CD81 (Abcam, Cat#ab109201, RRID: AB_10866464), and TSG101 (Abcam, Cat#ab125011, RRID: AB_10974262) were employed, and the bands were analyzed using ProteinSimple software (ProteinSimple, http://www.proteinsimple.com/fluorchem_e.html, RRID: SCR_013724).
Small RNA Sequencing
Previously isolated EVs were used for RNA extraction with the Exosomal RNA Isolation Kit (NORGEN, Thorold, ON, Canada). After evaluating RNA amount, purity, and fragment integrity, total RNA was first ligated with 3′ adapters using T4 RNA Ligase 2, followed by ligation with 5′ adapters using T4 RNA Ligase 1 (NEB, Phoenix, AZ, USA). Reverse transcription was performed with SuperScript II Reverse Transcriptase (THERMO, Shanghai, China) to produce cDNA, which was then amplified by PCR using High-Fidelity DNA Polymerase (NEB). The PCR products were purified and enriched through PAGE electrophoresis, followed by sequencing with Single-end 50 bp on the Illumina HiSeq 2500. To identify known and novel microRNAs, unique sequences with the length ranging from 18 to 26 nucleotides were mapped to species precursors in miRBase 22.0. Differentially expressed microRNAs were analyzed using a Student’s t-test.
Proteomic Analysis
Proteins from the EVs were extracted, quantified, and digested using the filter-aided proteome preparation method. The resulting peptides were desalted, lyophilized, reconstituted, and quantified. After chromatographic separation, the samples underwent LC-MS/MS analysis using a timsTOF Pro mass spectrometer (Bruker, Germany) to obtain mass spectrometry data for identification and quantitative analysis. Finally, protein cluster analysis, GO functional annotation, and KEGG pathway annotation were performed.
EVs Treatment
Rats were assigned to one of four treatment groups (n=6/group): control rats (CON), DSS-treated rats (DSS), DSS-treated rats administered rectally with EVs (50 μg/mL) extracted from the feces of DSS-treated rats (EV-DSS), and DSS-treated rats administered rectally with EVs extracted from the feces of cucurbitacin IIa-treated rats (EV-CUC). The experiment lasted 14 days, with DSS treatment applied during the last 7 days. At the end of the experiment, all rats were anesthetized and sacrificed for sample collection.
MicroRNA Determination by qPCR
Previously isolated EVs were used for RNA extraction with the Exosomal RNA Isolation Kit (NORGEN, Thorold, ON, Canada) and then cDNA was synthesized from the total RNA using the SuperScript First-Strand Synthesis System (Invitrogen, Shanghai, China). Real-time PCR was performed with advanced miRNA assays (THERMO). The primer sequences were as follows: Forward, 5′-GCGCGTGTAAACATCCTACAC-3′; Reverse, 5′-ATCCAGTGCAGGGTCCGAGG-3′; RT primer, 5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAGCTGA-3′. Synthetic cel-miR-39 was spiked into the total RNA as an internal control.
In Vivo microRNA Treatment
Rats were allocated to one of three treatment groups (n=6/group): control rats (CON), DSS-treated rats (DSS), and DSS-treated rats administered intravenously via tail vein injection with 60 mg/kg rno-miR-30b-5p agomir (RiboBio, Guangzhou, China) every other day over a 14-day period. At the end of the experiment, all rats were anesthetized and sacrificed for sample collection.
Statistical Analysis
All data was analyzed with one-way ANOVA followed by Student-Newman-Keuls post hoc test or two-tailed Student’s T-test using the SPSS Statistics 19.0 Software. All data was presented as mean ± SEM and P value less than 0.05 was used as the significance criterion.
Results
Cucurbitacin IIa Alleviated Colitis Symptoms in DSS-Treated Rats
We initially explored the effects of cucurbitacin IIa on DSS-induced colitis. The results indicated that DSS caused significant decreases in body weight, colon weight, and length, while cucurbitacin IIa alleviated these changes in rats with colitis (Figure 1A–D). Hematoxylin and eosin staining revealed severe morphological damage, including noticeable edema and irregular villus arrangement due to DSS treatment, which was mitigated by cucurbitacin IIa (Figure 1E). Cucurbitacin IIa also reduced the histological index of colitis induced by DSS (Figure 1F) and increased the number of goblet cells in the colon of DSS-treated rats (Figure 1G and H). Notably, cucurbitacin IIa reduced the relative expression of TNF-α and IL-1β genes induced by DSS, indicating its anti-inflammatory effect (Figure 1I and J).
Cucurbitacin IIa Altered the Morphology and Composition of EVs
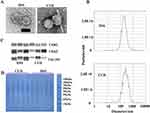
We then collected feces from DSS-treated and cucurbitacin IIa-treated rats to isolate and purify EVs. TEM confirmed the presence of EVs (Figure 2A). NTA revealed that the average diameter of EVs from DSS-treated and cucurbitacin IIa-treated rats was 194.3 nm and 261.8 nm, respectively (Figure 2B). We further validated the EVs by detecting the expression of their marker proteins. CD63, CD81, and TSG101 proteins were identified in the EVs (Figure 2C). Additionally, gel electrophoresis demonstrated that cucurbitacin IIa treatment increased the content of proteins with a molecular weight of approximately 35 kDa in the EVs (Figure 2D).
Cucurbitacin IIa Altered the Composition of microRNAs Encapsulated in Extracellular Vesicles
To investigate whether cucurbitacin IIa affects the composition of EVs, we first performed small RNA sequencing to identify the microRNAs encapsulated in the EVs. Venn diagram analysis revealed 45 unique microRNAs in the EVs from the feces of DSS-treated rats and 58 unique microRNAs in the EVs from the feces of cucurbitacin IIa-treated rats (Figure 3A). We observed that the expression of four microRNAs was significantly increased, while 16 microRNAs were significantly decreased in the CUD group compared to the DSS group (Figure 3B). Differentially expressed microRNAs between the two groups were grouped and classified using hierarchical clustering analysis (Figure 3C). Notably, the volcano plot showed that rno-miR-30b-5p was the most significantly affected microRNA due to cucurbitacin IIa treatment (Figure 3D). Further analysis confirmed that the relative expression of rno-miR-30b-5p was significantly higher in the CUD group compared to the DSS group (Figure 3E).
Cucurbitacin IIa Altered the Protein Profiles in Extracellular Vesicles
We then characterized and quantified proteins in the EVs from DSS-treated and cucurbitacin IIa-treated rats using proteomic analysis. Principal component analysis (PCA) revealed that the overall protein profiles in the DSS group were distinct from those in the CUD group (Figure 4A). Subcellular localization analysis indicated that most of these proteins were originally localized to extracellular spaces, the cytosol, and the plasma membrane (Figure 4B). Differentially expressed proteins (DEPs) between the two groups were grouped and classified using hierarchical clustering analysis (Figure 4C). Cucurbitacin IIa treatment resulted in 30 upregulated DEPs and 53 downregulated DEPs (Figure 4D). KEGG pathway analysis revealed that the DEPs were primarily involved in nutrient metabolism-related pathways, including pancreatic secretion, nitrogen metabolism, vitamin digestion and absorption, and fat digestion and absorption (Figure 4E). Gene ontology analysis further identified proteolysis as one of the major enrichment pathways for DEPs. Additionally, DEPs were enriched in pathways related to microvillus structure and regulation of microvillus length, as well as in the IgA immunoglobulin complex (Figure 4F).
Extracellular Vesicles From Cucurbitacin IIa-Treated Rats Alleviated Colitis Symptoms in DSS-Treated Rats
Since cucurbitacin IIa treatment altered both the composition of microRNAs and protein profiles in the EVs, we further investigated whether these EVs mediated the beneficial effects of cucurbitacin IIa on colitis. We found that EVs extracted from the feces of cucurbitacin IIa-treated rats mitigated the decreases in body weight, colon weight, and length in DSS-treated rats (Figure 5A–D). These EVs also alleviated colonic morphological damage and reduced the histological index of colitis caused by DSS treatment, whereas EVs from DSS-treated rats did not exhibit such effects (Figure 5E and F). Rats in the EV-CUC group had higher goblet cells in the colon compared to those in the DSS and EV-DSS groups (Figure 5G and H). Additionally, EVs from cucurbitacin IIa-treated rats decreased the relative expression of TNF-α and IL-1β genes induced by DSS (Figure 5I and J).
MiR-30b-5p Alleviated Colitis Symptoms in DSS-Treated Rats
To further investigate which components of EVs play a crucial role in alleviating colitis, we subjected the collected EVs to freezing and thawing followed by sonication to obtain lysed EVs. These lysed EVs were then treated with RNase to degrade RNA. We observed that, after these treatments, the EVs exhibited no anti-inflammatory effects on LPS- and TNF-α-treated Caco-2 cells (data not shown). Consequently, we explored whether changes in microRNA composition were critical for colitis alleviation. We treated rats with rno-miR-30b-5p agomir, which was significantly increased in the EVs by cucurbitacin IIa treatment. We found that rno-miR-30b-5p agomir alleviated decreases in body weight, colon weight, and length in DSS-treated rats (Figure 6A-D). Additionally, rno-miR-30b-5p agomir mitigated colonic morphological damage and reduced the histological index of colitis caused by DSS treatment (Figure 6E and F). However, rats treated with rno-miR-30b-5p agomir had no significant effects on goblet cell numbers in the colon (Figure 6G and H). Finally, rno-miR-30b-5p agomir decreased the relative expression of TNF-α and IL-1β genes induced by DSS (Figure 6I and J).
Discussion
The phytochemistry and pharmacology of cucurbitacin IIa have recently garnered increasing attention. Evidence highlights its various beneficial effects, including anti-inflammatory, anti-tumor, anti-bacterial, and immune-enhancing properties.1 The mechanisms underlying these effects involve the EGFR-MAPK signaling pathway, caspase-3-dependent apoptosis and autophagy, and survivin downstream of the JAK2/STAT3 signaling pathway.2,10,11 In this study, we found that cucurbitacin IIa exhibited anti-inflammatory effects and alleviated colitis symptoms in a rodent model of colitis. Importantly, we identified the pivotal role of microRNAs encapsulated in the EVs in mediating the beneficial effects of cucurbitacin IIa. These results suggest that cucurbitacin IIa holds promise as a potential therapeutic strategy for treating IBD.
Weight loss, chronic inflammation, and intestinal morphology impairment are major characteristics of colitis. We first observed that cucurbitacin IIa alleviated body weight loss, prevented reductions in colon weight and length, and mitigated morphological damage induced by DSS treatment. Additionally, cucurbitacin IIa treatment decreased the expression of TNF-α and IL-1β genes in colonic tissue, indicating its anti-inflammatory effects. Importantly, goblet cells and their secreted mucins play a crucial role in maintaining the intestinal barrier and supporting gut microbiota colonization.12 Cucurbitacin IIa protected goblet cells from DSS-induced reduction in rats, suggesting that its therapeutic efficacy may be associated with enhanced barrier function. However, whether cucurbitacin IIa directly targets goblet cells or indirectly affects their function remains to be explored.
The intestinal microenvironment is crucial for optimal gut function, including nutrition metabolism, barrier function, and immune system regulation. Dysbiosis of this microenvironment can promote the initiation and progression of intestinal diseases, such as IBD and functional gastrointestinal disorders.13 For decades, gut bacteria have been recognized as critical regulators of the intestinal microenvironment. Recently, growing evidence suggests that EVs secreted by live cells also play a significant role in dynamic interactions with dietary functional components and intestinal function.14,15 In the present study, we examined changes in EVs in fecal samples and found that cucurbitacin IIa not only affected the average diameter of the EVs but also altered their encapsulated protein content. Notably, purified EVs extracted from the feces of cucurbitacin IIa-treated rats alleviated colitis symptoms, similar to the effects observed with direct cucurbitacin IIa treatment. These findings help unravel the complexity of diet-intestine interactions and provide new insights into the mechanisms through which cucurbitacin IIa affects IBD. However, the promotional effects of cucurbitacin IIa on the release of EVs still require further confirmation.
EVs encapsulate a diverse array of molecules, including DNA, RNA, proteins, lipids, and peptidoglycan. Recent analyses of the protein components within EVs have highlighted their various functions. For instance, EV-associated proteins have been shown to promote wound healing and tissue repair,16 and the levels of proteins such as SerpinF2, CD14, and Cystatin C in the EVs are linked to systemic vascular events and heart failure.17 In the present study, we observed that cucurbitacin IIa altered the protein composition in the EVs from DSS-treated mice. Notably, these differentially expressed proteins were predominantly enriched in metabolic pathways and the IgA immunoglobulin complex. However, we did not specifically investigate the direct effects of these protein changes on colitis symptoms. Recently, there has been increasing attention to the role of small RNAs encapsulated in the EVs as novel mediators of interactions between host and external components. MicroRNAs, such as miR-200b-3p, encapsulated in EVs can interact with bacteria and influence the intestinal microenvironment in colitis.18 Importantly, in our study, the beneficial effects of cucurbitacin IIa on colitis diminished after the small RNAs in the EVs were degraded. These findings suggest that the alteration of RNAs, rather than proteins, encapsulated in EVs mediates the therapeutic effects of cucurbitacin IIa on colitis.
MicroRNAs play crucial roles in regulating the development and pathogenesis of IBD. In our study, we found that cucurbitacin IIa treatment significantly increased the levels of rno-miR-30b-5p, a microRNA encapsulated in the EVs from rats. This microRNA has been previously reported to regulate the expression of IL-10 and Toll-like receptor 4 in the pathogenesis of uveitis, suggesting its anti-inflammatory properties.19 Thus, we further confirmed the beneficial effects of rno-miR-30b-5p agomir treatment on colitis in rats. Beyond modulating host gene expression, microRNAs in the intestine can also interact with target genes in gut microbes, influencing their metabolism and providing protective effects against intestinal diseases.20,21 Future studies should investigate whether cucurbitacin IIa and rno-miR-30b-5p impact gut microbiota composition and whether changes in gut microbiota mediate their anti-inflammatory effects. Additionally, it remains to be determined whether rno-miR-30b-5p improves intestinal health by directly targeting host genes or indirectly interacting with gut microbes. Furthermore, it remains to be explored in future studies whether other factors, including gut microbiota and metabolites, may be related to the beneficial effects of cucurbitacin IIa.
Conclusion
In conclusion, we found that cucurbitacin IIa alleviated intestinal morphology impairment and inflammatory responses in DSS-induced colitis, suggesting its potential for managing IBD. Cucurbitacin IIa treatment also modified the composition of proteins and microRNAs encapsulated in the EVs secreted by the host, contributing to the alleviation of colitis. Notably, cucurbitacin IIa may exert its effects by promoting the expression of EV-encapsulated rno-miR-30b-5p. These findings enhance our understanding of cucurbitacin IIa’s mechanisms in intestinal health and offer new insights for developing therapeutic targets for IBD treatment.
Data Sharing Statement
Small RNA sequencing data have been deposited in the NCBI BioProject database (https://www.ncbi.nlm.nih.gov/bioproject/) under accession numbers PRJNA1143683. The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Funding
This work was supported by the Doctoral Development Foundation of LiHuiLi Hospital (No. 2023BSKY-ZYY).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Zeng YJ, Wang J, Huang QW, et al. Cucurbitacin IIa: a review of phytochemistry and pharmacology. Phytother Res. 2021;35:4155–4170. doi:10.1002/ptr.7077
2. He J, Wang Y, Xu LH, et al. Cucurbitacin IIa induces caspase-3-dependent apoptosis and enhances autophagy in lipopolysaccharide-stimulated RAW 264.7 macrophages. Int Immunopharmacol. 2013;16:27–34. doi:10.1016/j.intimp.2013.03.013
3. Zhang Y, Feng D, Zeng Y, et al. Xuedan sustained release pellets ameliorate dextran sulfate sodium-induced ulcerative colitis in rats by targeting gut microbiota and MAPK signaling pathways. Front Pharmacol. 2022;13:833972. doi:10.3389/fphar.2022.833972
4. Zhu L, Gu P, Shen H. Protective effects of berberine hydrochloride on DSS-induced ulcerative colitis in rats. Int Immunopharmacol. 2019;68:242–251. doi:10.1016/j.intimp.2018.12.036
5. Higashiyama M, Hokari R. New and emerging treatments for inflammatory bowel disease. Digestion. 2023;104:74–81. doi:10.1159/000527422
6. Liu H, Sun JR, Wang MK, et al. Intestinal organoids and organoids extracellular vesicles for inflammatory bowel disease treatment. Chem Eng J. 2023;465:142842.
7. Chen L, Ou Q, Kou X. Extracellular vesicles and their indispensable roles in pathogenesis and treatment of inflammatory bowel disease: a comprehensive review. Life Sci. 2023;327:121830. doi:10.1016/j.lfs.2023.121830
8. Dong XY, Liu YY, Yang XB, et al. Extracellular vesicle miRNAs as key mediators in diet-gut microbiome-host interplay. Trends Food Sci Tech. 2023;136:268–281. doi:10.1016/j.tifs.2023.05.005
9. Mun D, Kang MKY, Shin M, et al. Alleviation of DSS-induced colitis via bovine colostrum-derived extracellular vesicles with microRNA let-7a-5p is mediated by regulating Akkermansia and -hydroxybutyrate in gut environments. Microbiol Spectr. 2023;11(6). doi:10.1128/spectrum.00121-23
10. Zhang J, Song Y, Liang Y, et al. Cucurbitacin IIa interferes with EGFR-MAPK signaling pathway leads to proliferation inhibition in A549 cells. Food Chem Toxicol. 2019;132:110654. doi:10.1016/j.fct.2019.110654
11. Boykin C, Zhang G, Chen YH, et al. Cucurbitacin IIa: a novel class of anti-cancer drug inducing non-reversible actin aggregation and inhibiting survivin independent of JAK2/STAT3 phosphorylation. Br J Cancer. 2011;104:781–789. doi:10.1038/bjc.2011.10
12. Liu J, Lu YF, Wu Q, et al. Oleanolic acid reprograms the liver to protect against hepatotoxicants, but is hepatotoxic at high doses. Liver Int. 2019;39:427–439. doi:10.1111/liv.13940
13. Liang X, Dai N, Sheng K, et al. Gut bacterial extracellular vesicles: important players in regulating intestinal microenvironment. Gut Microbes. 2022;14:2134689. doi:10.1080/19490976.2022.2134689
14. Liu L, Liang L, Yang C, et al. Extracellular vesicles of Fusobacterium nucleatum compromise intestinal barrier through targeting RIPK1-mediated cell death pathway. Gut Microbes. 2021;13:1–20. doi:10.1080/19490976.2021.1902718
15. Diaz-Garrido N, Badia J, Baldoma L. Microbiota-derived extracellular vesicles in interkingdom communication in the gut. J Extracell Vesicles. 2021;10:e12161. doi:10.1002/jev2.12161
16. Roefs MT, Sluijter JPG, Vader P. Extracellular vesicle-associated proteins in tissue repair. Trends Cell Biol. 2020;30:990–1013. doi:10.1016/j.tcb.2020.09.009
17. Zhang YN, Vernooij F, Ibrahim I, et al. Extracellular vesicle proteins associated with systemic vascular events correlate with heart failure: an observational study in a dyspnoea cohort. PLoS One. 2016;11:e0148073. doi:10.1371/journal.pone.0148073
18. Shen Q, Huang Z, Ma L, et al. Extracellular vesicle miRNAs promote the intestinal microenvironment by interacting with microbes in colitis. Gut Microbes. 2022;14:2128604. doi:10.1080/19490976.2022.2128604
19. Sun Y, Guo D, Liu B, et al. Regulatory role of rno-miR-30b-5p in IL-10 and toll-like receptor 4 expressions of T lymphocytes in experimental autoimmune uveitis in vitro. Mediators Inflamm. 2018;2018:2574067. doi:10.1155/2018/2574067
20. He L, Zhou X, Liu Y, et al. Fecal miR-142a-3p from dextran sulfate sodium-challenge recovered mice prevents colitis by promoting the growth of Lactobacillus reuteri. Mol Ther. 2022;30:388–399. doi:10.1016/j.ymthe.2021.08.025
21. Zhou X, Liu Y, Xiong X, et al. Intestinal accumulation of microbiota-produced succinate caused by loss of microRNAs leads to diarrhea in weanling piglets. Gut Microbes. 2022;14:2091369. doi:10.1080/19490976.2022.2091369
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Fibromodulin Ablation Exacerbates the Severity of Acute Colitis
Halasi M, Grinstein M, Adini A, Adini I
Journal of Inflammation Research 2022, 15:4515-4526
Published Date: 8 August 2022
Cucurbitacin E Alleviates Colonic Barrier Function Impairment and Inflammation Response and Improves Microbial Composition on Experimental Colitis Models

Zhan F, Song W, Fan Y, Wang F, Wang Q
Journal of Inflammation Research 2024, 17:2745-2756
Published Date: 6 May 2024
Mir-218-5p from Extracellular Vesicles of Endometrium in Patients with Recurrent Implantation Failure Impairs Pre-Implantation Embryo Development

Cai L, Lv M, Wei J, Liu C, Li Y, Liao Z, Li T, Zhang H, Xi L, Sui C
International Journal of Nanomedicine 2025, 20:5661-5679
Published Date: 1 May 2025
Zanthoxylum bungeanum-Derived Nanobiotics for Effective Against Ulcerative Colitis in Mouse Model
Gong Q, Sun C, Jiang T, Guo Y
International Journal of Nanomedicine 2025, 20:6317-6331
Published Date: 20 May 2025
IL-17A as a Key Mediator of Pulmonary-Intestinal Immune Interactions in a Mouse Model of Asthma and Colitis
Wu C, Hu X, Mo Z, Meng Y, Du Y, Duan Y, Zeng Z, Shan J, Li J, Zhang N, Ma Y, Wang H, Liu C, Zhang G, Foster PS, Xu H, Li F, Yang M
Journal of Inflammation Research 2025, 18:8199-8216
Published Date: 21 June 2025