Back to Journals » Clinical Ophthalmology » Volume 19
Dynamic Muscle Stimulation of the Periorbital Area for Improvement of Blinking in Dry Eye Patients
Authors Chelnis JG, Chelnis A
Received 23 December 2024
Accepted for publication 18 March 2025
Published 26 March 2025 Volume 2025:19 Pages 1057—1071
DOI https://doi.org/10.2147/OPTH.S513989
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
James G Chelnis,1,2 Alexandra Chelnis1
1Manhattan Face and Eye, New York, NY, USA; 2New York Eye and Ear Infirmary of Mount Sinai, New York, NY, USA
Correspondence: James G Chelnis, Manhattan Face and Eye, 150 W 58th St #1C, New York, NY, 10019, USA, Tel +1212-484-9707, Email [email protected]
Purpose: To investigate whether Dynamic Muscle Stimulation technology (DMSt) in the peri-orbital area improves blinking quality in subjects with lower lid laxity and dry eye disease (DED) due to Meibomian Gland Dysfunction (MGD).
Patients and Methods: Eligible subjects had lower lid laxity and DED due to MGD. Treatment consisted of DMSt administered 4 times at 1-week intervals. Outcome measures were tested before each treatment and at the follow-up (FU) 4 weeks after the final treatment. The main hypothesis was a decrease in the proportion of subjects with lower lid laxity, defined as abnormal lower lid distraction test (LLDT) or abnormal snap-back test (SBT). Outcomes related to DED comprised the modified meibomian gland score (mMGS), tear breakup time (TBUT), and symptoms of DED (OSDI). Other outcomes included the Margin to Reflex Distance 1 and 2 (MRD1 and MRD2), estimation of blink rate, blink quality, and eyelid appearance.
Results: 30 subjects completed FU. LLDT decreased from 11.1 (SD 2.2) mm to 5.3 (SD 1.3) mm (P< 0.0001). The proportion of subjects with normal LLDT and SBT increased from 3% to 80% and from 30% to 93%, respectively (p< 0.0001). Lower lid laxity decreased from 100% at BL to 23% at FU (p< 0.0001). MRD2 gradually decreased from 5.5 (SD 0.9) mm at BL to 5.0 (SD 0.4) mm at FU (P< 0.001). TBUT, mMGS, and OSDI changed by +286%, − 78%, and − 53%, respectively (P< 0.0001). The proportion of subjects with normal eyelid appearance, blink quality, blink rate and eyelid closure increased from 0 to 63% (p< 0.0001), 0 to 73% (p< 0.0001), 36% to 93% (p< 0.0001) and 73% to 100% (p< 0.01), respectively. No adverse events occurred.
Conclusion: In DED patients, DMSt in the peri-orbital area decreases lower lid laxity and improves blinking quality. These, in turn, may be useful for managing signs and symptoms of DED due to MGD.
Plain Language Summary: Evaporative dry eye disease (DED) is one of the most common pathologies of the ocular surface. This multi-factorial disease is mostly due to poor function of meibomian glands within the eyelids (MGD). Patients with DED due to MGD suffer from discomfort, visual disturbances, and a deterioration in the quality of life. The condition is often accompanied by abnormal blinking, as a result of lid laxity and reduction of muscle mass around the eyelids. Traditionally, lid laxity can be surgically corrected, however this intervention carries some risks and recovery can be slow. A non-surgical approach would be useful for shortening the recovery time and reducing those risks. One candidate is trans-cutaneous electrical stimulation, using Dynamic Muscle Stimulation technology (DMSt) developed by Lumenis Be. This technology, adopted from aesthetics, improves skin elasticity and increases muscle tone, both of which deteriorate with age. Hence, we reasoned that in subjects with lid laxity such treatment could be helpful for restoring lid function. In turn, this would improve blinking quality and reduce signs and symptoms of ocular surface disease. In a prospective study, 30 subjects with lower lid laxity and moderate to severe DED due to MGD were treated with DMSt applied below the lower eyelids. Treatment consisted of four sessions at 1-week intervals. One month after the last treatment, the number of subjects with lower lid laxity decreased by 80%. In 8 subjects with incomplete closure of eyelids, this abnormality completely disappeared. Eyelid appearance and blinking quality improved by 60% and 70% each. The number of subjects with a normal blinking rate increased by about 60%. In addition, there were clinically significant improvements in both signs and symptoms of DED. No complications were observed. We conclude that this novel approach is safe and useful as an adjunct solution for management of evaporative DED.
Keywords: dry eye disease, meibomian gland dysfunction, lower lid laxity, neuromuscular stimulation
Introduction
Dry Eye Disease (DED) is recognized as a multifactorial disease of the ocular surface characterized by a loss of homeostasis of the tear film, and accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play etiological roles. 1 Among the many factors which can lead to this loss of homeostasis are abnormalities in eyelid position and mechanics, such as lower lid laxity, and impairment of eye blinking. 1 In healthy individuals, the rate of spontaneous blinking is highly variable and depends on many factors such as task, fatigue, age, and experimental conditions. 2 However, it is widely accepted that in DED patients the blinking rate is consistently higher than that of the normal population, 3–5 possibly due to irritation of the ocular surface caused by an unstable tear film. 4,6 Conversely, a low blinking rate (for example, due to prolonged use of computers or other high-concentration tasks) increases tear film evaporation, and thus constitutes a major risk factor for DED. 6–8 Not only is the rate of blinking implicated in DED, but also the quality. For example, incomplete blinks (where the upper and lower lids do not contact each other) are associated with DED symptoms, decreased tear breakup-time, dropout of meibomian glands, and reduced expressibility. 9,10 Another example is eyelid malposition, resulting in compromised lid seal during sleep, which was found in over 60% of DED patients compared to only 20% in healthy individuals. 11
One of the factors most affecting the quality of blinking is lid laxity, characterized by eyelid margins that are easily distracted away from the globe. The condition is mostly due to natural aging,12 possibly the result of collagen loss in the eyelid skin.13 Several studies have found that lid laxity is closely associated with abnormal tear film parameters and symptoms of DED.12,14,15 Another factor which could affect lid laxity and the quality of blinking is the integrity and health of the orbicularis oculi muscle (OOM). Reduction in muscle mass and strength of skeletal muscles is part of normal aging.16 A reduction in OOM muscle mass and strength is a central contributor to eyelid malposition typically seen in older patients, such as seen in ectropion and entropion. Specifically, these age-dependent changes in the OOM have been found to encompass decreased muscle fiber density and capillary coverage.17 Okuda et al reported that the OOM is thinner in older subjects, compared to younger subjects.18 In Asian subjects, the OOM has been found not only to thin with age but also to gradually loosen from bony attachments.19
Lower lid laxity is typically evaluated with the snap-back test and/or lower lid distraction test.20 In the snap-back test (SBT), the lower lid is pulled away from the globe. Once released, the time required for the lid to passively return to its original position is measured. If the snap-back is not immediate (< 1 sec), the test is considered abnormal.21 In the lid distraction test (LLDT), the distance that the eyelid can be pulled away from the globe is measured. Distances over 6 mm are considered abnormal.21 Traditionally, lower lid laxity is surgically corrected.21 The post-operative period typically features swelling, bruising, and discomfort.22 Most symptoms and signs associated with this condition recover within several weeks, but the entire recovery can take several months.22 Additionally, as with any surgery, patients face certain risks with treatment. Complications may include corneal abrasions, orbital hemorrhages, infections, eyelid malposition (e.g., lower lid retraction, lagophthalmos, ectropion), strabismus, exposure keratopathy, epiphora, worsening of pre-existing ocular surface disease, changes in eyelid height and contour, hypertrophic scarring, and dermal pigmentation.21
An alternative approach, that does not involve surgery, would be useful for shortening the recovery time and reducing risks for those that are poor candidates for surgery due to frailty, comorbidities, or difficulty discontinuing blood thinning medication. One candidate is transcutaneous electrical stimulation (TCES) in the periocular area. To date, TCES has been rarely used in facial areas. Kavanagh and colleagues reported that static TCES, where the stimulation is transmitted via spatially fixed conductive pads, increased the thickness and muscle tone of the zygomatic major muscle.23 Mäkelä and her group found that in patients with paralyzed facial muscles (frontalis, zygomatic major, or orbicularis oculi), TCES restored muscle function.24 In these cases, the stimulation was transmitted via conductive pads spatially fixed on the skin, hence static. A few studies examined the efficacy of dynamic stimulation, where the stimulation electrodes were moved back and forth over facial regions.25,26 Both studies found that this approach improved skin tightness, skin elasticity, and reduced wrinkles. Dynamic stimulation is more effective compared to the static approach, since it allows motor units to recover from their refractory period between stimulations.
The use of TCES for correcting lower lid laxity was not examined before. If this proves to be useful for reducing lid laxity, it would be valuable to see if it improves blink quality and triggers improvement in signs and symptoms of DED as well. The purpose of this preliminary investigation is to examine the potential merits of this approach with a dynamic version of TCES.
Materials and Methods
Participants
Participants were enrolled from August 2023 to July 2024. Eligible participants were men and women aged 22 or older, with lower lid laxity and signs and symptoms of DED due to meibomian gland dysfunction (MGD). The main inclusion criteria included subjects with lower lid laxity as clinically judged with LLDT and SBT, moderate to severe DED symptoms (OSDI ≥ 23), and TBUT < 5 seconds in both eyes. Subjects with the following conditions were excluded from the study: any ocular or eyelid surgery within 3 months, moderate or severe floppy lid syndrome, corneal dystrophy, exophthalmos, thyroid eye disease, ocular chemical injury or burn, limbal stem cell deficiency, facial nerve palsy, blepharospasm, hemifacial spasm, corneal neuropathy, pregnant or nursing women. An informed consent was obtained from all participants.
Study Design
This was a prospective, single site, interventional, open-label clinical study approved by an Institutional Review Board (Sterling IRB, # 10866). The trial was registered in ClinicalTrials.gov (NCT05945069). The study complied with the declaration of Helsinki.
Treatment
Subjects were treated with dynamic TCES, hereafter referred as Dynamic Muscle Stimulation (DMSt). The full schedule of treatment comprised 4 sessions, at 1-week intervals. Prior to each treatment session, the study investigator adjusted the DMSt settings (power, frequency and pulse duration) so as to obtain twitching of fiber muscles in the OOM. Then, DMSt was applied bilaterally, 5 minutes per side, on the peri-orbital skin. Treatment was applied in a repetitive and slow back and forth C-shape motion, from the anterior lacrimal crest along the orbital rim and up to the zygomatic-frontal suture (Figure 1). The infra-orbital nerve foramen was avoided.
Outcome Measures
Unless noted otherwise, all outcome measures were evaluated at baseline (BL) immediately before the first treatment session (Tx1), before every subsequent treatment session (Tx2, Tx3 and Tx4) and at a single follow-up (FU) visit conducted one month after the last treatment session. The primary outcome measures were the lower lid distraction test (LLDT) and the snap-back test (SBT). LLDT and SBT were performed as described in Milbratz-Moré et al.20 LLDT greater than 6 mm was defined as abnormal. In SBT, times shorter than 1 second were defined as abnormal. Lower lid laxity was determined if either LLDT or SBT were abnormal. The study hypothesis was that, following exposure to DMSt, the proportion of subjects with lower lid laxity decreases. Other outcome measures included mMGS, OSDI, TBUT, MRD1 and MRD2, corneal sensitivity with Esthesiometry, eyelid gap, eyelid appearance, subjectively estimated blink rate and blink quality, and discomfort level during the procedure, as described below.
mMGS (Modified Meibomian Gland Score)
Fifteen (15) meibomian glands along the lower lid (5 nasal + 5 central + 5 temporal) were gently expressed. Each gland was graded 0 (clear liquid meibum), 1 (cloudy liquid meibum) 2 (inspissated meibum), or 3 (blocked gland). mMGS was then evaluated as the sum of 15 grades (range: 0 to 45). Moderate to severe MGD is defined by an mMGS ≥ 32 and a decrease in mMGS indicates an improvement in MGD.
OSDI (Ocular Surface Disease Index)
Dry eye symptoms were self-evaluated with the OSDI questionnaire (range: 0 to 100). OSDI above 23 is consistent with moderate to severe DED. A decrease in OSDI indicates an improvement in DED symptoms.
TBUT (Tear Breakup Time)
FUL-GLO® fluorescein sodium ophthalmic strip (0.6 mg) was applied to the inferior tarsal conjunctiva. The subject was asked to blink a few times to distribute the dye over the cornea. Once positioned at the slit lamp, the examiner viewed the eye of the subject through a slit lamp using broad beam cobalt blue illumination and a yellow barrier filter. Then, the subject was asked to keep the eyelids open without blinking. A stopwatch was started as soon as the subject opened the eyes and was stopped at the first sign of breakup in the precorneal fluorescein-stained tear layer. For each eye, 3 consecutive readings were taken, and the average value was recorded. TBUT below 5 seconds are consistent with moderate to severe DED. An increase in TBUT indicates an improvement in MGD.
MRD2 (Margin to Reflex 2)
A penlight was held between the examiner’s eyes and directed at the patient’s eyes. With the patient gazing in the primary position, the distance from the corneal light reflex to the central portion of the lower-eyelid margin was measured. A normal value is approximately 5 mm.27
MRD1 (Margin to Reflex 1)
Same as MRD2, but when the distance from the corneal light reflex to the central portion of the upper-eyelid margin was measured. Normal values range between 4 and 5 mm.27
Corneal Sensitivity
Evaluated with a Cochet-Bonnet esthesiometer (range: 1 to 6 cm). Subjects with DED have significantly lower values, compared to subjects with healthy ocular surface.28
Lagophthalmos
The study investigator asked the subject to close both eyes (unforced eye closing). If no gap was observed between the eyelid margins, lagophthalmos was absent and eyelid closure was defined as complete. Otherwise, the level of lagophthalmos between eyelids was estimated by measuring the vertical height of the palpebral fissure during eye closure with the slit lamp ruler. A decrease indicates an improvement in blink quality.
Qualitative Blink Rate and Blink Quality
During normal conversation with the subject, the study investigator estimated whether, according to his experience, the blinking rate was low, within normal range, or high. According to his experience and taking account of rate, completeness, and pattern of blinking, the study investigator graded the blink quality as normal, mild, moderate or severe.
Eyelid Appearance
Using biomicroscopy at the slit lamp, the study investigator graded the appearance of eyelids as normal or featuring mild, moderate or severe levels of disease.
Discomfort Level
Immediately after each treatment (Tx1, Tx2, Tx3, and Tx4), the subject was asked to mark the discomfort level on a 100 mm long Visual Analog Scale (VAS), where the anchor was “What was the degree of pain/discomfort experienced during the procedure?”, and the left and right stems were “None” and “Intolerable”, respectively. Discomfort level (range: 0 to 100) was then evaluated as the distance of the subject’s mark from the left stem.
Statistical Analysis
Statistical analyses were done with excel or JMP (SAS). For variables measured in both eyes, intra-subject correlation was removed by averaging the values of the two eyes. Descriptive statistics of continuous variables were expressed with means and standard deviations. For categorical variables, descriptive statistics were expressed as counts (N) and percentages (%).
Statistical significance (α) was set to 0.05. For repeated measures across time, the null hypothesis of no longitudinal change within subjects was tested with a multiple analysis of variance (F-test). For comparison of categorical variables at BL and any of the time points, the null hypothesis of no difference was tested with a chi-square test if in the contingency table there were no cells with an expected frequency of less than 5, or with a Fisher’s exact test if otherwise.29 Statistical significances are abbreviated with N/S (Not significant, p>0.05), * (p<0.05), ** (p<0.01), *** (p<0.001), or **** (p<0.0001).
Results
Participant Flow
Thirty-one subjects were screened between August 2023 to July 2024. Participation of one subject was terminated after a single treatment session due to exposure to Covid. No other subject was lost to follow-up. Overall, 30 subjects (60 eyes) were included in the analysis.
Demographics
Age was 67.0±12.1 years (min: 28.5, max: 84.7). Of the 30 subjects, 24 (80%) were women. Of the 30 subjects, 23 (76.7%), 4 (13.3%), 2 (6.7%) and 1 (3.3%) were Caucasian, Hispanic/Latino, African, or Asian/Pacific, respectively.
Treatment
Thirty subjects were treated with DMSt (Lumenis Be, Yokneam, IL). Per subject, settings were adjusted to elicit twitches of the orbicularis oculi while maintaining tolerability. Intensities ranged between 5–10 Watts; Pulse duration was between 160 and 250 microseconds; Frequency was either 1.56 or 3.31 Hz.
Baseline Values
Table 1 summarizes the means and standard deviations of continuous variables at BL. On average, LLDT was 11.1 ±2.2 mm. In 29 of 30 subjects 97% LLDT was abnormal (> 6mm). One subject had a borderline value of 6 mm. For MRD1 and MRD2, averages were 3.0 ± 1.4 mm and 6.0 ± 1.0 mm, respectively. Outcome measures related to ocular surface disease were consistent with moderate to severe DED: 2.4 ±0.8 sec for TBUT, 31.2 ±7.3 for mMGS, and 59.7 ±20.3 for OSDI, respectively.
 |
Table 1 Baseline Values of Continuous Variables |
Table 2 summarizes the frequencies (N) and proportions (%) of categorical variables at BL. SBT was abnormal in 70% of the subjects. Eyelid appearance was moderate or severe in 53% and 47% of the subjects, respectively. Qualitative blink quality at rest was mild, moderate or severe in 13%, 80% and 7% of the subjects, respectively. Lagophthalmos was present in 27% of the subjects. The blink rate was abnormally low in 23% of the subjects, within normal range in 37% of subjects, or abnormally high in 40% of subjects. Overall, 30 subjects had either abnormal LLDT or abnormal SBT (or both), indicating lower lid laxity in 100% of the subjects.
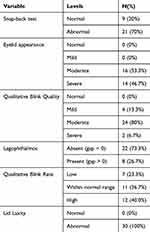 |
Table 2 Baseline Values of Categorical Variables |
Changes of Continuous Outcome Measures
Figure 2 shows several examples of subjects before treatment and immediately after the 2nd treatment. All changes are summarized in Table 3.
 |
Table 3 Change of Continuous Variables |
 |
Figure 2 Before-After photos of patients treated with DMSt. “After” photos were taken immediately after the 2nd treatment. |
Figure 3 illustrates outcome measures related to the lower lid laxity: LLDT (Figure 3A) gradually decreased by 14%, 30%, 44%, and 54% at Tx2, Tx3, Tx4, and FU, respectively (P<0.0001). The proportion of subjects with normal LLDT (Figure 3B, light red bars) increased from 3% at BL/Tx1 to 10% at Tx2 (N/S), 30% at Tx3 (p<0.05), 53% at Tx4 (p<0.0001) and 80% at FU (p<0.0001). The proportion of subjects with normal SBT (Figure 3B, light blue bars) increased from 30% at BL/Tx1 to 57% at Tx2 (p<0.05), 70% at Tx3 (p<0.01), 90% at Tx4 (p<0.0001) and 93% at FU (p<0.0001). MRD2 (Figure 3C) gradually decreased by 8%, 12%, 15% and 17% at Tx2, Tx3, Tx4, and FU, respectively (P<0.001). Overall, the number of subjects with lower lid laxity (Figure 3D) decreased from 30 (100% of the subjects) at BL/Tx1 to 28 (93.3%) at Tx2 (N/S), 22 (73.3%) at Tx3 (p<0.01), 14 (46.7%) at Tx4 (p<0.0001) and 7 (23.3%) at FU (p<0.0001). For simplicity, hereafter the 23 subjects with no lower lid laxity at the follow-up are referred to as group A, and the 7 subjects with residual lower lid laxity are referred to as group B. In a sub-analysis, all outcome measures related to DED due to MGD were slightly better in group A compared to group B, albeit the differences were not always large enough for statistical significance: TBUT was 9.4±2.3 sec in group A versus 7.9±1.2 sec in group B (p<0.05); mMGS was 6.3±4.2 in group A versus 8.5±5.2 in group B (p=0.33); and OSDI was 26.3±12.6 versus 33.3±23.8 in group B (p=0.48).
Figure 4 illustrates outcome measures related to DED due to MGD, which all improved significantly: mMGS (Figure 4A) decreased by 10%, 34%, 59%, and 78% at Tx2, Tx3, Tx4, and FU, respectively (P<0.0001). TBUT (Figure 4B) increased by 63%, 192%, 245% and 286% at Tx2, Tx3, Tx4, and FU, respectively (P<0.0001). OSDI (Figure 4C) decreased by 6%, 21%, 35% and 53% at Tx2, Tx3, Tx4, and FU, respectively (P<0.0001). Esthesiometry (Figure 3D) increased by 4%, 5%, 8% and 10% at Tx2, Tx3, Tx4 and FU, respectively (P<0.001). There was no change in MRD1 (P=0.36, not shown).
Changes of Categorical Outcome Measures
Changes in categorical and subjectively assessed outcome measures are summarized in Table 4 and Figure 5. For simplicity, in this figure we display only the proportions of subjects with normal observations. Normal eyelid appearance (white bars) increased from 0 at BL and Tx2 to 3.3% at Tx3 (N/S), 40% at Tx4 (p<0.001), and 63% at FU (p<0.0001). Normal qualitative blink quality at rest (light gray bars) increased from 0 at BL and Tx2 to 13% at Tx3 (p<0.05), 37% at Tx4 (p<0.001) and 73% at FU (p<0.0001). Normal qualitative blink rate at rest (dark gray bars) increased from 37% at BL to 60% at Tx2 (N/S), 80% at Tx3 (p<0.001), 83% at Tx4 (p<0.001) and 93% at FU (p<0.0001). Absence of lagophthalmos (black bars) increased from 73% at BL to 90% at Tx2 (N/S), 93% at Tx3 (p<0.05), 97% at Tx4 (p<0.05) and 100% at FU (p<0.001).
 |
Table 4 Change of Categorical Variables |
Discussion
The current study investigated the benefits of Dynamic Muscle Stimulation technology in the periocular area for decreasing lower lid laxity in subjects with moderate to severe DED due to MGD. In 100% of the subjects, LLDT gradually and consistently decreased over time. At the follow-up, LLDT was more than halved compared to the baseline. While 97% of the subjects had an abnormal (> 6 mm) at baseline, at the follow-up only 20% remained with an abnormal value. The proportion of subjects with normal SBT also gradually increased, from 30% at the baseline to 93% at the follow-up. Overall, the proportion of subjects with lower lid laxity (either or both abnormal LLDT and abnormal SBT) decreased from 100% at baseline to 23.3% at the follow-up. MRD2 also showed desirable improvements in lid function and appearance. Further studies may allow improved identification of subjects that would benefit most prior to treatment. One such application could be the non-surgical treatment of ectropion and entropion. This was outside the bounds of this study, but as the limits of improvements in LLDT with this technology are further elucidated, it should prove to be a point of interest.
Other improvements were observed in eyelid appearance, corneal sensitivity, and the blink rate and blink quality estimated by the study investigator. Outcome measures related to the severity of DED due to MGD also showed significant improvements, with TBUT increasing by 4-fold, mMGS decreasing by almost 80%, and OSDI symptoms decreasing by about 50%. The number of subjects with moderate to severe TBUT (≤ 5 sec), moderate to severe mMGS (≥32), and moderate to severe OSDI (≥23) decreased from 30 (100%) to 1 (3%), from 16 (53%) to 0, and from 30 (100%) to 17 (56%), respectively. Since the treatment area did not include the upper eyelids, as expected MRD1 did not change.
Taken together, these results indicate that peri-ocular treatment with dynamic muscle stimulation reduced lower lid laxity, improved blinking quality and rate, and also improved signs and symptoms of DED due to MGD. Interestingly, in subjects with no lower lid laxity at the follow-up (group A), all outcome measures related to DED due to MGD were better, compared to subjects with residual lower lid laxity (group B), although the differences were not always large enough for statistical significance (probably due to the small number of subjects in group B). This result indicates that the decrease in lid laxity, leading to improvement in blink rate and quality, could indirectly result in improved signs and symptoms of DED due to MGD. However, a larger study is required to definitely demonstrate a causal relationship between lid laxity, blink quality, and signs/symptoms of DED.
Additionally, the improvements in corneal sensitivity may highlight the relationship between improved lid laxity, preservation of the tear lake, and corneal nerve health. Sensitivity in this case was recorded by esthesiometry and indirectly, through OSDI scores and the presence of related symptoms.
The mechanism(s) of action underlying these promising results is not clear. One possibility is that DMSt in the peri-orbital area could reduce lower lid laxity by activation of fibroblasts, increased neo-collagenesis, and improved skin elasticity/tightening. This could indicate that muscle stimulation is actually stimulation of numerous soft tissues. Indeed, previous studies have found that electrical impulses increased the expression of a growth factor (FGF) that controls the proliferation and migration of fibroblasts.30,31 Subjects treated with transcutaneous electrical nerve stimulation experienced effective proliferation of fibroblasts, and production, maturation and organization of collagen fibers.32 Better blinking could also be due to improved function of the OOM. Previous studies have shown that transcutaneous electrical stimulation of large muscles improves their toning, firmness and strength.33,34 In facial muscles, transcutaneous electrical stimulation increased the thickness of the zygomatic major muscle by almost 20%, due to an increase in resting tone.23,35 Aging in the lower eyelid and midface also features deflation and descent of neighboring fat pads, including the sub-orbicularis oculi fat (SOOF) pad, malar, nasolabial, medial cheek, and lateral cheek fat pads. These changes are accompanied by bony volume loss, and numerous other soft tissue changes to the ligaments, muscle, and so on.36 Dynamic muscle stimulation may serve as a nonsurgical avenue to counter some of these age-related changes, and help lift and restore volume to its more desirable position by leading to increased muscle tone and subsequent tension of the superficial musculo-aponeurotic system (SMAS). With respect to muscle stimulation, a dynamic approach such as the one used in this study is expected to be more effective compared to static stimulation, because it allows muscle fibers and motor neurons to recover from their refractory period, between successive stimulations.
In turn, the reduction in lower lid laxity could improve the efficiency of blinking and, thus, improve tear film stability. Indeed, several studies have found that floppy eyelid syndrome may be accompanied with various ocular surface disorders such as ocular surface inflammation,37 tear dysfunction,14,38 punctate keratitis,39,40 blepharitis,41 atrophy of meibomian glands,12,41 Demodex in meibomian glands,41,42 and conjunctivitis.38,40 Although floppy eyelid syndrome primarily features symptoms and signs of the upper eyelids, its primary finding is lid laxity and poor opposition to the globe. It may be a similar enough model so as to aid in relating the improvements we observed in DED-related outcomes to improvements in the laxity of any eyelid. Randomized controlled studies, in which the relationship between lower lid laxity and tear stability is directly investigated, are required to support this possibility. Aging is also associated with a decrease in skin blood flow, resulting in the accumulation of oxidants and skin damage.45 Several studies have established that neuromuscular electrical stimulation increases blood flow.43,44,46 Thus, another possible benefit of DMSt is the improvement in blood flow.
There were several limitations to this study. First, since the study examined a novel approach there was no preliminary estimation of size effect, and the study was not powered. Second, the study did not include a control group. Hence, it is possible that at least part of the improvements observed in this study are due to placebo or Hawthorne effects (the latter, the process by which a subject is aware of being followed and, consequently, changes his/her routine behavior or hygiene habits, thereby affecting the outcome). Future studies with well-defined controlled groups are required for elaborating the contribution of DMSt. Second, some of the exploratory outcomes, such as blink quality and blink rate, were qualitatively estimated by the study investigator. As any subjective evaluation, results could be biased especially since the study was not controlled. Use of quantitative tools, for example high-speed cameras and image processing software, would be useful to more accurately determine the extent of improvements in blinking quality and rate, using quantitative methods as described previously.3,47–49 Other limitations of the current study include a small sample size and a modest follow-up duration. Further studies with larger sample size, longer follow-up, control group, additional sites and quantitative measurements of blinking rate and quality are needed to better evaluate the merits of this novel approach, to better understand the mechanism of action, and to demonstrate the advantage of dynamic over static electrical stimulation.
Conclusion
Dynamic Muscle Stimulation technology (DMSt) reduces lower lix laxity and helps to improve blink quality, meibomian gland function, and dry eye symptoms. Unlike two other technologies in this space – IPL and RF – this technology can help address deficits in eyelid position and movement, particularly useful for older patients or those with floppy or mispositioned eyelids.
Data Sharing Statement
De-identified participant generated or analyzed during this study are available from the corresponding author upon reasonable request, for a period of 2 years after publication.
Acknowledgments
This study was funded by Lumenis Be.
Disclosure
The authors report no conflicts of interest in this study.
References
1. Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15(3):276–283. doi:10.1016/j.jtos.2017.05.008
2. Doughty MJ. Consideration of three types of spontaneous eyeblink activity in normal humans: during reading and video display terminal use, in primary gaze, and while in conversation. Optom Vis Sci. 2001;78(10):712–725. doi:10.1097/00006324-200110000-00011
3. Tsubota K, Hata S, Okusawa Y, Egami F, Ohtsuki T, Nakamori K. Quantitative videographic analysis of blinking in normal subjects and patients with dry eye. Arch Ophthalmol. 1996;114(6):715–720. doi:10.1001/archopht.1996.01100130707012
4. Nakamori K, Odawara M, Nakajima T, Mizutani T, Tsubota K. Blinking is controlled primarily by ocular surface conditions. Am J Ophthalmol. 1997;124(1):24–30. doi:10.1016/s0002-9394(14)71639-3
5. Cruz AA, Garcia DM, Pinto CT, Cechetti SP. Spontaneous eyeblink activity. Ocul Surf. 2011;9(1):29–41. doi:10.1016/s1542-0124(11)70007-6
6. Himebaugh NL, Begley CG, Bradley A, Wilkinson JA. Blinking and tear break-up during four visual tasks. Optom Vis Sci. 2009;86(2):E106–E114. doi:10.1097/OPX.0b013e318194e962
7. Belmonte C, Nichols JJ, Cox SM, et al. TFOS DEWS II pain and sensation report. Ocul Surf. 2017;15(3):404–437. doi:10.1016/j.jtos.2017.05.002
8. Su Y, Liang Q, Su G, Wang N, Baudouin C, Labbé A. Spontaneous eye blink patterns in dry eye: clinical correlations. Invest Ophthalmol Vis Sci. 2018;59(12):5149–5156. doi:10.1167/iovs.18-24690
9. Wang MTM, Tien L, Han A, et al. Impact of blinking on ocular surface and tear film parameters. Ocul Surf. 2018;16(4):424–429. doi:10.1016/j.jtos.2018.06.001
10. Jie Y, Sella R, Feng J, Gomez ML, Afshari NA. Evaluation of incomplete blinking as a measurement of dry eye disease. Ocul Surf. 2019;17(3):440–446. doi:10.1016/j.jtos.2019.05.007
11. Korb DR, Blackie CA, Nau AC. Prevalence of compromised lid seal in symptomatic refractory dry eye patients and asymptomatic patients. Invest Ophthalmol Vis Sci. 2017;58:2696.
12. Chhadva P, McClellan AL, Alabiad CR, Feuer WJ, Batawi H, Galor A. Impact of eyelid laxity on symptoms and signs of dry eye disease. Cornea. 2016;35(4):531–535. doi:10.1097/ICO.0000000000000786
13. Tyers AG. Aging and the ocular adnexa: a review. J R Soc Med. 1982;75(11):900–902. doi:10.1177/014107688207501116
14. Liu DT, Di Pascuale MA, Sawai J, Gao YY, Tseng SC. Tear film dynamics in floppy eyelid syndrome. Invest Ophthalmol Vis Sci. 2005;46(4):1188–1194. doi:10.1167/iovs.04-0913
15. Mastrota KM. Impact of floppy eyelid syndrome in ocular surface and dry eye disease. Optom Vis Sci. 2008;85(9):814–816. doi:10.1097/OPX.0b013e3181852777
16. Roubenoff R, Hughes VA. Sarcopenia: current concepts. J Gerontol A Biol Sci Med Sci. 2000;55(12):M716–M724. doi:10.1093/gerona/55.12.m716
17. Fukada K, Kajiya K. Age-related structural alterations of skeletal muscles and associated capillaries. Angiogenesis. 2020;23(2):79–82. doi:10.1007/s10456-020-09705-1
18. Okuda I, Irimoto M, Nakajima Y, Sakai S, Hirata K, Shirakabe Y. Using multidetector row computed tomography to evaluate baggy eyelid. Aesthetic Plast Surg. 2012;36(2):290–294. doi:10.1007/s00266-011-9829-2
19. Okuda I, Akita K, Komemushi T, Imaizumi K, Jinzaki M, Ohjimi H. Basic consideration for facial aging: age-related changes of the bony orbit and orbicularis oculi muscle in East Asians. Aesthet Surg J. 2023;43(4):408–419. doi:10.1093/asj/sjac318
20. Milbratz-Moré GH, Pauli MP, Lohn CLB, Pereira FJ, Grumann AJ. Lower eyelid distraction test: new insights on the reference value. Ophthalmic Plast Reconstr Surg. 2019;35(6):574–577. doi:10.1097/IOP.0000000000001392
21. Murri M, Hamill EB, Hauck MJ, Marx DP. An update on lower lid blepharoplasty. Semin Plast Surg. 2017;31(1):46–50. doi:10.1055/s-0037-1598632
22. Nieć M, Olejarz Z, Nowak K, et al. Factors influencing the length of the recovery period after blepharoplasty: a review of the latest data. J Educ Health Sport. 2024;59:183–208. doi:10.12775/JEHS.2024.59.012
23. Kavanagh S, Newell J, Hennessy M, Sadick N. Use of a neuromuscular electrical stimulation device for facial muscle toning: a randomized, controlled trial. J Cosmet Dermatol. 2012;11(4):261–266. doi:10.1111/jocd.12007
24. Mäkelä E, Venesvirta H, Ilves M, et al. Facial muscle reanimation by transcutaneous electrical stimulation for peripheral facial nerve palsy. J Med Eng Technol. 2019;43(3):155–164. doi:10.1080/03091902.2019.1637470
25. Gold MH, Biron J. Improvement of wrinkles and skin tightening using TriPollar® radiofrequency with dynamic muscle activation (DMA™). J Cosmet Dermatol. 2020;19(9):2282–2287. doi:10.1111/jocd.13620
26. Omatsu J, Yamashita T, Mori T, et al. Neuromuscular electrical stimulation for facial wrinkles and sagging: the 8-week prospective, split-face, controlled trial in Asians. J Cosmet Dermatol. 2024;23(10):3222–3233. doi:10.1111/jocd.16403
27. Lima Lang MP, Marinho DR, Procianoy F. The influence of luminous intensity on the eyelid aperture and measurement of the margin reflex distance. Orbit. 2022;41(3):311–314. doi:10.1080/01676830.2021.1892770
28. Xu KP, Yagi Y, Tsubota K. Decrease in corneal sensitivity and change in tear function in dry eye. Cornea. 1996;15(3):235–239. doi:10.1097/00003226-199605000-00002
29. Kim HY. Statistical notes for clinical researchers: Chi-squared test and Fisher’s exact test. Restor Dent Endod. 2017;42(2):152–155. doi:10.5395/rde.2017.42.2.152
30. Sebastian A, Syed F, Perry D, et al. Acceleration of cutaneous healing by electrical stimulation: degenerate electrical waveform down-regulates inflammation, up-regulates angiogenesis and advances remodeling in temporal punch biopsies in a human volunteer study. Wound Repair Regen. 2011;19(6):693–708. doi:10.1111/j.1524-475X.2011.00736.x
31. Koca Kutlu A, Ceçen D, Gürgen SG, Sayın O, Cetin F. A comparison study of growth factor expression following treatment with transcutaneous electrical nerve stimulation, saline solution, povidone-iodine, and lavender oil in wounds healing. Evid Based Complement Alternat Med. 2013;2013:361832. doi:10.1155/2013/361832
32. Machado AF, Santana EF, Tacani PM, Liebano RE. The effects of transcutaneous electrical nerve stimulation on tissue repair: a literature review. Can J Plast Surg. 2012;20(4):237–240. doi:10.1177/229255031202000415
33. Bax L, Staes F, Verhagen A. Does neuromuscular electrical stimulation strengthen the quadriceps femoris? A systematic review of randomised controlled trials. Sports Med. 2005;35(3):191–212. doi:10.2165/00007256-200535030-00002
34. Porcari JP, Miller J, Cornwell K, et al. The effects of neuromuscular electrical stimulation training on abdominal strength, endurance, and selected anthropometric measures. J Sports Sci Med. 2005;4(1):66–75.
35. Donath AS, Glasgold RA, Glasgold MJ. Volume loss versus gravity: new concepts in facial aging. Curr Opin Otolaryngol Head Neck Surg. 2007;15(4):238–243. doi:10.1097/MOO.0b013e32825b0751
36. Ugradar S, Kim JS, Massry G. A review of midface aging. Ophthalmic Plast Reconstr Surg. 2023;39(2):123–131. doi:10.1097/IOP.0000000000002282
37. Sward M, Kirk C, Kumar S, Nasir N, Adams W, Bouchard C. Lax eyelid syndrome (LES), obstructive sleep apnea (OSA), and ocular surface inflammation. Ocul Surf. 2018;16(3):331–336. doi:10.1016/j.jtos.2018.04.003
38. Schwartz LK, Gelender H, Forster RK. Chronic conjunctivitis associated with ‘floppy eyelids’. Arch Ophthalmol. 1983;101(12):1884–1888. doi:10.1001/archopht.1983.01040020886010
39. Culbertson WW, Tseng SC. Corneal disorders in floppy eyelid syndrome. Cornea. 1994;13(1):33–42. doi:10.1097/00003226-199401000-00007
40. Goldberg R, Seiff S, McFarland J, Simons K, Shorr N. Floppy eyelid syndrome and blepharochalasis. Am J Ophthalmol. 1986;102(3):376–381. doi:10.1016/0002-9394(86)90014-0
41. van den Bosch WA, Lemij HG. The lax eyelid syndrome. Br J Ophthalmol. 1994;78(9):666–670. doi:10.1136/bjo.78.9.666
42. Van Nouhuys HM, Van den Bosch WA, Lemij HG, Mooy CM. Floppy eyelid syndrome associated with demodex brevis. Orbit. 1994;13(3):125–129. doi:10.3109/01676839409031147
43. Wang JS, Chen SY, Lan C, Wong MK, Lai JS. Neuromuscular electric stimulation enhances endothelial vascular control and hemodynamic function in paretic upper extremities of patients with stroke. Arch Phys Med Rehabil. 2004;85(7):1112–1116. doi:10.1016/j.apmr.2003.11.027
44. Clarke Moloney M, Lyons GM, Breen P, Burke PE, Grace PA. Haemodynamic study examining the response of venous blood flow to electrical stimulation of the gastrocnemius muscle in patients with chronic venous disease. Eur J Vasc Endovasc Surg. 2006;31(3):300–305. doi:10.1016/j.ejvs.2005.08.003
45. Franzoni F, Plantinga Y, Femia FR, et al. Plasma antioxidant activity and cutaneous microvascular endothelial function in athletes and sedentary controls. Biomed Pharmacother. 2004;58(8):432–436. doi:10.1016/j.biopha.2004.08.009
46. Williams KJ, Ravikumar R, Gaweesh AS, et al. A review of the evidence to support neuromuscular electrical stimulation in the prevention and management of venous disease. Adv Exp Med Biol. 2017;906:377–386. doi:10.1007/5584_2016_128
47. Mitchell T, Murri M, Pflugfelder SC. Video viewing blink rate in normal and dry eyes. Eye Contact Lens. 2021;47(8):442–444. doi:10.1097/ICL.0000000000000791
48. Oganov A, Yazdanpanah G, Jabbehdari S, Belamkar A, Pflugfelder S. Dry eye disease and blinking behaviors: a narrative review of methodologies for measuring blink dynamics and inducing blink response. Ocul Surf. 2023;29:166–174. doi:10.1016/j.jtos.2023.05.011
49. Srivastav S, Basu S, Singh S. Tear film changes in symptomatic versus asymptomatic video display terminal users following computer challenge test. Ocul Surf. 2023;30:53–56. doi:10.1016/j.jtos.2023.08.003
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Autologous Platelet‐Rich Plasma Drops for Evaporative Dry Eye Disease from Meibomian Gland Dysfunction: A Pilot Study
Murtaza F, Toameh D, Chiu HH, Tam ES, Somani S
Clinical Ophthalmology 2022, 16:2199-2208
Published Date: 6 July 2022
Pulsed Light Therapy in the Management of Dry Eye Disease: Current Perspectives
Barbosa Ribeiro B, Marta A, Ponces Ramalhão J, Marques JH, Barbosa I
Clinical Ophthalmology 2022, 16:3883-3893
Published Date: 24 November 2022
Prevalence of Dry Eye Disease Among Individuals Scheduled for Cataract Surgery in a Norwegian Cataract Clinic
Graae Jensen P, Gundersen M, Nilsen C, Gundersen KG, Potvin R, Gazerani P, Chen X, Utheim TP, Utheim ØA
Clinical Ophthalmology 2023, 17:1233-1243
Published Date: 27 April 2023
A Randomized, Controlled Trial Comparing Tearcare® and Cyclosporine Ophthalmic Emulsion for the Treatment of Dry Eye Disease (SAHARA)
Ayres BD, Bloomenstein MR, Loh J, Chester T, Saenz B, Echegoyen J, Kannarr SR, Perez VL, Rodriguez TC, Dickerson Jr JE
Clinical Ophthalmology 2023, 17:3925-3940
Published Date: 18 December 2023
Evaluation of Perfluorohexyloctane Eyedrops in Habitual Contact Lens Wearers
Geffen DI, Pennell G
Clinical Ophthalmology 2024, 18:3179-3183
Published Date: 7 November 2024





