Back to Journals » Journal of Inflammation Research » Volume 18
Decrease of Resolvin D1 and E1 in Patients with Chronic Epilepsy, Implicating Dysfunction of Neuroinflammation Resolution
Authors Li H, Dong Z, Yang Y, Sun Q, Lu F, Li R, Zhou W, Gui W, Gao R, Wang Y
Received 9 February 2025
Accepted for publication 16 June 2025
Published 27 June 2025 Volume 2025:18 Pages 8541—8551
DOI https://doi.org/10.2147/JIR.S521679
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Adam Bachstetter
Hanli Li,1,2 Zhong Dong,2 Yujing Yang,3 Qibing Sun,2 Fengqing Lu,2 Ran Li,2 Weifeng Zhou,1 Wei Gui,3 Rupan Gao,4 Yu Wang2
1School of Clinical Medicine, Anhui Medical College, Hefei, People’s Republic of China; 2Department of Neurology, the First Affiliated Hospital of Anhui Medical University, Hefei, People’s Republic of China; 3Department of Neurology, The First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei, People’s Republic of China; 4Department of Hematology, Zhongshan Hospital Fudan University, Shanghai, People’s Republic of China
Correspondence: Rupan Gao, Department of Hematology, Zhongshan Hospital Fudan University, Shanghai, People’s Republic of China, Email [email protected] Yu Wang, Department of Neurology, The First Affiliated Hospital of Anhui Medical University, Hefei, People’s Republic of China, Email [email protected]
Objective: Inflammation resolution is mediated by specialized pro-resolving lipid mediators (SPMs). It’s of high interest to understand the alterations of SPMs in chronic epilepsy.
Methods: Sixty-five patients with chronic epilepsy, 43 healthy controls and 43 patients with non-inflammatory neurological disorders were enrolled in this study. Plasma and cerebrospinal fluid (CSF) levels of SPMs were measured using liquid chromatography-tandem mass spectrometry (LC-MS-MS). Moreover, Resolvin D1 (RvD1) and resolvin E1 (RvE1), as well as pro-inflammatory factors were analyzed by enzyme-linked immunosorbent assay (ELISA).
Results: LC-MS-MS found several lipid mediators were altered, especially plasma (P = 0.014) and CSF (P = 0.010) RvE1 levels were decreased in patients with chronic epilepsy. Furthermore, ELISA results showed that in plasma and CSF, RvD1 and RvE1 levels were both lower in patients with chronic epilepsy, while pro-inflammatory factors were higher than in controls. Moreover, plasma RvE1 level was independently negatively associated with the National Hospital Seizure Severity Scale scores (&Bgr; = − 0.00636, P < 0.001). While plasma RvD1 level was correlated with Mini-Mental State Examination scores (R = 0.284, P = 0.022).
Conclusion: This study demonstrated decreased levels of RvD1 and RvE1 both in plasma and CSF in patients with chronic epilepsy, suggesting impaired resolution of inflammation in chronic epilepsy. Targeting RvD1 and RvE1 may be a novel direction worthy of further research in the treatment of chronic epilepsy.
Keywords: chronic epilepsy, inflammation, specialized pro-resolving lipid mediators, resolvin D1, resolvin E1
Introduction
Epilepsy, a chronic neurological disorder characterized by recurrent epileptic seizures, is often accompanied by impaired cognition and mood disorders.1,2 Although a variety of antiseizure medications (ASMs) are clinically available for epilepsy treatment, approximately one-third of the epilepsy develop into drug-resistant epilepsy.3 Increasing evidences suggest that pro-inflammatory factors are increased in plasma/serum, cerebrospinal fluid (CSF) and brain tissue in patients with chronic epilepsy,4–7 and the associated neuroinflammation is regarded as a consequence of epileptic seizures as well as an important intrinsic promoting mechanism for the development of epilepsy.8 It is assumed that neuroinflammation persists in chronic epilepsy due to a deficiency of endogenous resolution of inflammation, thereby leading to neuronal network hyperexcitability and recurrent epileptic seizures.9–11
Typically, inflammation resolution balances the inflammatory responses. Recent studies have indicated that autoimmune and chronic inflammatory diseases might be the result of the failure of inflammation resolution mediated by a group of lipid mediators called specialized pro-resolving lipid mediators (SPMs), which are composed of resolvins, lipoxins, protectins and maresins.12 In immune cells, SPMs are endogenously formed from omega-3 (n-3) and omega-6 (n-6) polyunsaturated fatty acids (PUFAs).13 Despite the established link between neuroinflammation and epilepsy, the role of SPMs in this process remains unclear. Increasing evidences suggest that the synthesis of SPMs is impaired and the metabolism of SPMs is altered in patients with some chronic brain inflammation diseases, and it has been evidenced that the lipid metabolism, especially SPMs metabolism, of the brain has emerged as a key process of neuroinflammation resolution in psychiatric and neurodegenerative disorders.14 Resolvin D1 (RvD1) and Resolvin E1 (RvE1) are two representative resolvin proteins in the SPMs family, which are biosynthesized from docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), respectively. By alleviating neuroinflammation, they have shown significant therapeutic effects in the treatment of Parkinson’s disease (PD),15 Alzheimer’s disease (AD)16 and chronic pain.17 However, to date, the possible changes of SPMs in plasma and CSF and their pathological role in epilepsy remain unclear.
In this study, we aim to elucidate the alterations of SPMs in plasma and CSF in patients with chronic epilepsy, especially the two major resolvins, RvD1 and RvE1. Besides, the levels of pro-inflammatory factors in plasma and CSF were also measured in patients with chronic epilepsy. We then explored the association of resolvins and clinical characteristics in patients with chronic epilepsy.
Materials and Methods
Study Design and Patient Characteristics
The plasma and CSF of 65 patients with chronic epilepsy were collected consecutively from September 2023 to November 2024 through the Department of Neurology, the First Affiliated of Anhui Medical University, China. As plasma controls, the plasma of 43 age- and sex-matched healthy individuals were collected during the same period from the physical examination center in the same hospital. And the CSF of 43 age- and sex-matched patients with non-inflammatory neurological disorders were collected as CSF controls in the Department of Neurology. Epilepsy was diagnosed by two independent epileptologists according to the criteria of the International League Against Epilepsy (ILAE),18 and the age range was 18–61 years. Chronic epilepsy was defined as epilepsy of at least one year duration19 in this study. Plasma and CSF samples of patients were collected on admission during interictal episode (minimum of 24 hours post-seizure). Patients with inflammatory disease, recent infection, active tumor or use of anti-inflammatory and antibiotic drugs within one month were all excluded. All study participants or their guardians were informed of the study protocol and potential risks and provided written consent for the study. The study was approved by the ethics committee of the First affiliated Hospital of Anhui Medical University, and was conducted in accordance with the Helsinki Declaration.
Clinical and Laboratory Data Collection
Clinical informations on patients were collected through patient interviews using standardized questionnaire and detailed electronic medical records. Brain magnetic resonance imaging (MRI) and 24 hours ambulatory electroencephalogram (EEG) were recorded in all patients. The epileptic details including duration of epilepsy, number of types of ASMs and the epileptic seizure types were obtained from clinical assessments by epileptologists based on clinical data, results of ambulatory EEG and brain imaging. The demographic and laboratory data, including gender, age, blood leukocyte count, neutrophil–lymphocyte ratio (NLR), albumin, triglyceride (TG), total cholesterol (TC), CSF leukocyte count and CSF protein of each participant were detected on the day of blood and CSF sampling. The selection of NLR, albumin, TG and TC was based on their established roles in systemic inflammation and neurological disorders.20,21 Seizure severity was evaluated according to the National Hospital Seizure Severity Scale (NHS3) ranging from 1 to 27,22 Besides, the Mini Mental State Examination (MMSE) test was used to assess cognitive function of the patients.23
Blood and CSF Collection and Lipid Mediator Measurements
Venous blood samples were collected in an EDTA anticoagulant-containing tube (5 mL), and the samples were centrifuged at 12000 rpm at 4 °C for 10 min. CSF samples were obtained by lumbar puncture and collected in polypropylene tubes (1 mL), then centrifuged at 12000 rpm at 4 °C for 10 min. All the plasma and CSF samples were stored at −80°C until measurement.
Liquid Chromatography–Tandem Mass Spectrometry (LC-MS-MS)
Due to resource constraints and ethical considerations, we initially conducted a preliminary LC-MS-MS analysis on 8 patients and 6 controls. Subsequently, we analyzed the entire cohort using the enzyme-linked immunosorbent assay (ELISA) to validate the obtained results. The plasma and CSF samples from 8 patients with chronic epilepsy and 6 controls were randomly selected from the sample cohort to perform quantifications of all lipid mediators (LMs) using LC-MS-MS method as previously described.24,25 Multiple LMs were detected, and these detected LMs were SPMs that included RvD1, RvD2, RvD3, RvD5, RvE1, lipoxin A4 (LXA4), LXB4, Maresin 1 (MaR1), neuroprotectin D1 (PD1), PDX, pro-inflammatory LMs (leukotriene B4, LTB4), prostaglandins (PGD2, PGE2 and PGF2α), their precursors (DHA, EPA and AA) and the intermediate products in the metabolic pathways which included 4-hydroxy-docosahexaenoic acid (4-HDHA), 7-HDHA, 14-HDHA, 16-HDHA, 17-HDHA, 20-HDHA, 5-hydroxy-eicosapentaenoic acid (5-HEPE), 11-HEPE, 12-HEPE, 15-HEPE, 18-HEPE, 5-hydroxyeicosatetraenoic acid (5-HETE), 8-HETE, 11-HETE,12-HETE, 15-HETE, 20-HETE et al. The multiple LMs and their relationships in synthesis and metabolism were outlined in Supplementary Figure 1. RvE1 and RvD1, as the identified SPMs, were further analyzed by ELISA.
Detection of Resolvins and Cytokines by ELISA
The levels of RvD1 and RvE1 in plasma and CSF of all subjects were measured by human resolvin D1 ELISA kit and human resolvin E1 ELISA kit (Cayman Chemical, USA) according to manufacturer’s instructions, the levels of cytokines including interleukin-1β (IL-1β), IL-6 and tumor necrosis factor-α (TNF-α) in plasma and CSF of patients and controls were analyzed by using ELISA kit (Jianglai Biological, China), following the manufacturer’s instructions.
Statistical Analysis
All data were expressed as mean ± standard error of the mean (SEM). Orthogonal partial least squares-discriminant analysis (OPLS-DA) of LMs was performed using R version 3.5.1. Heat maps were visualized using ComplexHeatmap (2.13.1) and R software. Data were analyzed using SPSS 25.0 statistical software. Data distributions were tested for normality. An independent samples t-test and Pearson’s correlation were used for continuous variables and for categorical variables, Chi-square test was used to test the difference. Non-normally distributed data were assessed with Mann–Whitney U-test for the two groups. Besides, multivariate linear regression was used to further examine associations between resolvins and NHS3 scores of patients while adjusting for possible confounding factors.
Results
Baseline Characterization of Patients with Chronic Epilepsy and Controls
The basic demographic and laboratory characteristics of patients with chronic epilepsy and controls are shown in Table 1. In total, we analyzed 65 plasma and CSF samples from patients (age from 18 to 61, 37 males, 28 females), 43 plasma samples from controls (age from 21 to 58, 24 males, 19 females) and 43 CSF samples from another controls (age from 20 to 57, 23 males, 20 females). There was no significant difference in age, gender, blood leukocyte count, TG, TC, CSF leukocyte count or CSF protein between epilepsy and controls. Patients with chronic epilepsy displayed higher blood NLR level, and lower albumin level than controls. From the chronic epilepsy cohort, 23 had only generalized-onset tonic-clonic seizures (GTCS), and 42 had focal seizures (11 individuals) or focal to bilateral tonic-clonic seizures (31 individuals) (FS). Of the patients receiving treatment with ASMs, 18 received monotherapy, 47 received polytherapy.
 |
Table 1 Baseline Characteristics of the Study Population |
The Plasma and CSF LMs Were Altered in Patients with Chronic Epilepsy
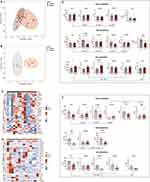
To reveal the potential differences in LMs profiles between patients with chronic epilepsy (n = 8) and controls (n = 6), we first analyzed bioactive SPMs, their intermediate derivatives, and fatty acid precursors from plasma and CSF samples using LC-MS-MS. Based on published criteria, a total of 38 distinct LMs from endogenous substrates DHA, EPA and AA were quantified and analyzed by LC-MS-MS, and the data were then statistically analyzed (Supplementary Table 1). And the identification of key SPMs, including RvD1 and RvE1, as shown in Supplementary Figure 2. The OPLS-DA plot of LMs showed a separation between controls and chronic epilepsy clusters in plasma (Figure 1A) and CSF (Figure 1B), respectively. Furthermore, heats map derived by clustering differentially lipid mediators from plasma (Figure 1C) and CSF (Figure 1D), described that the levels of several LMs was altered significantly in patients with chronic epilepsy as compared with controls. Specifically, in plasma (Figure 1E), patients with chronic epilepsy had significantly lower levels of EPA metabolome, including RvE1 (P = 0.014) and 11-HEPE (P = 0.020), as compared with controls. For AA metabolome, plasma level of 8-HETE (P = 0.019) significantly increased in patients with chronic epilepsy as compared with controls. Meanwhile, in CSF (Figure 1F), for DHA metabolome, level of 14-HDHA (P = 0.037) significantly decreased in patients with chronic epilepsy as compared with controls. And for EPA metabolome, patients with chronic epilepsy had significantly lower level of RvE1 (P = 0.010), as compared with controls. Furthermore, for AA metabolome, level of 12-HETE (P = 0.009) significantly decreased in patients with chronic epilepsy as compared with controls. However, for DHA level, there was no significant difference either in plasma or in CSF between patients with chronic epilepsy and controls. Finally, the CSF levels of AA (P = 0.022) and EPA (P = 0.002) were lower in patients with chronic epilepsy than in controls. Notably, RvD1 was not detected in plasma and there was no significant difference in CSF RvD1 (P = 0.683) between the two groups.
Lower Levels of RvD1 and RvE1 in Plasma and CSF From Patients with Chronic Epilepsy by ELISA
Based on the findings of LC-MS-MS analysis, further analysis of RvD1 and RvE1 in plasma as well as in CSF from 65 patients with chronic epilepsy and 43 controls was performed by ELISA (Figure 2A). In agreement with LC-MS-MS results, RvE1 levels in both plasma (P < 0.001) and CSF (P = 0.001) were significantly lower in patients with chronic epilepsy compared with controls. As for RvD1, its level in plasma (P = 0.035) as well as in CSF (P < 0.001) were significantly lower in patients with chronic epilepsy as compared to controls.
Changes of Pro-Inflammatory Cytokines in Patients with Chronic Epilepsy
To further determine the differences in the levels of inflammatory markers between patients with chronic epilepsy and controls (Figure 2B), we analyzed the levels of pro-inflammatory cytokines including IL-1β, IL-6, TNF-α in plasma and CSF samples from all subjects. The plasma levels of IL-1β (P <0.001) and TNF-α (P = 0.024) and the CSF levels of IL-1β (P <0.001) and IL-6 (P = 0.005) in patients with chronic epilepsy were significantly higher than in healthy controls. While no difference was found in the levels of plasma IL-6 (P = 0.066) and CSF TNF-α (P = 0.134) between two groups.
Correlations Between Resolvins and Disease Severity in Patients with Chronic Epilepsy
The levels of RvD1 and RvE1 either in plasma or in CSF were not associated with age, gender, seizure types and number of ASMs (Supplementary Table 2). As for epilepsy severity, we found that NHS3 scores were inversely correlated with plasma levels of RvD1 (R = −0.291, P = 0.019) and RvE1 (R = −0.301, P = 0.015) and CSF level of RvE1 (R = −0.325, P = 0.008) (Figure 3A–D). Furthermore, multivariate linear regression analysis was conducted with NHS3 scores as a dependent variable, and age, gender, blood leukocyte count, NLR, Albumin, TG, CSF leukocyte count, CSF protein, plasma and CSF levels RvD1 and RvE1 as covariates, revealing that only plasma RvE1 level was negatively correlated with NHS3 scores (Β = −0.00636, P < 0.001) (Supplementary Table 3). Moreover, only plasma level of RvD1 (R = 0.284, P = 0.022) positively correlated with MMSE scores (Figure 3E–H).
Discussion
In this study, ELISA results showed that the levels of RvD1and RvE1 in plasma as well as in CSF were lower in patients with chronic epilepsy than in controls, and results from LC-MS-MS analysis of a small sample of the cases also confirmed lower plasma and CSF levels of RvE1 in patients with chronic epilepsy, suggesting a dysfunction of inflammation resolution in chronic epilepsy. Notably, the LC-MS-MS method failed to detect plasma level of RvD1, which is consistent with most previous studies in other population.26,27 The differences in the detection results may reflect the differences in the sensitivity of different detection methods. A possible explanation for this is that the level of RvD1 in human plasma is very low and may be below the detection limits of some laboratories. Therefore, it is understandable that some SPMs were inconsistently detected across studies using the LC-MS-MS.28
PUFAs and their bioactive metabolites of DHA and EPA play a critical role in the inflammation regulation.12 Animal studies demonstrated that n-3 PUFAs could raise seizure threshold29 while in clinical studies, n-3 PUFAs supplementation to patients with epilepsy have produced conflicting results,30 and two randomized clinical trials both found no significant benefits of daily supplementation despite the level of n-3 PUFAs was obviously increased in these patients.31,32 Thus, we hypothesize that the underlying cause of the poor clinical efficacy of n-3 PUFAs supplementation on epilepsy was a failure of producing sufficient levels of SPMs derived from n-3 PUFAs, rather than the insufficient bioavailability of n-3 PUFAs in the body. In addition, LC-MS-MS analysis found alterations in SPMs precursors (AA and EPA) and intermediates in patients with chronic epilepsy, and these results were similar to those previously reported in patients with inflammation.24,27 Therefore, in our study, reduced levels of RvD1, RvE1 and other LMs in plasma and CSF indicate that the peripheral and central synthetic pathways of SPMs in chronic epilepsy do not proceed normally, and these alterations may be associated with brain inflammation aggravating epilepsy.
It is increasingly evidenced that neuroinflammation has a fundamental role in the development of epilepsy.8–11 Amount of clinical studies have found that the peripheral blood and CSF levels of proinflammatory cytokines, such as IL-1β, IL-6 and TNF-α, are significantly elevated in chronic epilepsy.4–6 Consistent with literature reports, we found that the levels of proinflammatory cytokines IL-1β and TNF-α in plasma, as well as IL-1β and IL-6 in CSF were significantly elevated in interictal phase in patients with chronic epilepsy. On the other hand, we found for the first time that levels of multiple SPMs in peripheral blood and central nervous system were altered, especially the levels of RvD1 and RvE1, which accelerate the resolution of inflammation, were reduced in patients with chronic epilepsy. Overall, these results suggest that, in chronic epilepsy, the balance between pro-inflammatory cytokine induced inflammatory response and SPMs induced inflammation resolution is disrupted. SPMs regulate the resolution processes of the inflammatory response by antagonizing the action of inflammatory mediators, thereby terminating acute inflammation and restoring homeostasis.13 While the inflammation resolution is disrupted due to the insufficiency or absence of SPMs, acute inflammation will develop into chronic inflammation.33 Thus, it is reasonable to speculate that the insufficient levels of peripheral and central RvD1 and RvE1 may be an important cause of persistent inflammatory brain changes in chronic epilepsy. In addition, our results showed that the peripheral blood NLR level was increased and the serum albumin was decreased in patients with chronic epilepsy, which is consistent with our previous reports.20
Experimental studies have shown that RvD1 or RvE1 treatment protected against neuroinflammation in different neurological diseases via decreasing inflammatory factors, reducing microglial activation and alleviating neutrophil accumulation.15–17 In clinical studies, plasma or CSF RvD1 levels were low in PD patients,15 and the CSF RvD1 level was significantly positively correlated with MMSE scores in AD patients.25 Here, we found that plasma RvD1 levels were positively correlated with MMSE scores in patients with chronic epilepsy, which indicates that RvD1 is closely associated with cognitive function in chronic epilepsy. It is reported that cognitive impairment associated with seizures is a significant risk for development of epilepsy.1,34 In addition, emerging evidences support the critical role of innate immunity and neuroinflammation in epilepsy, especially in epilepsy with cognitive impairment.35,36 Our study further demonstrated the association between epilepsy and cognition and suggested that early detection of plasma RvD1 in patients with epilepsy can assess the risk of cognitive impairment as early as possible. Resolvins have been shown to be correlated with the severity of neuroinflammatory disease.24,25 In this study, Spearman correlation test revealed that the plasma and CSF levels of RvE1 and the plasma level of RvD1 were positively correlated with NHS3 scores, and multiple linear regression analysis further revealed that plasma level of RvE1 was independently negatively associated with NHS3 scores, suggesting the potential role of plasma RvE1 as a biomarker for predicting epilepsy severity. Emerging evidence from clinical studies reveals that reduced biosynthesis of SPMs, especially of resolvins, are closely correlated with the disease progression in neurodegenerative disorders.25,37 Furthermore, experimental supplementation with RvE1 demonstrated therapeutic benefits, by alleviating neuroinflammation and ultimately improving the disease outcomes of the experimental model.16 Although there are reports indicating that the inflammatory process can significantly aggravate the severity of epileptic seizures,6 our study is the first to reveal the association between resolvins, particularly plasma RvE1 levels, and the severity of epileptic seizures. These findings suggest that targeted modulation of RvE1 may alleviate seizure severity, a discovery that could pave the way for innovative therapeutic strategies against chronic epilepsy. In general, we found that both central and peripheral RvD1 and RvE1 decreased in patients with chronic epilepsy, and in particular, the decrease of peripheral resolvins were closely related to the severity of epileptic seizures and cognitive impairment, suggesting that dysfunction of neuroinflammation resolution may contribute to the pathogenesis of epilepsy mainly through peripheral pathways.
There are some limitations to this study that need to be addressed. The sample size of this study was small and all the subjects included were from the same hospital in-patient unit, which may have selection bias, and patient attributes (such as age and comorbidities) may have affected the results. Therefore, further expansion of the cohort study is needed. Although LC-MS-MS is regarded as a best quantitation method for measuring LMs, there are still variations in the concentrations of SPMs reported, even several LMs could not be detected in our small sample of cases as the measurable minimum limits of LMs are not known, thus, RvD1 and RvE1 were mainly measured by ELISA in this study. In addition, experimental model studies are needed to elucidate how resolvins are involved in the pathogenesis of epilepsy.
Conclusion
In conclusion, plasma and CSF levels of RvD1 and RvE1 is decreased in chronic epilepsy, which might be due to the potential dysregulation of n-3 PUFA metabolism, thereby damaging the biosynthesis of SPMs, or due to the increased consumption of SPMs. Decreased plasma RvE1 is an independent risk factor for the severity of epileptic seizures, and decreased plasma RvD1 is correlated with the impairment of cognitive function in chronic epilepsy. Therefore, this study suggests an impaired resolution of neuroinflammation due to the reduction of RvD1 and RvE1 in chronic epilepsy. Targeting RvD1 and RvE1 may be a novel direction worthy of research in the treatment of chronic epilepsy.
Data Sharing Statement
An unauthorized version of the Chinese MMSE was used by the study team without permission, however this has now been rectified with PAR. The MMSE is a copyrighted instrument and may not be used or reproduced in whole or in part, in any form or language, or by any means without written permission of PAR (www.parinc.com).
Ethics Approval
The study protocol was reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Anhui Medical University (Approval No. PJ2023-11-21).
Acknowledgments
We acknowledge the support from the Department of Neurology and the Department of Health Management Center, The First Affiliated Hospital of Anhui Medical University, Hefei, China.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study is supported by the National Natural Science Foundation of China (Yu Wang, Grant No. 82071460) and Health Research Program of Anhui (Hanli Li, Grant No. AHWJ2024Aa30201).
Disclosure
The authors report no competing interests.
References
1. Hermann BP, Struck AF, Busch RM, Reyes A, Kaestner E, McDonald CR. Neurobehavioural comorbidities of epilepsy: towards a network-based precision taxonomy. Nat Rev Neurol. 2021;17(12):731–746. doi:10.1038/s41582-021-00555-z
2. Löscher W, Stafstrom CE. Epilepsy and its neurobehavioral comorbidities: insights gained from animal models. Epilepsia. 2023;64(1):54–91. doi:10.1111/epi.17433
3. Beghi E. The epidemiology of epilepsy. Neuroepidemiology. 2020;54(2):185–191. doi:10.1159/000503831
4. Ichiyama T, Nishikawa M, Yoshitomi T, Hayashi T, Furukawa S. Tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-6 in cerebrospinal fluid from children with prolonged febrile seizures. Comparison with acute encephalitis/encephalopathy. Neurology. 1998;50(2):407–411. doi:10.1212/WNL.50.2.407
5. Librizzi L, Vila Verde D, Colciaghi F, et al. Peripheral blood mononuclear cell activation sustains seizure activity. Epilepsia. 2021;62(7):1715–1728. doi:10.1111/epi.16935
6. Vieira ELM, de Oliveira GNM, Lessa JMK, et al. Peripheral leukocyte profile in people with temporal lobe epilepsy reflects the associated proinflammatory state. Brain Behav Immun. 2016;53:123–130. doi:10.1016/j.bbi.2015.11.016
7. Iyer A, Zurolo E, Spliet WG, et al. Evaluation of the innate and adaptive immunity in type I and type II focal cortical dysplasias. Epilepsia. 2010;51(9):1763–1773. doi:10.1111/j.1528-1167.2010.02547.x
8. Aronica E, Crino PB. Inflammation in epilepsy: clinical observations. Epilepsia. 2011;52(Suppl 3):26–32. doi:10.1111/j.1528-1167.2011.03033.x
9. Vezzani A, Maroso M, Balosso S, Sanchez MA, Bartfai T. IL-1 receptor/Toll-like receptor signaling in infection, inflammation, stress and neurodegeneration couples hyperexcitability and seizures. Brain Behav Immun. 2011;25(7):1281–1289. doi:10.1016/j.bbi.2011.03.018
10. Zhang Z, Li Y, Jiang S, Shi FD, Shi K, Jin WN. Targeting CCL5 signaling attenuates neuroinflammation after seizure. CNS Neurosci Ther. 2023;29(1):317–330. doi:10.1111/cns.14006
11. Giansante G, Marte A, Romei A, et al. Presynaptic L-type Ca(2+) channels increase glutamate release probability and excitatory strength in the Hippocampus during chronic neuroinflammation. J Neurosci. 2020;40(36):6825–6841. doi:10.1523/JNEUROSCI.2981-19.2020
12. Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510(7503):92–101. doi:10.1038/nature13479
13. Serhan CN, Levy BD. Resolvins in inflammation: emergence of the pro-resolving superfamily of mediators. J Clin Invest. 2018;128(7):2657–2669. doi:10.1172/JCI97943
14. Ponce J, Ulu A, Hanson C, et al. Role of specialized pro-resolving mediators in reducing neuroinflammation in neurodegenerative disorders. Front Aging Neurosci. 2022;14:780811. doi:10.3389/fnagi.2022.780811
15. Krashia P, Cordella A, Nobili A, et al. Blunting neuroinflammation with resolvin D1 prevents early pathology in a rat model of Parkinson’s disease. Nat Commun. 2019;10(1):3945. doi:10.1038/s41467-019-11928-w
16. Hamlett ED, Hjorth E, Ledreux A, Gilmore A, Schultzberg M, Granholm AC. RvE1 treatment prevents memory loss and neuroinflammation in the Ts65Dn mouse model of Down syndrome. Glia. 2020;68(7):1347–1360. doi:10.1002/glia.23779
17. Xu ZZ, Zhang L, Liu T, et al. Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nat Med. 2010;16(5):592–7,1pfollowing7. doi:10.1038/nm.2123
18. Scheffer IE, Berkovic S, Capovilla G, et al. ILAE classification of the epilepsies: position paper of the ILAE commission for classification and terminology. Epilepsia. 2017;58(4):512–521. doi:10.1111/epi.13709
19. Kumar S, Singh MB, Shukla G, et al. Effective clinical classification of chronic epilepsy into focal and generalized: a cross sectional study. Seizure. 2017;53:81–85. doi:10.1016/j.seizure.2017.11.002
20. Li H, Yang Y, Hu M, et al. The correlation of temporal changes of neutrophil-lymphocyte ratio with seizure severity and the following seizure tendency in patients with epilepsy. Front Neurol. 2022;13:964923. doi:10.3389/fneur.2022.964923
21. Berger S, Raman G, Vishwanathan R, Jacques PF, Johnson EJ. Dietary cholesterol and cardiovascular disease: a systematic review and meta-analysis. Am J Clin Nutr. 2015;102(2):276–294. doi:10.3945/ajcn.114.100305
22. Cramer JA, French J. Quantitative assessment of seizure severity for clinical trials: a review of approaches to seizure components. Epilepsia. 2001;42(1):119–129. doi:10.1046/j.1528-1157.2001.19400.x
23. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi:10.1016/0022-3956(75)90026-6
24. Kooij G, Troletti CD, Leuti A, et al. Specialized pro-resolving lipid mediators are differentially altered in peripheral blood of patients with multiple sclerosis and attenuate monocyte and blood-brain barrier dysfunction. Haematologica. 2020;105(8):2056–2070. doi:10.3324/haematol.2019.219519
25. Do KV, Hjorth E, Wang Y, et al. Cerebrospinal fluid profile of lipid mediators in Alzheimer’s disease. Cell Mol Neurobiol. 2023;43(2):797–811. doi:10.1007/s10571-022-01216-5
26. Toewe A, Balas L, Durand T, Geisslinger G, Ferreirós N. Simultaneous determination of PUFA-derived pro-resolving metabolites and pathway markers using chiral chromatography and tandem mass spectrometry. Anal Chim Acta. 2018;1031:185–194. doi:10.1016/j.aca.2018.05.020
27. Fosshaug LE, Colas RA, Anstensrud AK, et al. Early increase of specialized pro-resolving lipid mediators in patients with ST-elevation myocardial infarction. EBioMedicine. 2019;46:264–273. doi:10.1016/j.ebiom.2019.07.024
28. Calder PC. Eicosapentaenoic and docosahexaenoic acid derived specialised pro-resolving mediators: concentrations in humans and the effects of age, sex, disease and increased omega-3 fatty acid intake. Biochimie. 2020;178:105–123. doi:10.1016/j.biochi.2020.08.015
29. Zhao YC, Wang CC, Li XY, et al. Supplementation of n-3 PUFAs in adulthood attenuated susceptibility to pentylenetetrazol induced epilepsy in mice fed with n-3 PUFAs deficient diet in early life. Mar Drugs. 2023;21(6):354. doi:10.3390/md21060354
30. Taha AY, Burnham WM, Auvin S. Polyunsaturated fatty acids and epilepsy. Epilepsia. 2010;51(8):1348–1358. doi:10.1111/j.1528-1167.2010.02654.x
31. DeGiorgio CM, Miller P, Meymandi S, Gornbein JA. n-3 fatty acids (fish oil) for epilepsy, cardiac risk factors, and risk of SUDEP: clues from a pilot, double-blind, exploratory study. Epilepsy Behav. 2008;13(4):681–684. doi:10.1016/j.yebeh.2008.08.001
32. Bromfield E, Dworetzky B, Hurwitz S, et al. A randomized trial of polyunsaturated fatty acids for refractory epilepsy. Epilepsy Behav. 2008;12(1):187–190. doi:10.1016/j.yebeh.2007.09.011
33. Leuti A, Fazio D, Fava M, Piccoli A, Oddi S, Maccarrone M. Bioactive lipids, inflammation and chronic diseases. Adv Drug Deliv Rev. 2020;159:133–169. doi:10.1016/j.addr.2020.06.028
34. Bell B, Lin JJ, Seidenberg M, Hermann B. The neurobiology of cognitive disorders in temporal lobe epilepsy. Nat Rev Neurol. 2011;7(3):154–164. doi:10.1038/nrneurol.2011.3
35. Dong X, Fan J, Lin D, et al. Captopril alleviates epilepsy and cognitive impairment by attenuation of C3-mediated inflammation and synaptic phagocytosis. J Neuroinflammation. 2022;19(1):226. doi:10.1186/s12974-022-02587-8
36. Dong X, Hao X, Xu P, et al. RNA sequencing analysis of cortex and hippocampus in a kainic acid rat model of temporal lobe epilepsy to identify mechanisms and therapeutic targets related to inflammation, immunity and cognition. Int Immunopharmacol. 2020;87:106825. doi:10.1016/j.intimp.2020.106825
37. Wang X, Zhu M, Hjorth E, et al. Resolution of inflammation is altered in Alzheimer’s disease. Alzheimers Dement. 2015;11(1):40–50.e1–2. doi:10.1016/j.jalz.2013.12.024
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.