Back to Journals » Research and Reports in Urology » Volume 17
Delayed Presentation of Mixed Testicular Tumor 19 Years After Orchiopexy: A Case Report and Literature Review
Authors Prihadi JC, Haruman SP , Tambun R, Yustira RS
Received 25 February 2025
Accepted for publication 4 July 2025
Published 14 July 2025 Volume 2025:17 Pages 225—231
DOI https://doi.org/10.2147/RRU.S521544
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Guglielmo Mantica
Johannes Cansius Prihadi,1 Sean Peter Haruman,1 Renaningtyas Tambun,2 Renandha Septaryan Yustira3
1Department of Surgery, Urology Division, School of Medicine and Health Sciences, Atma Jaya Catholic University of Indonesia/Atma Jaya Hospital, Jakarta, Indonesia; 2Department of Pathological Anatomy, St. Carolus Hospital, Jakarta, Indonesia; 3Department of Urology, St. Carolus Hospital, Jakarta, Indonesia
Correspondence: Johannes Cansius Prihadi, Department of Surgery, Urology Division, School of Medicine and Health Sciences, Atma Jaya Catholic University of Indonesia/Atma Jaya Hospital, Jl. Pluit Selatan Raya No. 19, RT.21/RW.8, Penjaringan, North Jakarta, DKI Jakarta, Jakarta, 14440, Indonesia, Tel +62216694366, Email [email protected]
Background: Testicular cancer is a relatively rare malignancy, accounting for 1% of solid tumors in adult males and 5% of all urological tumors. Orchidopexy has been shown to lower the risk of testicular cancer, but it does not prevent it.
Case Presentation: A 20-year-old adult male presented with a painful and palpable mass in his right testicle. The patient had orchidopexy at six months old but had been asymptomatic until his recent hospital visit. There was no previous history of cancer. Tumor markers including AFP and β-HCG were found to be elevated. Preoperative ultrasonograghy (USG) showed a solid and cystic mass of the right testis with increased vascularization. Following these findings, the patient underwent an orchidectomy. Histopathology examination revealed mixed type NSGCT of embryonal and choriocarcinoma. However, after completing four cycles of chemotherapy, a CT scan evaluation showed an enlarged mass in the abdomen, which was confirmed to be a mature teratoma.
Conclusion: In patients with a history of cryptorchidism, orchidopexy does not rule out the possibility of testicular cancer. Orchidopexy did reduce the risk of seminoma, but not nonseminomatous germ cell tumor, which in our case continued to develop a growing teratoma on the abdomen. It is essential to conduct long-term monitoring of patients who have undergone orchiopexy due to their risk of developing testicular cancer.
Keywords: NSGCT, testicular cancer, mixed germ cell tumor, cryptorchidism, radical orchidectomy, orchidopexy, undescended testis
Introduction
Testicular cancer is a relatively rare malignancy, accounting for 1% of solid tumors in adult males and 5% of all urological tumors. This cancer is the most prevalent in men aged 20 to 40. Approximately 95% of testicular neoplasms are germ cell tumors (GCT), which consist of seminoma and nonseminomatous (NSGCT).1,2 The history of cryptorchidism is considered to be the biggest factor in the development of testicular cancer. The risk of developing testicular cancer will increase by 3.6–7.4 times higher than the general population, and as many as 2–6% of men with a history of cryptorchidism will develop testicular cancer.3,4
In patients with cryptorchidism, prepubertal orchidopexy has been found to reduce the incidence of testicular cancer. However, even after an orchidopexy, the risk for testicular cancer is significantly higher than in healthy males, therefore patients must remain cautious.4
This study used SCARE criteria, we report a case of embryonal carcinoma and choriocarcinoma in an adult patient with a history of orchidopexy.5
Case Report
A 20-year-old adult male presented with the chief complaint of right testicular enlargement accompanied by pain for one month. The patient had not taken any medication for this complaint. The mass was also growing in size. The patient had a history of orchidopexy on the right testicle at the age of 6 months for cryptorchidism and had no symptoms after then. There were no other health issues or a family history of cancer in the patient.
On physical inspection, the right testicle appeared larger than the left, while the rest appeared normal. A solid mass with the size of a ping pong ball was palpated in the right testicle, whereas the left testicle was within normal limits. The transillumination test resulted in a negative result. After that, an ultrasound of both testes revealed a solid and cystic mass of the right testis measuring 65 × 34.5 mm (Figure 1).
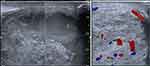 |
Figure 1 An ultrasound of the right testicle revealed a mass and increased vascularization. |
The complete blood count and blood biochemistry results were within normal limits. Analysis of serum testicular tumor markers; alpha-fetoprotein (AFP) 1291 ng/mL (normal: 0.89–8.78 ng/mL), beta-human chorionic gonadotropin (β-HCG) 924 mIU/mL (normal: 0–5mIU/mL) as presented in Table 1.
 |
Table 1 The Tumor Markers During Treatment |
A right high ligation orchidectomy was performed on the patient. The testes were 6.5 × 4.5 × 6.5 cm in size, grayish-brown in color, and had a gray mass with necrosis and hemorrhage on dissection, with a wall thickness of 1–1.5 cm (Figure 2). The spermatic cord was 6.5 cm long and had a diameter of 0.5–1.5 cm. There were no complication intra- or post-operatively. The patient was discharged on POD 3 and returned two months later for his chemotherapy.
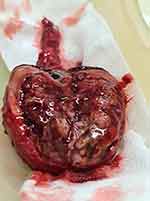 |
Figure 2 Macroscopic appearance of the right testicle. |
On histopathological examination, the mass was identified as a mixed germ cell tumor of embryonal carcinoma and choriocarcinoma, as well as lymphovascular invasion (Figure 3). There was no tumor invasion in the epididymis, tunica albuginea, or tunica vaginalis. An abdominal CT scan before to chemotherapy showed a large solid mass 16 × 10 × 8.5 cm in size in the aortico-caval to the right parailiac region, pressing the aorta to the left and slightly crossing the midline to the left and causing moderate obstructive right hydronephrosis and hydroureter (Figure 4).
 |
Figure 4 Abdominal CT scan before chemotherapy showed a large solid mass 16×10 x 8.5 cm in size. |
Before starting chemotherapy using BEP regimen, serum tumor markers AFP, β-HCG, and LDH (lactate dehydrogenase) were detected at 276 ng/mL, 105 mIU/mL, and 216 U/L, respectively. During the second cycle of chemotherapy, the patient experienced grade 4 hematological toxicity, which was manageable with supportive care. One month after finishing four cycles of BEP, the patient was checked for serum tumor markers and an abdominal CT scan as a follow-up. AFP, β-HCG, and LDH were found to be 6.88 ng/mL, 1.2 mIU/mL, and 202 U/L respectively. When the abdominal CT scan was compared to the previous CT scan, a solid mass with cystic components was seen in the midline para aortocaval at the level of vertebra L1 to the right para iliac at the level of vertebra S1 17 × 16 × 9.3 cm in size, pushing the aorta to the left and the right ureter to the anterior (Figure 5). Therefore, the patient underwent a biopsy due to an unresectable mass. The histopathological findings indicated that it was a mature teratoma.
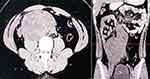 |
Figure 5 Abdominal CT scan after chemotherapy showed solid mass with cystic components 17×16 x 9.3 cm in size. |
Discussion
The patient was diagnosed with mixed type NSGCT embryonal and choriocarcinoma in our case. This variety is extremely rare, accounting for only 1–3% of all occurrences.6,7
There is no conclusive evidence that orchidopexy can prevent cryptorchidism-related GCT of the testicles. According to one study hypothesis, undescended testes exposed to higher temperatures trigger aberrant apoptosis, which changes gonocytes into intratubular germ cell neoplasms (ITGCN), which can eventually lead to cancer. The presence of placental-like alkaline phosphatase (PLAP)-positive germ cells in cryptorchid testes backed up this idea. PLAP is usually discovered when gonocyte maturation into type A spermatogenesis fails, resulting in inadequate germ cell development.8
In this case, the patient had orchidopexy at the age of six months. Patients who have orchidopexy at a given age have a lower relative risk, according to studies. A retrospective study stated that individuals receiving orchidopexy before the age of 13 had a relative risk of 2.23, whereas those above the age of 13 had a relative risk of 5.4.9 According to a literature review, individuals who had orchidopexy when they were 10 to 12 years old had a 2 to 6 times lower relative risk than those who had it when they were older than 12 years old or not at all.10 As a result, many recommended orchidopexy at a younger age, and in modern practice according to consensus, orchidopexy can be done as early as 6 months and as late as 18 months because delaying it can influence fertility.11
It took 20 years for this patient to be diagnosed with testicular cancer after undergoing orchidopexy. We found similarities with the previous study, in which testicular cancer was found after 21 years of orchidopexy.12 The average age interval from orchidopexy to the formation of testicular cancer was around 17.6 years in a retrospective study, with the shortest period being 5 years and the longest being 30 years. Patients receiving orchidopexy above the age of 13 years had a greater rate of cancer formation, with an average age of around 14 years.13 A study also reported that a 23-year-old patient had NSGCT despite having an orchidopexy when he was 5 years old.14 Another retrospective study found that 49 patients with a history of orchidopexy had an average time interval of 18 years before a germ cell tumor developed in the ipsilateral testis.15 The time interval between orchidopexy and the development of GCT varies greatly and is determined by the age of the orchidopexy. When compared to earlier studies, our patients’ age interval was more or less comparable.
Furthermore, we investigated whether tumor type affects cancer onset rates. Unfortunately, we only found one study that reported 73 cases of germ cell tumors with a prior history of orchidopexy and discovered that patients with seminoma had a longer time interval between orchidopexy and orchidectomy, with an interval of 16.4 years, compared to 8.9 and 9.8 years for embryonal and teratoma types, respectively.16 According to the latest literature, NSGCT has more vascularization than seminoma, allowing them to grow faster.2 As a result, the type of tumor affects the rate of cancer progression.
In this case, the type of cancer that develops after orchidopexy is NSGCT. According to a previous study of 43 patients who had orchidopexy after puberty, as many as 40% developed seminoma, followed by embryonal tumors (25%), and teratocarcinoma (19%). Another study that compared patients who had orchidopexy and those who did not discover that the group without orchidopexy had a higher rate of seminoma (71.4%), whereas those who had orchidopexy were dominated by teratocarcinoma (35%), embryonal (33%), and seminoma (29%).10 A retrospective study of 34 testicular cancer patients, 9 of whom had orchidopexy and the remaining did not have their cryptorchidism corrected. According to the findings of this study, NSGCT was very dominant in patients who had undergone orchidopexy (78%), whereas seminoma was prevalent in patients who did not correct their cryptorchidism (68%).17 Similar outcomes are seen in other case studies.12,14,18,19 A review concluded that orchidopexy reduced the relative risk of seminoma but did not increase the relative incidence of NSGCT.20 These findings corroborate our case report, in which a patient had NSGCT after orchidopexy as well.
Radical orchidectomy, in combination with chemotherapy, radiation, or retroperitoneal lymph node dissection (RPLND), is the standard treatment for testicular GCT, depending on the tumor type and severity. The relevance of examining tumor markers before orchidectomy rather than afterward can result in undertreatment with possibly poor outcomes or overtreatment with significant toxicity.21 Getting the right dose of chemotherapy to maximize patient outcomes is a critical part of advanced GCT management. The choice of RPLND (±) adjuvant chemotherapy or primary chemotherapy (±) after RPLND chemotherapy for mixed germ cell tumor grade IIB is still debatable, yet both have a 95% survival rate.2 According to the European Association of Urology’s standards for treatment stage IIB, if the patient has a negative tumor marker, it can be observed for 6 weeks before checking the tumor marker again, or nerve-sparing RPLND can be done first, followed by chemotherapy for two cycles. If the tumor marker is positive, three cycles of chemotherapy (BEP) were administered followed by removal of the remaining tumor.22 After first-line chemotherapy, between 38% and 68% of patients had tumor residuals more than 1 cm, indicating that they should have post-chemotherapy surgery.23–25
Teratoma in our case can be included as growing teratoma syndrome (GTS), which defined by the following criteria: enlargement of metastatic masses during or after chemotherapy given for NSCGT, normal level of serum tumor markers, and histopathologically confirmed mature teratoma in a resected metastatic site in a patient with NSGCT.26 GTS is a rare condition with incidence ranging from 1.9% to 7.6%. According to a retrospective review in a single-center study, the most common site of metastasis was retroperitoneum (66%), lung (24%), and brain (5%).27 GTS should be surgically resected because they are resistant to both chemotherapy and radiation therapy.28,29 In a recent study, patients with unresectable disease had clinical benefits with interferon or salvage chemotherapy (TIP) followed by autologous stem cell transplantation (ASCT). Thus, these therapies seem to be promising but need further research.27
In this instance even though the patient received orchiopexy at a young age, it is evident that a clinical suspicion of any ambiguous scrotal ultrasound and long-term monitoring are crucial. We observed a number of comparable cases, including a recent case where a 24-year-old adult male presented with complaint mild discomfort of the left scrotal and ultrasound showed large amount of fluid collection in the left scrotal containing multiple thick echogenic septations and was described as complex hydrocele. But when the patient underwent surgery, scrotal exploration refuted the presence of a hydrocele instead it revealed the presence of a multicystic testicular tumor distorting the testicular parenchyma.30 Understanding the importance of long-term monitoring and being mindful of testicular cancer (TC) survivors’ quality of life is crucial in a case of 20-year-old adult with bilateral testicular lesions where he underwent tight radical orchiectomy and left testis-sparing surgery (TSS). Bilateral testicular tumors are very rare, accounting for 1%–5% of all testicular germ-cell tumors (TGCTs). This paper emphasizes the importance of having compliance with follow-up protocols and long-term monitoring of TC patients, given the general propensity to psychosocial distress and the risk of physical difficulties deriving from both the curative treatments and the hormonal replacement therapy.31 This is very similar to this case, in which we must be conscious of the necessity of self-examination and the rarity of the condition as well as the significance of long-term monitoring because of the disease’s protracted course.
In this case report, we have a limitation that it is a single case report, which restricts the broader application of the findings and underscores the importance of conducting further research with larger groups.
Conclusion
Even though orchidopexy was performed as soon as possible, it does not rule out the development of testicular cancer, as in our case. NSGCT is the most common type of testicular cancer found after orchidopexy, but because this type has a high percentage of post-chemotherapy residual mass, follow-up is required to determine the possibility of further action. It is essential to conduct long-term monitoring of patients who have undergone orchiopexy due to their risk of developing testicular cancer.
Acknowledgments
We would like to express our gratitude to Dr Albertus Ardian Prawidyanto, SpRad in the Department of Radiology of St Carolus Hospital who has helped us a lot in this study.
Informed Consent
We have obtained informed consent from the patient for the writing and publication of this case report, including all relevant clinical details and accompanying images. Institutional approval was not required for the publication of this case report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sandikci F, Cimen S, Cimen SG, et al. Bilateral intra-abdominal testicular tumor: case report. Inte J Surg Case Rep. 2018;49:102. doi:10.1016/J.IJSCR.2018.06.027
2. Partin AW, Dmochowski RR, Kavoussi LR, Peters CA. Neoplasm of Testis. In: Campbell-Walsh-Wein Urology.
3. Ferguson L, Agoulnik AI. Testicular cancer and cryptorchidism. Front Endocrinol. 2013;4(MAR). doi:10.3389/FENDO.2013.00032
4. Salonia A, Bettocchi C, Carvalho J, et al. Sexual and reproductive health EAU guidelines. European Association of Urology. 2021. Available from: https://uroweb.org/guideline/sexual-and-reproductive-health/.
5. Agha RA, Borrelli MR, Farwana R, et al. The SCARE 2018 statement: updating consensus surgical case report (SCARE) guidelines. Inter J Surg. 2018;60:132–136. doi:10.1016/J.IJSU.2018.10.028
6. Stamatiou K, Papadopoulos P, Perlepes G, et al. Mixed germ cell tumor of the testicle with ravdomuosarcomatous component: a case report. Cases J. 2009;2(1):9299. doi:10.1186/1757-1626-2-9299
7. Gaddam SJ, Chesnut GT. Testicle Cancer. StatPearls; 2020.
8. Mohamed AO, Murtagh K, Kockelbergh R, ElMalik K. Testicular surveillance post-orchidopexy and its impact on early diagnosis of testicular cancer. Indian J Surg Oncol. 2020;11(3):513–517. doi:10.1007/S13193-020-01169-5
9. Pettersson A, Richiardi L, Nordenskjold A, Kaijser M, Akre O, Institutet K. Age at surgery for undescended testis and risk of testicular cancer. New England J Med. 2009;356(18):1835–1841. doi:10.1056/NEJMOA067588
10. Wood HM, Elder JS. Cryptorchidism and testicular cancer: separating fact from fiction. J Urol. 2009;181(2):452–461. doi:10.1016/j.juro.2008.10.074
11. Radmayr C, Bogaert G, Dogan HS, et al. EAU guidelines: paediatric urology | uroweb. European Association of Urology. 2021. Available from: https://uroweb.org/guideline/paediatric-urology/#6.
12. Kubota M, Terada N, Ito K, et al. A 45,X/46,XY male with orchidopexy diagnosed with mixed germ cell tumor after 21-year follow-up. Urology Case Reports. 2017;13:120–122. doi:10.1016/J.EUCR.2017.04.001
13. Saini AK, Regmi S, Seth A, Narayan R, Singh P, Dogra PN. Outcome analysis of tumors in undescended testes--a single center experience of 15 years. Urology. 2013;82(4):852–856. doi:10.1016/J.UROLOGY.2013.05.050
14. Manassero F, Cuttano MG, Morelli G, Salinitri G, Spurio M, Selli C. Mixed germ cell tumor after bilateral orchidopexy in persistent Müllerian duct syndrome with transverse testicular ectopia. Urologia Internationalis. 2004;73(1):81–83. doi:10.1159/000078809
15. Jones JA, Hobaugh CW, Salvas DB, Bennett RM, Godoy G, Donohue JP. Urology and andrology open journal outcomes in patients with testicular maldescent and germ cell neoplasia: a retrospective assessment and review of the literature outcomes in patients with testicular maldescent and germ cell neoplasia: a retrospective assessment and review of the literature retrospective study. Urol Androl Open J. 2018;2(1):8–18. doi:10.17140/UAOJ-2-113
16. Martin DC. Germinal cell tumors of the testis after orchiopexy. J Urol. 1979;121(4):422–424. doi:10.1016/S0022-5347(17)56808-1
17. Jones BJ, Thornhill JA, O’Donnell B, et al. Influence of prior orchiopexy on stage and prognosis of testicular cancer. Europ Urol. 1991;19(3):201–203. doi:10.1159/000473619
18. Nanpo Y, Yamauchi T. [Testicular tumor following orchiopexy: a case report]. Acta Urologica Japonica. 2006;52(8):655–659. Italian
19. Minato N, Yamaguchi Y, Koga M, Sugao H, Hoshi M, Mori H. [Testicular cancer with inguinal lymph node metastasis in a patient with prior orchiopexy for undescended testis: a case report]. Acta Urologica Japonica. 2011;57(11):643–647. Italian
20. Batra NV, DeMarco RT, Bayne CE. A narrative review of the history and evidence-base for the timing of orchidopexy for cryptorchidism. J Pedia Urol. 2021;17(2):239–245. doi:10.1016/j.jpurol.2021.01.013
21. Feldman DR. State-of-the-art management of germ cell tumors. Am Soc Clin Oncol Educ Book. 2018;38(38):319. doi:10.1200/EDBK_201139
22. Algaba F, Bokemeyer C, Boormans J, et al. Testicular cancer eau guidelines on. Euro Urolog. 2020.
23. Honecker F, Aparicio J, Berney D, et al. ESMO consensus conference on testicular germ cell cancer: diagnosis, treatment and follow-up. Ann Oncol. 2018;29(8):1658–1686. doi:10.1093/ANNONC/MDY217
24. Krege S, Beyer J, Souchon R, et al. European consensus conference on diagnosis and treatment of germ cell cancer: a report of the second meeting of the European germ cell cancer consensus group (EGCCCG): part I. Europ Urol. 2008;53(3):478–496. doi:10.1016/j.eururo.2007.12.024
25. Krege S, Beyer J, Souchon R, et al. European consensus conference on diagnosis and treatment of germ cell cancer: a report of the second meeting of the European germ cell cancer consensus group (EGCCCG): part II. Europ Urol. 2008;53(3):497–513. doi:10.1016/j.eururo.2007.12.025
26. Logothetis CJ, Samuels ML, Trindade A, Johnson DE. The growing teratoma syndrome. Cancer. 1982;50(8):1629–35.
27. Acikgoz Y, Bal O, Ergun Y, Oksuzoglu B, Yildiz B, Doǧan M. Systemic treatment options for growing teratoma syndrome: a single-center experience with a comprehensive review of the literature. J Cancer Res Ther. 2021;17(1):75–79. doi:10.4103/jcrt.JCRT_568_19
28. Gorbatiy V, Spiess P, Pisters L. The growing teratoma syndrome: current review of the literature. Indian J Urol. 2009;25(2):186–189. doi:10.4103/0970-1591.52910
29. Tanaka K, Toyokawa G, Tagawa T, et al. Successful treatment of growing teratoma syndrome of the lung by surgical resection: a case report and literature review. Anticancer Res. 2018;38(5):3115–3118. doi:10.21873/anticanres.12571
30. Symeonidis EN, Sountoulides P, Asouhidou I, et al. Be cautious of “complex hydrocele” on ultrasound in young men. Arch Ital Urol Androl. 2020;92(1):61–63. doi:10.4081/aiua.2020.1.61
31. Symeonidis EN, Tsifountoudis I, Anastasiadis A, et al. Synchronous bilateral testicular cancer with discordant histopathology occurring in a 20-year-old patient: a case report and review of the literature. Urologia. 2023;90(2):434–441. doi:10.1177/03915603211028556
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


