Back to Journals » International Journal of General Medicine » Volume 18
Does Control Nutritional Status (CONUT) Score Predict Early and Long-Term Mortality at the Initiation of Maintenance Hemodialysis?
Authors Selen T , Ulusal Okyay G, Ayerden Ebinç F, Merhametsiz Ö, Şahin H , Aylı MD
Received 8 April 2025
Accepted for publication 2 July 2025
Published 7 July 2025 Volume 2025:18 Pages 3775—3786
DOI https://doi.org/10.2147/IJGM.S533080
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor David E. Stec
Tamer Selen,1 Gülay Ulusal Okyay,2 Fatma Ayerden Ebinç,2 Özgür Merhametsiz,3 Hatice Şahin,2 Mehmet Deniz Aylı2
1Nephrology Department of Health Sciences University Eskişehir City Hospital, Eskişehir, Turkey; 2Nephrology Department of Health Sciences University Etlik City Hospital, Ankara, Turkey; 3Nephrology Department of Yeniyüzyıl University Private Gaziosmanpaşa Hospital, İstanbul, Turkey
Correspondence: Tamer Selen, Nephrology Department of Health Sciences University Eskişehir City Hospital, Eskişehir, Turkey, Tel +905545474576, Email [email protected]
Purpose: Controlled Nutritional Status (CONUT) score is strongly associated with mortality in various patient groups. This study aimed to assess the CONUT score’s ability to predict mortality in Hemodialysis (HD) patients.
Patients and Methods: Data from 243 patients who started HD between 2012 and 2020 were analyzed retrospectively. Laboratory test results within the first month after dialysis initiation were recorded and CONUT scores were calculated.
Results: Over a mean follow-up duration of 32.2 months, the association between early (within six months) and late mortality was assessed. During this period, 119 patients (48.8%) died. Non-survivors had higher CONUT scores. The optimal cut-off for the CONUT score was 5.5. Sensitivity and specificity for predicting mortality were 62.2% and 61.23%, respectively. The optimal cut-off for early mortality was 6.5. Sensitivity and specificity for predicting early mortality were 68% and 69.3%, respectively. The CONUT score demonstrated moderate predictive accuracy for mortality (AUC 0.665) and higher accuracy for early mortality (AUC 0.729), highlighting its clinical utility in risk stratification.
Conclusion: CONUT score at the beginning of HD is a reliable tool in predicting overall mortality in these patients, but it is especially useful in predicting early mortality.
Keywords: CONUT score, hemodialysis, malnutrition, mortality
Introduction
Protein-energy malnutrition (PEM) is a nutritional and metabolic disorder characterized by the simultaneous loss of body protein and energy reserves. It is especially common among patients with end-stage renal disease (ESRD), resulting from factors such as hypercatabolic states, uremic toxins, malnutrition, and inflammation.1 PEM has been linked to increased cardiovascular mortality,2 diminished quality of life, and elevated morbidity and mortality rates.3,4 Although clinical guidelines underscore the importance of nutritional screening in this patient population, evidence regarding the efficacy of specific assessment tools remains limited.5 Various scoring systems have been developed; however, many are time-consuming and depend on multiple clinical and laboratory parameters.6,7
The CONUT (Controlling Nutritional Status) score is a practical and reliable tool for assessing nutritional status, using biomarkers such as total cholesterol, serum albumin, and lymphocyte count. It effectively measures protein reserves, lipid metabolism, and immune function.8 Total cholesterol, a major component of the CONUT score, reflects nutritional status in dialysis patients. Due to the phenomenon known as reverse epidemiology, where higher cholesterol levels paradoxically correlate with better survival in dialysis patients, a decrease in cholesterol leads to an increased CONUT score, which is associated with poorer survival outcomes.9 Research has shown a strong association between the CONUT score and mortality across various clinical conditions. A high CONUT score is a significant independent prognostic factor for morbidity and survival in diseases such as cancer,10–12 lymphoma,13 multiple myeloma,14 thalassemia,15 and acute heart failure.16 In patients with ESRD undergoing peritoneal dialysis, a higher CONUT score independently predicts all-cause mortality17 and is associated with increased risk of technical failure, cardiovascular disease, and mortality.18 Given the high mortality risk in patients undergoing HD, it is crucial to identify effective and easily accessible tools for predicting outcomes. Few studies have explored the association between CONUT scores and mortality in hemodialysis (HD) patients.19–21 There are limitations to current research in this area. First, previous studies do not provide information on early and late mortality. In addition, most of these studies were cross-sectional and lacked longitudinal follow-up data.1,19,20 To address these gaps, we aimed to conduct this retrospective cohort study. We investigated the prognostic value of high CONUT scores in predicting mortality in ESRD patients newly starting HD.
Materials and Methods
Study Population and Design
This retrospective, single-center, observational cohort study aimed to evaluate the predictive value of the CONUT score for all-cause mortality in patients with ESRD newly initiated on HD. The study was approved by the medical ethics committee of Ankara Etlik City Hospital (reference number: AESH-BADEK-2024-890) and was conducted in accordance with the principles of the Declaration of Helsinki.
Adult patients with end-stage renal disease (ESRD), aged 18 years and older, who started planned outpatient HD in our hospital between January 2012 and February 2020 were screened for inclusion. Exclusion criteria included hospitalization within three months prior to the first HD session, conditions affecting blood test results (liver disease, active malignancies, hematological disorders, or autoimmune diseases), and missing data. A total of 243 eligible patients were followed until death, transplantation, or the last follow-up on February 1, 2020. Data were collected from electronic records and patient files. Basic demographic and clinical information, including age, gender, dialysis initiation date, and comorbidities (eg, hypertension, diabetes, and atherosclerotic disease), were documented. Laboratory test results within the first month after dialysis initiation were recorded, including complete blood count, fasting blood sugar, creatinine, urea, uric acid, calcium (Ca), phosphorus (P), albumin (Alb), total cholesterol, low-density lipoprotein (LDL) cholesterol, triglycerides, and C-reactive protein (CRP). These tests are performed according to standard protocols in our HD unit, and samples are collected before dialysis and analyzed on the Roche Cobas c 702 (Roche Diagnostics, Mannheim, Germany) autoanalyzer. CONUT scores were calculated based on serum albumin, lymphocyte count, and total cholesterol levels, using the values shown in Figure 1.
 |
Figure 1 CONUT score calculation. |
Statistical Analyses
Statistical analyses were performed using SPSS version 24 (SPSS Inc., Chicago, IL, USA). A two-sided p-value of < 0.05 was considered statistically significant. The Kolmogorov–Smirnov test was used to assess the distribution of numerical data. For normally distributed continuous data, comparisons were made using Student’s t-test, with results presented as mean ± standard deviation (SD). Non-normally distributed data were analyzed using the Mann–Whitney U-test, with results reported as median (min-max). Categorical variables were compared using the chi-square test or Fisher’s exact test, with results expressed as counts and percentages. Spearman rank correlation was used to examine the relationship between the CONUT score and selected parameters. The primary endpoint was all-cause mortality following HD initiation, with potential predictors assessed via univariate and multivariate Cox regression analyses (model 1). A separate model (model 2) was created to evaluate the independent contribution of the CONUT score to mortality risk, considering its correlation with albumin, cholesterol, and lymphocyte counts. Patient survival rates were estimated using the Kaplan-Meier method, with comparisons made using the Log rank test. Receiver operating characteristic (ROC) curve analysis and area under the curve (AUC) were used to assess the CONUT score’s predictive ability. Univariate and multivariate Cox regression survival analyses were used to predict longterm mortality. For the multivariate analysis, the possible factors identified in the univariate analysis were included in the logistic regression analysis to determine independent predictors of early mortality in hemodialysis patients. A 5% type-I error level was used to infer statistical significance.
Results
The baseline characteristics of the 243 patients included in the study are shown in Table 1. The mean age was 60.1 ± 15.34 years, with 123 (50.4%) male patients and 37 (15.2%) diagnosed with diabetes. During a median follow-up of 32.2 (min-max 1–97) months, 119 (48.8%) patients died.
 |
Table 1 Baseline Characteristics of the Study Population and Univariate Comparisons of Survivors and Non-Survivors |
Patients were divided into two groups based on mortality, and group comparisons are detailed in Table 1. Non-survivors were older (68 vs 56 years, p < 0.001) and had a higher prevalence of cardiovascular disease (8% vs 0%, p = 0.002). Non-survivors also had lower levels of urea (100 mg/dL vs 116 mg/dL, p = 0.012), serum creatinine (5.30 mg/dL vs 6.24 mg/dL, p = 0.002), albumin (3.2 g/dL vs 3.5 g/dL, p < 0.001), triglycerides (123 mg/dL vs 146 mg/dL, p = 0.032), and LDL cholesterol (96 mg/dL vs 115 mg/dL, p = 0.014). Conversely, they had higher CRP levels (22.8 mg/L vs 8.26 mg/L, p < 0.001) and a higher CONUT score (6 vs 5, p < 0.001) compared to survivors.
The results of the regression analysis for overall mortality are shown in Table 2. In Model 1, each 1-year increase in age was associated with a 1.030-fold increase in mortality risk. Each 1-unit increase in CRP corresponded to a 1.005-fold increase in mortality risk. Conversely, each 1 g/dL increase in albumin was linked to a 1.572-fold reduction in mortality risk, and each 1 mg/dL increase in total cholesterol was associated with a 1.991-fold decrease in mortality risk. In Model 2, which included the CONUT score, a 1-year increase in age was associated with a 1.029-fold increase in mortality risk, and each 1-unit increase in CRP was linked to a 1.005-fold increase in risk. Additionally, each 1-unit increase in the CONUT score was associated with a 1.188-fold increase in mortality risk.
 |
Table 2 Analyses of Univariate and Multivariate Cox Regression Survival Analysis |
Receiver operating characteristic (ROC) analysis was conducted to evaluate the predictive accuracy of the CONUT score for mortality. The optimal cut-off value for the CONUT score was identified as 5.5, with an area under the ROC curve (AUC) of 0.665 (95% CI: 0.596–0.733, p < 0.001) (Figure 2). The sensitivity for predicting mortality was 62.2%, and the specificity was 61.23%. The positive predictive value was 60%, and the negative predictive value was 62.8%. Kaplan-Meier survival analysis based on the CONUT cut-off value of 5.5 is shown in Figure 3 (log-rank p < 0.001). During a follow-up period of up to 97 months, patients with a CONUT < 5.5 estimate 64.2 months (57–71.3); CONUT ≥5.5 estimate 41.6 months (35.5–47.7).
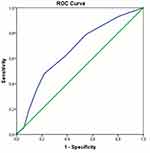 |
Figure 2 ROC curve for CONUT score. AUC 0.665 (CI 95% 0.596–0.733) and p<0.001. Statistically significant cut-off value 5.5 (sensitivity 62.2%, specificity 61.23%). |
Patients were divided into two groups: early mortality (within 6 months) and long-term mortality. Patients who developed early mortality had lower body weight, arterial hypertension rates, serum creatinine, calcium, and uric acid levels, as well as higher platelet and white blood cell counts, CRP levels, and CONUT scores. Comparisons between the groups are detailed in Table 3.
 |
Table 3 Baseline Characteristics of the Study Population According to Early Mortality |
The results of the regression analysis for early mortality are shown in Table 4. In Model 1, each 1 kg decrease in body weight was associated with a 1.048-fold increase in mortality risk, and each 1-unit decrease in serum albumin was linked to a 1.639-fold increase in risk. In Model 2, each 1 kg decrease in body weight was associated with a 1.045-fold increase in mortality risk, and each 1-unit increase in the CONUT score was associated with a 1.396-fold increase in risk. ROC analysis to assess the predictive accuracy of the CONUT score for early mortality identified an optimal cut-off value of 6.5. The area under the ROC curve (AUC) was 0.729 (95% CI: 0.628–0.830, p < 0.001) (Figure 4). Sensitivity for early mortality was 68%, and specificity was 69.3%.
 |
Table 4 Analyses of Univariate and Multivariate Regressions for Early Mortality |
 |
Figure 4 Early mortality ROC curve CONUT. AUC 0.729 (CI 95% 0.628–0.830) and p<0.001. Statistically significant cut-off value 6.5 (sensitivity 68%, specificity 69.3%). |
Discussion
In this study, we assessed the efficacy of the CONUT score in predicting mortality in patients newly started on maintenance HD. Of the 243 patients included, 119 (48.8%) died during a median follow-up of 32 months, underscoring the vulnerability of this population. Our analysis identified independent associations between age, CONUT score, albumin levels, total cholesterol, and CRP levels at dialysis initiation and the risk of death during follow-up. These findings suggest that the CONUT score may serve as a useful tool in clinical practice for assessing both overall mortality and the risk of early death in patients starting HD.
In our cohort, nonsurvivors were significantly older (mean age 68 years) than survivors (mean age 56 years), with age identified as a predictor of death. This aligns with other studies,22 as aging increases mortality risk in dialysis patients due to more comorbidities, poor physical health, malnutrition, and weakened immunity.23 Dialysis may also be less effective in older individuals, with treatment adherence difficulties further contributing to the risk.24
Previous analyses have shown that low serum total cholesterol, lymphocyte counts, and albumin levels—the main components of the CONUT score—are linked to malnutrition and independently predict mortality in HD patients.25,26 Albumin, a key marker of nutritional status, is closely linked to inflammation, malnutrition, and comorbidities in dialysis patients.27,28 As a negative acute-phase reactant, its levels are influenced by proinflammatory cytokines, which promote breakdown and suppress synthesis.29 Low albumin is associated with increased mortality in HD patients,30,31 and as albumin levels decrease, the CONUT score increases. Consistent with this, our study found that nonsurvivors had lower albumin levels and higher CONUT scores, both significant mortality predictors. Given our cohort of stable patients who recently initiated planned HD, these results likely reflect pre-dialysis care, suggesting that the CONUT score effectively identifies malnutrition and inflammation in new dialysis patients. On the other hand, our analysis showed that for every 1 mg/dL increase in total cholesterol, there was a 1.008-fold reduction in mortality risk. This aligns with the concept of “reverse epidemiology” in dialysis patients, unlike in the general population.9 Our findings support this well-established concept.
Serum C-reactive protein (CRP) is a marker of inflammation, with high CRP levels at HD initiation predicting both all-cause and cardiovascular mortality.32–34 In our study, CRP levels were higher in nonsurvivors, with each 1-unit increase in CRP linked to a 1.005-fold rise in mortality risk.
Only a few studies have explored the link between CONUT scores and mortality in HD patients. Babic et al (2021) were the first to show that higher CONUT scores were associated with increased mortality in maintenance HD patients.19 Aydin et al (2022) extended this finding to a larger cohort of new HD patients, showing a similar association.20 Our study also focused on new HD patients but with a larger sample size, older patients, and longer follow-up. These differences may explain the variations in predictive accuracy of CONUT scores across studies. Takagi et al (2022) examined the association between CONUT scores and mortality in both HD and PD patients, but the different risks for inflammation and malnutrition between the two groups may limit generalizability.10,21 Our study is the first to demonstrate that CONUT scores may predict both overall and early mortality in new HD patients.
The CONUT score has moderate predictive accuracy for mortality risk, but its use alone is limited. While not perfect, its performance suggests it may help identify patients at higher risk of death. With a sensitivity of 62.2% and specificity of 61.3%, the CONUT score may distinguish between survivors and nonsurvivors in a moderate proportion of cases. The positive predictive value of 60% and negative predictive value of 62.8% further demonstrate that the score provides meaningful prognostic insights. Specifically, patients with a CONUT score below 5.5 had significantly longer survival compared with those with a score of 5.5 or higher. Additionally, patients with a CONUT score below 6.5 had significantly longer first six-month survival compared with those with a score of 6.5 or higher. This early mortality prediction is of particular clinical importance, as the initial months of dialysis are known to carry the highest mortality risk.35 Our findings suggest that the CONUT score may be a useful tool for identifying patients at higher nutritional risk. Although this study did not include dietary interventions, the CONUT score could potentially guide personalized nutritional support and early interventions in clinical practice to improve patient outcomes. Future studies are needed to explore the effectiveness of such targeted nutritional interventions based on CONUT assessment.
Our study has several limitations. It was a single-center, retrospective analysis with a relatively small sample size. The small number of patients makes it difficult to generalize the study. Additionally, baseline anthropometric measurements, such as body composition and waist circumference, were not included. We also did not assess long-term changes in nutritional status, as our analysis was limited to baseline scores. Data were collected only at the start of dialysis, and we did not evaluate how changes in nutritional status may have influenced prognosis after dialysis initiation. Finally, since the study focused specifically on HD patients, the findings may not be generalizable to all populations with ESRD.
Conclusion
This study suggests that the CONUT score, reflecting both malnutrition and inflammation, may serve as a valuable tool for predicting both short-term and long-term mortality in hemodialysis patients. The findings suggest that lower albumin levels, higher CRP, and a higher CONUT score are strong indicators of poor prognosis. The ability of the CONUT score to provide predictive insights into mortality and identify patients at higher risk highlights its potential clinical utility in the management of dialysis patients. However, further research with larger datasets and longitudinal studies is needed to confirm these findings and optimize the use of the CONUT score in clinical practice.
Ethics Statement
The study was approved by the medical ethics committee of Ankara Etlik City Hospital (reference number: AESH-BADEK-2024-890) and was conducted in accordance with the principles of the Declaration of Helsinki. Due to the retrospective design of the study, informed consent was not obtained from the patients. However, all data were anonymized, and patient confidentiality was strictly maintained in accordance with ethical standards.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Takagi K, Takahashi H, Miura T, et al. Prognostic value of the controlling nutritional status (CONUT) score in patients at dialysis initiation. Nutrients. 2022;14(11):2317. doi:10.3390/nu14112317
2. Hory B, Robino C, Albadawy M, Islam MS. NUTRIPEPA 2: impact d’une membrane adsorbante sur le syndrome PEW. Néphrol Thérap. 2014;10(5):314. doi:10.1016/j.nephro.2014.07.062
3. Hanna RM, Ghobry L, Wassef O, Rhee CM, Kalantar-Zadeh K. A practical approach to nutrition, protein-energy wasting, sarcopenia, and cachexia in patients with chronic kidney disease. Blood Purif. 2020;49(1–2):202–211. doi:10.1159/000504240
4. Yuksel E, Aydin E. The relationship between serum vitamin D levels and health-related quality of life in peritoneal dialysis patients. Int Urol Nephrol. 2021;54(4):927–936. doi:10.1007/s11255-021-02951-2
5. Ikizler TA, Burrowes JD, Byham-Gray LD, et al. KDOQI clinical practice guideline for nutrition in CKD: 2020 update. Am J Kidney Dis. 2020;76(3 Suppl 1):S1–S107. doi:10.1053/j.ajkd.2020.05.006
6. Kalantar-Zadeh K, Kopple JD, Block G, Humphreys MH. A malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients. Am J Kidney Dis. 2001;38(6):1251–1263. doi:10.1053/ajkd.2001.29222
7. Beto JA, Bansal VK, Hart J, McCarthy M, Roberts D. Hemodialysis prognostic nutrition index as a predictor for morbidity and mortality in hemodialysis patients and its correlation to adequacy of dialysis. J Ren Nutr. 1999;9(1):2–8.
8. Kokura Y, Kimoto K, Okada Y, Kawakita S. The controlling nutritional status score as a functional prognostic marker in patients with acute stroke: a multicenter retrospective cohort study. Nutrition. 2020;110889:79–80.
9. Kalantar-Zadeh K, Block G, Humphreys MH, Kopple JD. Reverse epidemiology of cardiovascular risk factors in maintenance dialysis patients. Kidney Int. 2003;63(3):793–808. doi:10.1046/j.1523-1755.2003.00803.x
10. Takagi K, Buettner S, Ijzermans JNM, Wijnhoven BPL. Systematic review on the controlling nutritional status (CONUT) score in patients undergoing esophagectomy for esophageal cancer. Anticancer Res. 2020;40(10):5343–5349. doi:10.21873/anticanres.14541
11. Peng L, Meng C, Li J, et al. The prognostic significance of controlling nutritional status (CONUT) score for surgically treated renal cell cancer and upper urinary tract urothelial cancer: a systematic review and meta-analysis. Eur J Clin Nutr. 2021;76(6):801–810. doi:10.1038/s41430-021-01014-0
12. Kheirouri S, Alizadeh M. Prognostic potential of the preoperative controlling nutritional status (CONUT) score in predicting survival of patients with cancer: a systematic review. Adv Nutr. 2021;12:234–250.
13. Baysal M, Bas V, Demirci U, et al. The utility of CONUT score in diffuse large B cell lymphoma patients. Niger J Clin Pract. 2021;24(8):1194–1199. doi:10.4103/njcp.njcp_429_20
14. Kamiya T, Ito C, Fujita Y, et al. The prognostic value of the controlling nutritional status score in patients with multiple myeloma. Leuk Lymphoma. 2020;61(8):1894–1900. doi:10.1080/10428194.2020.1749608
15. Akgun Cagliyan G. Is CONUT score a predictor of morbidity in patients with adult transfusion dependent beta thalassemia? Transfus Apher Sci. 2021;60(4):103126. doi:10.1016/j.transci.2021.103126
16. Kato T, Yaku H, Morimoto T, et al. Association with controlling nutritional status (CONUT) score and in-hospital mortality and infection in acute heart failure. Sci Rep. 2020;10(1):3320. doi:10.1038/s41598-020-60404-9
17. Yang Y, Zhou H, Zhang P, Chao W, Zou Y, Yang M. Evaluation of objective nutritional indexes as predictors of worse outcomes in peritoneal dialysis patients. Nutrition. 2020;79-80:110963. doi:10.1016/j.nut.2020.110963
18. Zhou H, Chao W, Cui L, Li M, Zou Y, Yang M. Controlling nutritional status (CONUT) score as immune-nutritional predictor of outcomes in patients undergoing peritoneal dialysis. Clin Nutr. 2020;39(8):2564–2570. doi:10.1016/j.clnu.2019.11.018
19. Babić G, Krečak I, Rožanković Čobanov VESNA, Petković S, Gulin M. The controlling nutritional status (Conut) score might predict survival in maintenance hemodialysis patients. Acta Medica Croatica. 2021;75(2):123–130.
20. Aydın FY, Yüksel E, Aydın E. Prognostic significance of controlling nutritional status (CONUT) score in hemodialysis patients. Anatolian Curr Med J. 2022;4(2):197–201. doi:10.38053/acmj.1076364
21. Naini AE, Karbalaie A, Abedini M, Askari G, Moeinzadeh F. Comparison of malnutrition in hemodialysis and peritoneal dialysis patients and its relationship with echocardiographic findings. J Res Med Sci. 2016;21(1):78. doi:10.4103/1735-1995.189695
22. Erdoğmuş S, Kaymakamtorunları F. Factors associated with mortality in maintenance hemodialysis patients: a single-center data from East Anatolian Region of Turkey. J Ankara Univer Facul Med. 2020;73(3).
23. Chan GC, Kalantar-Zadeh K, Ng JK, et al. Frailty in patients on dialysis. Kidney Int. 2024;106(1):35–49. doi:10.1016/j.kint.2024.02.026
24. Hughes CM. Medication non-adherence in the elderly: how big is the problem? Drugs Aging. 2004;21(12):793–811. doi:10.2165/00002512-200421120-00004
25. Reddan DN, Klassen PS, Szczech LA, et al. White blood cells as a novel mortality predictor in haemodialysis patients. Nephrol Dial Transplant. 2003;18(6):1167–1173. doi:10.1093/ndt/gfg066
26. Obialo CI, Okonofua EC, Nzerue MC, Tayade AS, Riley LJ. Role of hypoalbuminemia and hypocholesterolemia as copredictors of mortality in acute renal failure. Kidney Int. 1999;56(3):1058–1063. doi:10.1046/j.1523-1755.1999.00622.x
27. Nitta K, Tsuchiya K. Recent advances in the pathophysiology and management of protein-energy wasting in chronic kidney disease. Ren Replace Ther. 2016;2(1):4. doi:10.1186/s41100-016-0015-5
28. Friedman AN, Fadem SZ. Reassessment of albumin as a nutritional marker in kidney disease. J Am Soc Nephrol. 2010;21(2):223–230. doi:10.1681/ASN.2009020213
29. Honda H, Qureshi AR, Heimburger O, et al. Serum albumin, C-reactive protein, interleukin 6, and fetuin A as predictors of malnutrition, cardiovascular disease, and mortality in patients with ESRD. Am J Kidney Dis. 2006;47(1):139–148. doi:10.1053/j.ajkd.2005.09.014
30. Iseki K, Kawazoe N, Fukiyama K. Serum albumin is a strong predictor of death in chronic dialysis patients. Kidney Int. 1993;44(1):115–119. doi:10.1038/ki.1993.220
31. Owen WF, Lew NL, Liu Y, Lowrie EG, Lazarus JM. The urea reduction ratio and serum albumin concentration as predictors of mortality in patients undergoing hemodialysis. N Engl J Med. 1993;329(14):1001–1006. doi:10.1056/NEJM199309303291404
32. Iseki K, Tozawa M, Yoshi S, Fukiyama K. Serum C-reactive protein (CRP) and risk of death in chronic dialysis patients. Nephrol Dial Transplant. 1999;14(8):1956–1960. doi:10.1093/ndt/14.8.1956
33. Takahashi R, Ito Y, Takahashi H, et al. Combined values of serum albumin, C-reactive protein and body mass index at dialysis initiation accurately predicts long-term mortality. Am J Nephrol. 2012;36(2):136–143. doi:10.1159/000339940
34. Yeun JY, Levine RA, Mantadilok V, Kaysen GA. C-reactive protein predicts all-cause and cardiovascular mortality in hemodialysis patients. Am J Kidney Dis. 2000;35(3):469–476.
35. Lafrance JP, Rahme E. The risk of early mortality after dialysis initiation is associated with comorbidity burden. Kidney Int. 2008;73(8):1016–1023.
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Development and Validation of Prediction Models for All-Cause Mortality and Cardiovascular Mortality in Patients on Hemodialysis: A Retrospective Cohort Study in China
Yang M, Yang Y, Xu Y, Wu Y, Lin J, Mai J, Fang K, Ma X, Zou C, Lin Q
Clinical Interventions in Aging 2023, 18:1175-1190
Published Date: 28 July 2023
Current Knowledge of Beta-Blockers in Chronic Hemodialysis Patients
Haddiya I, Valoti S
International Journal of Nephrology and Renovascular Disease 2023, 16:223-230
Published Date: 12 October 2023
The Relationship Between Fracture and Mortality in a Chinese Maintenance Hemodialysis Patients Cohort
Liu X, Liu Z, Niu Y, Zhang K, Zhang X, Yu C
Journal of Multidisciplinary Healthcare 2024, 17:2031-2038
Published Date: 1 May 2024
Independent Association Between Malnutrition Inflammation Score and C Reactive Protein/Albumin Ratio in Hemodialysis Patients
Tur K, Güçlü A
Journal of Inflammation Research 2024, 17:9325-9333
Published Date: 22 November 2024
The Association Between Lifestyle and All-Cause Mortality in Patients Undergoing Maintenance Hemodialysis: A 3-year Prospective, Observational Study
Zhang L, Zhang S, Tang X
Journal of Multidisciplinary Healthcare 2025, 18:1721-1729
Published Date: 20 March 2025


