Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 18
Deucravacitinib Improved Interstitial Pneumonia Along with KL-6 Reduction in a Patient with Psoriasis: A Case Report
Authors Mima Y , Ohtsuka T, Akiyama N, Norimatsu Y
Received 24 April 2025
Accepted for publication 5 July 2025
Published 8 July 2025 Volume 2025:18 Pages 1677—1681
DOI https://doi.org/10.2147/CCID.S536534
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Yoshihito Mima,1,2 Tsutomu Ohtsuka,2 Norimichi Akiyama,3 Yuta Norimatsu4
1Department of Dermatology, Tokyo Metropolitan Police Hospital, Tokyo, Japan; 2Department of Dermatology, International University of Health and Welfare Hospital, Tochigi, Japan; 3Department of Respiratory Medicine, Fujieda Municipal General Hospital, Shizuoka, Japan; 4Department of Dermatology, International University of Health and Welfare Narita Hospital, Chiba, Japan
Correspondence: Yoshihito Mima, Department of Dermatology, Tokyo Metropolitan Police Hospital, 4-22-1 Nakano, Nakano-ku Tokyo, 164-8541, Japan, Tel +81-03-5343-5611, Fax +81-03-5343-5612, Email [email protected]
Abstract: This case report describes a patient with psoriasis and interstitial pneumonia (IP) presenting with linear opacities who was treated with deucravacitinib, aiming to highlight the potential role of deucravacitinib in improving IP. Psoriasis is a chronic immune-mediated skin disease involving T helper (Th) 17 cells, often accompanied by systemic comorbidities. Deucravacitinib, a selective oral tyrosine kinase 2 (TYK2) inhibitor targeting interleukin (IL)-23 and type I interferons, has shown strong efficacy and safety in psoriasis treatment. Interstitial pneumonia (IP) is a group of lung diseases characterized by inflammation, fibrosis, and progressive respiratory decline. Cytokines play key roles in its pathogenesis. Emerging evidence suggests that psoriasis has higher risks of IP, possibly due to shared IL-23/IL-17 pathway. The patient showed marked improvement in skin and lung findings, along with KL-6 levels after deucravacitinib treatment. TYK2 mediates downstream signaling of key pro-fibrotic and pro-inflammatory cytokines involved in IP. Therefore, we consider that deucravacitinib may have contributed to the improvement of IP by blocking these signaling pathways, thereby suppressing chronic T cell–driven inflammation and fibrosis. Further accumulation of cases and continued research will be essential in advancing discussions on the clinical utility of TYK2 inhibitors in IP management.
Keywords: interleukin, KL-6, deucravacitinib, T helper cells, tyrosine kinase 2
Introduction
Psoriasis is a chronic immune-mediated inflammatory skin disease that significantly impairs patients’ quality of life.1,2 Clinically, it typically presents as well-demarcated erythematous plaques covered with silvery-white scales, commonly appearing on the scalp, buttocks, elbows, and knees.3 Psoriasis is associated with a wide range of systemic comorbidities, including psoriatic arthritis, cardiovascular disease, metabolic syndrome, obesity, diabetes, hypertension, autoimmune diseases, malignancies, inflammatory bowel disease, non-alcoholic fatty liver disease, and depression.4 Both T helper 1 (Th1) and Th17 cells play central roles in psoriasis pathogenesis. Interleukin (IL)-12 is crucial for Th1 differentiation and proliferation, promoting the production of interferon-γ and tumor necrosis factor (TNF)-α. Meanwhile, IL-23 is essential for the maintenance and expansion of Th17 cells.5 Accordingly, IL-12 and IL-23 are both considered key pathogenic mediators in psoriasis vulgaris.5 Activated Th1 and Th17 cells produce cytokines such as IL-17A, IL-17F, IL-22, and TNF-α, which induce keratinocyte hyperproliferation and inflammation in the epidermis and dermis.5–7
Recent studies have reported a higher prevalence of IP among patients with psoriasis.8 IP comprises a heterogeneous group of disorders characterized by inflammation and fibrosis of the lung parenchyma, often leading to progressive respiratory symptoms, pulmonary function decline, respiratory failure, and reduced quality of life.9 Etiological factors include connective tissue diseases, environmental exposures, drug-induced lung injury, radiation therapy, occupational hazards, and allergens.10 The pathogenesis of IP involves complex interactions among immune cells, cytokines, and growth factors.11–13 Lung injury induces the release of growth factors such as transforming growth factor-beta (TGF-β), which activates fibroblasts and promotes their differentiation into myofibroblasts.11 In patients with IP, excessive proliferation of myofibroblasts leads to abnormal deposition of extracellular matrix components, such as collagen, in the pulmonary interstitium.11 This drives tissue remodeling and results in irreversible structural changes. Krebs von den Lungen-6 (KL-6) is an immunological biomarker that reflects the severity and progression of IP.14 Elevated KL-6 levels are associated with an increased risk of acute exacerbation and mortality, and are considered a poor prognostic indicator.14 Moreover, the interleukin (IL)-23/IL-17 axis, which plays a central role in the pathogenesis of psoriasis, has also been implicated in the inflammatory and fibrotic processes of IP. Indeed, patients with psoriasis have a significantly higher prevalence of IP compared to non-psoriatic individuals, suggesting a potential immunological link between the two conditions mediated through the IL-23/IL-17 signaling pathway.8
Recently, in addition to injectable biologics which inhibit Th17-mediated keratinocyte activation by targeting IL-17 and IL-23, deucravacitinib, a novel oral therapy, has been introduced.15,16 Deucravacitinib is a selective oral tyrosine kinase 2 (TYK2) inhibitor.16 TYK2, a member of the Janus kinase (JAK) family, mediates the signaling of IL-12, IL-23, and type I interferons.17 By selectively inhibiting TYK2, deucravacitinib disrupts these cytokine pathways, thereby suppressing keratinocyte hyperproliferation and inflammation, making it an effective treatment for psoriasis vulgaris.16,17 The efficacy of this treatment has been demonstrated in two large Phase III trials comparing it to placebo and apremilast therapy in patients with moderate-to-severe psoriasis.16 Common adverse effects include nasopharyngitis, upper respiratory tract infections, headache, diarrhea, and nausea. Unlike conventional JAK inhibitors, deucravacitinib has a favorable safety profile, with low rates of serious infections, thromboembolic events, and significant laboratory abnormalities including cytopenia.16
In the treatment of IP, in addition to conventional options such as antifibrotic agents like pirfenidone and nintedanib, as well as immunosuppressants including corticosteroids and azathioprine, JAK inhibitors have also been explored as potential therapeutic agents.18,19 Several case reports have suggested their clinical efficacy in this context.19 However, to date, no studies or case reports have demonstrated the efficacy of TYK2 inhibitors for IP.
Herein, we present the first case of a patient with psoriasis vulgaris and IP, who was treated with deucravacitinib, a selective TYK2 inhibitor. Notably, treatment resulted in marked improvement not only in KL-6 levels, but also in IP on CT findings.
Case Report
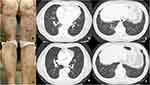
The patient was a 76-year-old man with a >5-year history of psoriasis who had been managed with topical betamethasone butyrate propionate and calcipotriol. Two years prior, computed tomography (CT) revealed mild linear and ground-glass opacities in the right lower lung field. As the patient remained asymptomatic, he was diagnosed with mild interstitial pneumonia (IP) requiring no active intervention. However, despite topical therapy, his psoriasis worsened over time, ultimately resulting in his referral to us. Physical examination revealed scaly, keratotic erythematous plaques on the trunk, extremities, and hairline without systemic symptoms such as joint pain, fatigue, or dyspnea (Figure 1a). Approximately 25% of the body surface area was affected and the Psoriasis Area and Severity Index (PASI) score was 25.4. Laboratory examination revealed slightly elevated Krebs von den Lungen (KL)-6 (592 U/mL), lactate dehydrogenase (283 U/l), and C-reactive protein (0.8 mg/dl) levels. Antinuclear antibody was negative, and no systemic symptoms indicative of connective tissue disease, such as joint pain or fever, were observed aside from the skin rash. CT tests 3 months prior to the initial visit confirmed the presence of linear and ground-glass opacities in the right lower lung field, which is consistent with prior findings (Figure 1b and c). When presented with the option of biologic agents or oral therapy, the patient expressed a preference for oral treatment, so we decided to administer deucravacitinib, an oral tyrosine kinase 2 (TYK2) inhibitor. The PASI score showed a marked improvement over time, decreasing to 15.2 at 1 month, 9.2 at 3 months, and reaching 1.8 at 8 months (Figure 1d). Throughout the course of treatment, the patient underwent careful monitoring for the potential exacerbation of IP. Notably, KL-6 levels showed significant improvement (277 U/mL), and follow-up CT at 8 months revealed marked resolution of the pulmonary opacities (Figure 1e and f).
Discussion
IP is a disease characterized by inflammation and fibrosis of the pulmonary interstitium. Following lung injury, growth factors such as transforming growth factor-beta (TGF-β) are released, leading to the activation and excessive proliferation of fibroblasts. This results in abnormal accumulation of extracellular matrix (ECM) components in the pulmonary interstitium, promoting tissue remodeling and ultimately causing irreversible structural changes. In IP, the progression to irreversible structural remodeling of the pulmonary interstitium is closely linked to the activation of T cells and their associated cytokines.12,13 Th2 cells secrete cytokines such as interleukin (IL)-13 and IL-31, which in turn activate myofibroblasts and promote collagen production, thereby accelerating fibrosis and contributing to disease progression.12 Th17 cells also play a role by cooperating with transforming growth factor-beta (TGF-β) to produce IL-17, which indirectly promotes fibrosis.15 Additionally, IL-6 not only contributes to local inflammation in the interstitium, but also facilitates TGF-β activation and promotes Th17 cell differentiation, further driving tissue remodeling and IP pathogenesis.11,13,20 Thus, the IL-23/IL-17 axis, which plays a central role in psoriasis, has also been implicated in the inflammation and fibrosis associated with IP. Patients with psoriasis have a significantly higher prevalence of comorbid IP compared to those without psoriasis, suggesting a potential immunological link between the two conditions mediated by the IL-23/IL-17 pathway.8
TYK2 mediates downstream signaling of several key pro-fibrotic and pro-inflammatory cytokines involved in IP, including IL-6, IL-13, IL-23, and IL-3121. Therefore, deucravacitinib—a selective TYK2 inhibitor—may suppress chronic T cell–driven inflammation and fibrosis by blocking these signaling pathways.11–13,20,21 Furthermore, because TYK2 is a key downstream molecule in the IL-23 receptor pathway in Th17 cells, its inhibition may suppress Th17 activation and reduce IL-17 production, potentially slowing the progression of pulmonary fibrosis.13,22 Inhibiting IL-6 signaling may further prevent Th17 differentiation and TGF-β activation, thereby mitigating lung inflammation and fibrosis and preventing irreversible tissue remodeling.11,20
KL-6 is a potential immunological biomarker that reflects the severity and progression of IP, with elevated levels being associated with an increased risk of acute exacerbation and higher mortality, making it a poor prognostic factor.14 Although IP and psoriasis share a common immunopathogenic pathway involving the IL-23/IL-17 axis, biologic agents targeting IL-23 or IL-17 have been associated with the onset or exacerbation of IP in some cases. Patients at higher risk for developing IP with these agents tend to be older and often present with elevated baseline KL-6 levels.23 In the assessment of interstitial pneumonia, quantitative analysis of CT findings is challenging, and evaluation often relies on visual interpretation of images before and after treatment. However, in the present case, deucravacitinib not only led to an improvement in IP on CT imaging, but also resulted in a significant reduction in KL-6 levels. These findings indicate that deucravacitinib may have played a crucial role in suppressing IP progression. By inhibiting immune cells and cytokines, deucravacitinib has the potential to regulate the inflammation and fibrosis of IP, thereby preventing the progression of irreversible tissue remodeling and reducing the risk of acute exacerbation and mortality.11–13,20–22
The pathogenesis of IP involves inflammatory cytokines such as IL-6, IL-13, IL-23, and IL-31, as well as growth factors such as TGF-β.11,12,20–22 Therefore, JAK inhibitors have previously been explored as a potential therapeutic option for IP, and there have been several reports of cases in which JAK inhibitors contributed to IP improvements.19 However, to date, no studies or case reports have demonstrated the efficacy of TYK2 inhibitors in the treatment of IP. Although a causal relationship between TYK2 inhibitor administration and the improvements in KL-6 levels and IP cannot be definitively established in this case, the temporal association between drug initiation and these clinical improvements suggests the potential therapeutic benefit of TYK2 inhibition in IP. This may represent the first case report to propose such a possibility. However, several limitations should be acknowledged. The reduction in KL-6 may have occurred as part of the natural course of the disease, or it may reflect spontaneous fluctuations in IP activity. While no other medications were altered during deucravacitinib therapy, the potential influence of concomitant drugs cannot be completely ruled out. In addition, histopathological confirmation of the IP subtypes was not obtained. To clarify whether the decrease in KL-6 represents a specific pharmacologic effect of TYK2 inhibition, prospective studies incorporating quantitative CT assessment and serial KL-6 measurements will be necessary. To evaluate both the therapeutic potential of TYK2 inhibitors for IP and their long-term pulmonary safety, further accumulation of similar cases and continued clinical investigation will be essential.
Conclusion
Herein, we report the case of a 76-year-old patient with psoriasis accompanied by interstitial lung opacities, in whom treatment with a TYK2 inhibitor led to marked improvement not only in skin lesions, but also in pulmonary findings. TYK2 mediates downstream signaling of key pro-fibrotic and pro-inflammatory cytokines involved in IP, including IL-6, IL-13, IL-23, and IL-31. Therefore, we propose that deucravacitinib, a selective TYK2 inhibitor, may have contributed to the improvement of IP by blocking these signaling pathways, thereby suppressing chronic T cell–driven inflammation and fibrosis. Further accumulation of cases and continued research will be essential in advancing discussions on the clinical utility of TYK2 inhibitors in IP management.
Data Sharing Statement
Additional data concerning this article may be requested from the corresponding author for reasonable reasons.
Informed Consent Statement
Written informed consent was obtained from the patient for the publication of this case report, including the clinical history, photographs, and radiological images. As this is a single case report, institutional ethical review by Tokyo Metropolitan Police Hospital was not applicable.
Funding
There is no funding to report.
Disclosure
Yoshihito Mima has received lecture fees from Bristol Myers Squibb, the distributor of deucravacitinib. Other authors declare no conflicts of interest related to this article.
References
1. Greb JE, Goldminz AM, Elder JT, et al. Psoriasis. Nat Rev Dis Primers. 2016;2:16082. doi:10.1038/nrdp.2016.82
2. Parisi R, Symmons DP, Griffiths CE, Ashcroft DM. Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team: global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377–385. doi:10.1038/jid.2012.339
3. Armstrong AW, Read C. Pathophysiology, clinical presentation, and treatment of psoriasis: a review. JAMA. 2020;323:1945–1960. doi:10.1001/jama.2020.4006
4. Takeshita J, Grewal S, Langan SM, et al. Psoriasis and comorbid diseases: epidemiology. J Am Acad Dermatol. 2017;76:377–390. doi:10.1016/j.jaad.2016.07.064
5. Alwan W, Nestle FO. Pathogenesis and treatment of psoriasis: exploiting pathophysiological pathways for precision medicine. Clin Exp Rheumatol. 2015;33:S2–S6.
6. Hawkes JE, Yan BY, Chan TC, Krueger JG. Discovery of the IL-23/IL-17 signaling pathway and the treatment of psoriasis. J Immunol. 2018;201:1605–1613. doi:10.4049/jimmunol.1800013
7. Yamanaka K, Yamamoto O, Honda T. Pathophysiology of psoriasis: a review. J Dermatol. 2021;48:722–731. doi:10.1111/1346-8138.15913
8. Kawamoto H, Hara H, Minagawa S, et al. Interstitial pneumonia in psoriasis. Mayo Clin Proc Innov Qual Outcomes. 2018;2:370–377. doi:10.1016/j.mayocpiqo.2018.07.006
9. Travis WD, Costabel U, Hansell DM, et al. An official American thoracic society/European respiratory society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188:733–748. doi:10.1164/rccm.201308-1483ST
10. Samarelli AV, Tonelli R, Marchioni A, et al. Fibrotic idiopathic interstitial lung disease: the molecular and cellular key players. Int J Mol Sci. 2021;22:8952. doi:10.3390/ijms22168952
11. Lederer DJ, Martinez FJ. Idiopathic pulmonary fibrosis. N Engl J Med. 2018;378:1811–1823. doi:10.1056/NEJMra1705751
12. Montero P, Milara J, Roger I, Cortijo J. Role of JAK/STAT in interstitial lung diseases; molecular and cellular mechanisms. Int J Mol Sci. 2021;22:6211. doi:10.3390/ijms22126211
13. Wilson MS, Madala SK, Ramalingam TR, et al. Bleomycin and IL-1beta-mediated pulmonary fibrosis is IL-17A dependent. J Exp Med. 2010;207:535–552. doi:10.1084/jem.20092121
14. Duan C, Gao L, Duan C, Gao L. KL-6 as an immunological biomarker predicts the severity, progression, acute exacerbation, and poor outcomes of interstitial lung disease: a systematic review and meta-analysis. Front Immunol. 2021;12:745233. doi:10.3389/fimmu.2021.745233
15. Ghoreschi K, Balato A, Enerbäck C, Sabat R. Therapeutics targeting the IL-23 and IL-17 pathway in psoriasis. Lancet. 2021;397:754–766. doi:10.1016/S0140-6736(21)00184-7
16. Armstrong AW, Gooderham M, Warren RB, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled Phase 3 POETYK PSO-1 trial. J Am Acad Dermatol. 2023;88:29–39. doi:10.1016/j.jaad.2022.07.002
17. Abduelmula A, Gooderham MJ. TYK2 inhibition: changing the treatment landscape for psoriasis? Expert Rev Clin Immunol. 2022;18:185–187. doi:10.1080/1744666X.2022.2008240
18. Bonella F, Spagnolo P, Ryerson C. Current and future treatment landscape for idiopathic pulmonary fibrosis. Drugs. 2023;83:1581–1593. doi:10.1007/s40265-023-01950-0
19. Huo R, Guo Q, Hu J, et al. Therapeutic potential of janus kinase inhibitors for the management of interstitial lung disease. Drug Des Devel Ther. 2022;16:991–998. doi:10.2147/DDDT.S353494
20. Epstein Shochet G, Brook E, Bardenstein-Wald B, Shitrit D. TGF-β pathway activation by idiopathic pulmonary fibrosis (IPF) fibroblast derived soluble factors is mediated by IL-6 trans-signaling. Respir Res. 2020;21:56. doi:10.1186/s12931-020-1319-0
21. Rusiñol L, Puig L. Tyk2 targeting in immune-mediated inflammatory diseases. Int J Mol Sci. 2023;24:3391. doi:10.3390/ijms24043391
22. Volpe E, Servant N, Zollinger R, et al. A critical function for transforming growth factor-β, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat Immunol. 2008;9:650–657. doi:10.1038/ni.1613
23. Miyagawa H, Hara H, Araya J. Characteristics of anti-IL-17/23 biologics-induced interstitial pneumonia in patients with psoriasis. PLoS One. 2021;16:e0245284. doi:10.1371/journal.pone.0245284
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.