Back to Journals » Clinical Ophthalmology » Volume 19
Ease of Use and Acceptability of an at-Home Vision Screening Kit in a Primarily Non-English Speaking, Underserved Population
Authors Do T , Nguyen M , Her K , Kuo B, Chau KL , Lu M, Lim MC
Received 15 November 2024
Accepted for publication 21 February 2025
Published 21 March 2025 Volume 2025:19 Pages 1021—1032
DOI https://doi.org/10.2147/OPTH.S504861
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Timothy Do, Michael Nguyen, Kara Her, Bryan Kuo, Kevin Leung Chau, Madeleine Lu, Michele C Lim
Department of Ophthalmology & Vision Science, University of California, Davis, Sacramento, CA, USA
Correspondence: Michele C Lim, Department of Ophthalmology & Vision Science, University of California, Davis, Tschannen Eye Institute, 4860 Y Street, Sacramento, CA, 95817, USA, Tel +1 (916) 734-6602, Fax +1 (916) 734-0411, Email [email protected]
Purpose: The study aims to investigate the ease of use, acceptability, and accuracy of a simple at-home vision screening kit in a non-English speaking socially disadvantaged population.
Design: This is a single site, prospective, cross-sectional study.
Methods: Patients at a clinic that provided free medical care to an urban, predominantly Asian, underserved population were invited to participate in this study. We designed a program for at-home vision assessment, which consisted of a simple at-home vision kit (Rosenbaum near card, Amsler grid) and instructions for use given in verbal and written form in the patient’s own language. Testing was performed in each eye during an in-person visit. Patients were then asked to test at-home and were later contacted by telephone to record the results. A survey questionnaire assessing the ease of use and acceptability of the vision kit was then administered.
Results: One hundred and one of 141 patients completed the study. Of those patients, 60.4% were female, 79.2% were Asian, 95% were born outside of the United States, 81.2% did not speak English as their primary language, 47.5% were not enrolled in health insurance, and 67.3% expressed difficulty in accessing eye care. The results of our survey (n = 101) demonstrated that 90% would continue utilizing the vision screening kit, 91.1% would recommend the vision screening kit to others, and 82.2% rated the vision screening kit as extremely easy to use. The Spearman’s rank correlation coefficient for the in-person VA logMAR scores and at-home VA logMAR scores was calculated to be ρ = 0.862, p < 0.0001 in the right eye and ρ = 0.834, p < 0.0001 in left eye.
Conclusion: Our tailored protocol and design of a simple at-home vision screening kit was widely accepted and easy to use by an underserved population in which English was not the primary spoken language. The kit demonstrated excellent correlation between in-person and at-home VA results.
Keywords: socioeconomic factors, language barriers, vision care accessibility, visual acuity repeatability, tele-ophthalmology
Introduction
Poor vision significantly decreases one’s quality of life and can result in billions of dollars in medical expenditures each year.1 In the United States, visual impairment and blindness affect an estimated 6.5 million adults above the age of 45.2 In addition to the growing burden of eye diseases, disparities exist in the access and utilization of eye care within the United States. Many studies have demonstrated that race, ethnicity, and socioeconomic status affect visual outcome, and these social inequalities contribute to disproportionate vision loss across different communities.3 Within Asian populations, and especially among females, older age and lower educational level have been identified as risk factors for moderate to severe visual impairment.4,5 They have also been reported to have lower rates of utilizing eye care services compared to White Americans.5 Additionally, prior studies have highlighted language barriers, lower socioeconomic status, and being foreign born as a determinants of low health literacy within the Asian population.6,7 The implications of language barriers not only contribute to poor adherence in medical interventions but also increase the risk of adverse events when delivering care.8
The COVID-19 pandemic was an unexpected event that forced the temporary closure of outpatient medical care in the United States in 2020. During the peak of the pandemic, thousands of ophthalmology office visits and surgeries were canceled, causing a delay in access to care which placed many patients at risk for visual loss.9 A survey administered to the public with chronic eye conditions illuminated the negative psychosocial impact of the pandemic lockdown and found that they had a fear of further sight loss due to delay in care.10 In addition, restrictions imposed by the pandemic made accessibility even more challenging for patients of low socioeconomic status, which exacerbated health disparities.11 In response to pandemic-related clinic closures, the medical community was forced to adapt and reconfigure methods for providing care to communities.
One such adaptation was patient-centered home visual acuity (VA) testing. Limited studies examined the use of at-home Snellen VA testing for patients and concluded that this type of screening could be effective,12–14 however, the use of such tools in a population with language barriers and poor access to medical care is not well studied.
The purpose of this study was to develop a patient-centered at-home vision screening kit that could easily be used by a primarily non-English-speaking population with barriers to medical care (under-insured or no medical insurance, language barriers, and poor socioeconomic status). Though initially created in response to the COVID-19 pandemic, the utility of the kit can be expanded to improve vision care accessibility to all patients with socioeconomic disadvantages by identifying and prioritizing patients with significant vision loss and expediting their access to an in-person eye care visit. For example, based on the reported screening results, the clinic could schedule in-person visits for patients according to severity of their visual loss.
The primary aim of this project was to determine whether our design and instructions for a vision screening kit were easy to use and whether it had high patient satisfaction in a non-English speaking, socially disadvantaged population. The secondary aim was to assess correlation between at-home and in-person VA testing.
Methods
This was a prospective, cross-sectional study conducted at a free medical clinic in an urban location that serves a primarily Asian population. The clinic is affiliated with the School of Medicine at the University of California, Davis whose Institutional Review Board reviewed and approved the study protocol. The research adhered to the tenets of the Declaration of Helsinki.
Study Participants
The inclusion criteria consisted of any patient seen in clinic between the ages of 18 to 90 who was interested in participating and able to provide informed consent. The exclusion criteria consisted of adults who were unable to consent, children, and prisoners.
At Home Vision Self-Test Kit
A vision self-test kit was designed by our team, which consisted of a Rosenbaum eye chart (Rosenbaum Near Point Reading Card, Optics Incorporated, Brunswick, Ohio), an eye occluder, and an Amsler grid. A key part of the kit was an informational packet written in the primary language of the patient, which explained the research study and provided instructions on how to use the kit in simple terminology. In addition, these instructions were also given verbally at the time of enrollment to confirm the participant’s understanding and to ensure that they were delivered in a culturally sensitive manner. Languages represented in this study were English, Mandarin, Cantonese, Vietnamese, Spanish, Mongolian, Taishanese, Hmong, Tagalog, Farsi, and Arabic. For Farsi and Arabic, the free clinic lacked a designated interpreter for those languages, so the study relied on caregivers or family members to assist with interpretation.
Enrollment Process and Data Collection
Informed consent was obtained in the patient’s primary language prior to initiating the research study. An interpreter, with fluency in the patient’s primary spoken language was assigned to the patient for the remainder of the study. Demographic information was collected, and a short survey was deployed to assess the barriers to accessing regular eye care. The demographic information we collected included age, gender, ethnicity, primary language, country of birth, primary residential address, insurance, and median household income. The patient’s history of hypertension, diabetes, and major ocular conditions were also collected. Snellen near VA measurements and Amsler grid testing were completed for both eyes by a member of our research team in the clinic.
Tailored Protocol for Vision Screening
During the enrollment process, we spent approximately 30 minutes during each encounter to explain the information sheet in the patient’s primary language. This consisted of step-by-step instructions on how to assess visual acuity and Amsler grid testing with the items provided in the kit. Patients were instructed to hold the Rosenbaum Pocket Eye chart and Amsler grid approximately 14 inches while testing each eye separately. Patients were also instructed to wear their usual corrective lenses for reading if applicable and to conduct the test in a well-lit environment. For the Amsler grid, patients were asked to fixate on the center black dot and to indicate whether surrounding lines are wavy, distorted, blurry, dark, or empty in any quadrant. After meticulously reviewing the instructions, we physically demonstrated to the patient the entire process of utilizing the self-test vision kit from start to finish. We then asked the patients if they had any additional questions prior to having them explain the instructions to us in their primary language. The informational packet also consisted of a visual acuity and an Amsler grid log for the patient to document their results. Patients were instructed to test their own vision at home approximately two weeks after the in-person visit. This timeframe was chosen to minimize the possibility of changes in vision from ocular disease. In addition, it was a reasonable timeframe in which patients could remember our instructions of how to utilize the kit. The patients were called approximately two weeks after the in-person clinic visit, and their self-reported visual acuity was collected. A survey was administered (in the patient’s primary spoken language) to assess the opinions and ease of use for the screening kit.
Socioeconomic Status
Socioeconomic status was assessed using the area deprivation index (ADI), which was created by the Health Resources and Services Administration. It is comprised of 17 education, employment housing-quality, and poverty parameters that were derived from the US Census Bureau data and American Community Survey data.15 The ADI is an updated index that is validated for a range of socioeconomic factors (income, education, employment, and housing quality) associated with health outcomes for use on the neighborhood level.16 This metric allows for rankings of neighborhoods based on their socioeconomic disadvantage within the state and ranks them in comparison with other areas. The primary residential address for each patient was inputted into the Neighborhood Atlas in 2021 to determine the ADI of their primary residence.15,17 The ADI scores within the state are ranked on a scale of 1 to 10, with 1 being the least disadvantaged neighborhood and 10 being the most disadvantaged neighborhood, which is associated with worse health outcomes.
To assess one form of transportation barrier to healthcare access, the distance between a patient’s primary residential address and the free clinic was calculated using Google Maps.18 The departure date was set on a Saturday at 9:00 AM to best predict the traffic conditions of driving to the clinic during the morning. The fastest route determined by Google Maps was used to measure the number of miles it would take for patients to commute to our clinic by car transportation.
Statistical Analysis
Descriptive statistics were used to analyze the baseline characteristics of the cohort. Percentages reported in the study may not total 100% due to rounding. The Snellen VA measurements obtained both in-office and at-home were converted into logMAR values for the purpose of statistical analysis. A sample size calculation indicated that 46 patients were needed to detect a logMAR difference of 0.1 between the in-office and at-home vision tests, assuming a paired t-test, standard deviation of 0.2, 2-sided a of 0.05, and 90% power. Additional subjects were invited to participate in the study to account for loss of follow-up. The data was analyzed separately between the right and the left eye. We then compared the two groups of in-office measurements and at-home measurements with the Wilcoxon signed rank test and p values <0.05 were considered statistically significant. A Bland-Altman plot was utilized to assess the agreement between in-office logMAR values and at-home logMAR values. The limits of agreement were reported for the 95% confidence interval, quantified as ± 1.96 standard deviations (SD) of the mean. According to prior literature, a difference of ± 0.2 logMAR from the mean represents 2 Snellen lines and was determined to be a clinically significant difference between both in-office VA and at-home VA.19,20 All statistical measurements were performed using GraphPad software, version 10.1.0 (264) (San Diego, CA).
Results
The enrollment period for the prospective cohort study occurred between June 1st, 2022, and November 1st, 2023. A total of 141 patients were recruited into the study during the enrollment period and 101 (71.6%) patients fully completed the study. Of the total number of patients recruited, 40/141 (28.4%) did not complete the study. Of those who did not complete the study, 1/40 (2.5%) patient had declined to continue the study upon telephone follow-up and expressed they had lost interest in the study. 3/40 (7.5%) patients reported that they lost the vision kit during the follow-up telephone call. The remaining 36/40 (90%) patients were lost to follow-up and could not be reached after at least 3 phone call attempts. In total, 202 eyes remained eligible for the study, 101 (50%) right eyes and 101 (50%) left eyes. The mean (range) days to follow-up telephone call was 20.3 (7–105) days.
Baseline Characteristics
Baseline characteristics are shown in Table 1. Of the 101 patients who fully completed the study, 61 (60.4%) were female. The mean (± SD) age of our patients who completed the study was 59.69 ± 14 years. Within our cohort, 80/101 (79.2%) identified as Asian. 96/101 (95%) of our patients reported that they were born outside of the United States. 82/101 (81.2%) patients did not speak English as their primary language. 49/101 (48.5%) patients did not report any past ocular history and 52/101 (51.5%) of patients reported having at least one ocular condition.
 |
Table 1 Baseline Characteristics of Study Participants |
Social Determinants of Health
Social determinants of health for study participants are shown in Table 2. The mean (± SD) ADI of our patients’ primary residential address was 9.5 ± 6.5 according to the Neighborhood Atlas 2021 and the ADI distribution is shown in Figure 1. The remaining 2/101 (1.9%) patients in our sample had a suppressed ADI because their primary residential address was in a densely populated area that was missing core component variables to generate a ranking.
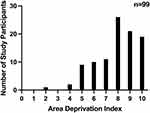 |
Figure 1 Frequency of Study Participants on the Area Deprivation Index. The area deprivation index value17 is shown on the x axis and the number of study participants is shown on the y axis. Sample size included 99 study participants, with 2 being omitted due to lack of data in their primary residential address generating a value on the ADI (n = sample size). |
 |
Table 2 Social Determinants of Health for Study Participants |
Accessibility to Eye Care Services
During the initial enrollment period, patients were surveyed (Supplemental S1) as to whether they faced difficulties in accessing eye care services and whether the COVID-19 pandemic had negatively impacted their access to care. Of the study group, 68/101 (67.3%) patients reported difficulty accessing regular eye care services and 51/101 (50.5%) patients reported that the COVID-19 pandemic had detrimentally influenced their ability to receive regular eye care services. Table 2 shows more detailed reasons for these difficulties. For the patients who reported difficulty in accessing eye care, 30/68 (44%) reported more than one contributing factor that prevented them from seeing an optometrist or ophthalmologist. For patients who reported that the COVID-19 pandemic negatively impacted their access to eye care service, 5/51 (9.8%) stated that more than one reason played a role in decreasing their provision of health services.
Survey Results
At the conclusion of the study, participants were surveyed (Supplemental S1) to assess the usability of the vision screening kit. They were requested to rate its ease of use on a scale ranging from 1 (extremely difficult) to 5 (extremely easy). Of the 101 subjects, 83/101 (82.2%) graded the vision kit a score of 5, 12/101 (11.9%) graded it a score of 4, and 6/101 (5.9%) graded it a score of 3. Participants were also asked if they plan to continue using this tool and if they would recommend this vision screening kit to their family or friends. Within our study group, 91/101 (90%) participants stated that they would continue using this vision screening kit and 92/101 (91.1%) participants expressed that they would recommend this vision screening kit to their family or friends.
Visual Acuity and Amsler Grid
For the right eye (n = 101), the mean (± SD) in-person VA logMAR score was 0.303 ± 0.268 and the mean (± SD) at-home VA logMAR score was 0.297 ± 0.274, p = 0.926 with no statistical difference between both variables. For the left eye (n = 101), the mean (± SD) in-person VA logMAR score was 0.274 ± 0.253 and the mean (± SD) at-home VA logMAR score was 0.270 ± 0.248, p = 0.838 with no statistical difference between both variables. The mean (± SD) absolute difference in logMAR score was 0.067 ± 0.114 and 0.072 ± 0.101 respectively. A Spearman’s rank correlation coefficient for the in-person VA logMAR scores and at-home VA logMAR scores was calculated to be ρ = 0.862 [95% CI, 0.799 to 0.906], p < 0.0001 in the right eye and ρ = 0.834 [95% CI, 0.760 to 0.886], p < 0.0001 in left eye.
The Bland-Altman plot of the right eye (Figure 2a) demonstrated that 96/101 (95%) eyes had agreement within 95% confidence interval [95% CI, −0.266 to 0.255]. 76/101 (75.2%) of eyes had ≤0.1 logMAR of difference between at-home VA and in-person VA. 95/101 (94.1%) of eyes had clinically significant agreement between the two variables as determined by ≤0.2 logMAR of difference from the mean difference. The Bland-Altman plot of the left eye (Figure 2b) demonstrated that 95/101 (94.1%) eyes had agreement within 95% confidence interval [95% CI, −0.247 to −0.240]. 73/101 (72.2%) of eyes had ≤0.1 logMAR of difference between at-home VA and in-person VA. 92/101 (91.1%) of eyes had clinically significant agreement between the two variables as shown by ≤0.2 logMAR of difference from the mean difference.
All patients were given a paper Amsler grid measured to be 6.5ʺ × 5.5ʺ as part of the vision screening kit. Of those who received the screening, 86/101 (85.1%) of patients were able to complete the screening at home upon the follow up telephone call while 15/101 (14.9%) of patients reported that they had misplaced the Amsler grid.
For the in-person Amsler grid screening in the right eye (n = 86), 84/86 (97.7%) eyes had normal findings and 2/86 (2.3%) eyes had abnormal findings where patients reported wavy lines in at least one quadrant on the Amsler grid. Of 84 eyes that had normal findings during the in-person screening, 82/84 (97.6%) eyes had reproducible normal findings during the at-home screening, while 2/84 (2.4%) eyes noted new abnormal findings during the at-home screening. Of those who had abnormal findings during the in-person screening, 2/2 (100%) eyes had reproducible abnormal findings in the same quadrant on the Amsler grid during the at-home screening. The overall reproducible rate of the Amsler grid screening in the right eye when comparing in-person results and at-home results were 97.7%.
For the Amsler grid screening in the left eye (n = 86), 80/86 (93%) eyes had normal findings and 6/86 (7%) had abnormal findings where patients reported wavy lines in at least one quadrant on the Amsler grid. Of the 80 eyes that had normal findings during the in-person screening, 78/80 (97.5%) eyes had reproducible normal findings during the at-home screening, while 2/80 (2.5%) eyes noted new abnormal findings during the at-home screening. Of the 6 eyes that had abnormal findings during the in-person screening, 6/6 (100%) had reproducible abnormal findings in the same quadrant on the Amsler grid during the at-home screening. The overall reproducible rate of the Amsler grid screening in the left eye when comparing in-person results and at-home results were 97.7%.
Discussion
This study demonstrates that a simple, paper, at-home vision screening kit was easy to use and well received by a primarily Asian, non-English-speaking population of patients who demonstrate barriers to health care access. To develop a kit that would be usable by a primarily non-English-speaking population, we wanted to incorporate tests that would provide high-yield information while remaining uncomplicated. A major advantage of the Rosenbaum eye chart is that numbers are more easily understood than the alphabet among non-English-speaking populations and patients can read the numbers in their primary spoken language.21 A prior study demonstrated that numbers had excellent concordance with alphabet charts and are a viable option for measuring best corrected visual acuity (BCVA) in individuals who are unfamiliar with the Roman alphabet.22 Additionally, VA remains one of the most important prognostic factors for visual outcomes, and determining a patient’s BCVA can aid providers in triaging patients that need to be evaluated in-person sooner.23 The Amsler grid serves as a useful tool for similar reasons. The patient is not required to read text that addresses potential issues related to language barriers. Additionally, the grid is an easy-to-use tool that can aid in screening for macular pathology and even severe stages of glaucoma.24,25 Both tools have relatively little cost, and the decision to use paper-based items was imperative in a population that may not have ready access to technology.
One noteworthy aspect of our study was the positive user experience reported by participants; they consistently expressed satisfaction with the ease of use and the overall comfort of the at-home screening process, which highlights that a user-friendly design is a key contributing factor to its success. User experience is a critical component in influencing the successful adoption and effectiveness of any healthcare tool or technology.26 In general, people are more likely to adopt new technologies or tools if there is a perceived value, improvement in quality of life, and if there is confidence in navigating or using the equipment.27 Poor implementation of new technology or health tools can be due to lack of user-friendliness.28 The user interface of the at-home vision screening tool was designed to be simple and intuitive, allowing participants to navigate the screening process without encountering complexities. Within our informational packet, we also tailored our directions by using simple language while avoiding jargon or complex terminology in explaining its utility. The visual acuity and Amsler grid results log also provided a simple framework for our patients to record the results. We believe that clear and concise instructions in the patient’s primary language, coupled with an uncomplicated setup, contributed to the positive experiences reported.
The survey responses from our study suggest that our patient population faces many barriers that limit their access to vision care. Earlier investigations demonstrated that a large percentage of participants reported high medical costs and lack of insurance as obstacles to accessing eye care.29 Similar to our study, lack of health insurance was found to be the most common reason for difficulty in accessing vision care. Many uninsured patients report that they cannot afford the high cost of coverage, and though there are opportunities through government programs, they may be unaware of these programs due to language barriers and application complexity.30,31 Other barriers such as lack of education and poor technological literacy were less commonly reported in this study group, though they are often seen in low-income populations and act in concert to impede access to eye care.32–34 Transportation barriers are an additional element of disadvantaged socioeconomic status. Previous research demonstrated that many patients relied on asking other family and friends to provide their transportation to eye clinics.33,35 In our study population, the average distance to commute to clinic was beyond the appropriate walking distance and similarly, patients expressed lack of transportation means as a barrier to accessing eye care. Many factors contribute to transportation barriers, including but not limited to distance, cost of traveling, as well as availability.36 The at-home vision kit could be useful for assessing patient visual status before asking a patient to travel long distances to come for an in-person visit. Finally, language-specific considerations are a factor that is crucial to our population of patients. Additional studies have shown that communication failures and difficulties related to medical interpretation made up 20% of barriers in eye care and further follow-up.37,38 Multilingual instructions, coupled with culturally competent outreach efforts, are necessary to ensure that language diversity does not impede the adoption and effectiveness of at-home vision screening.39 We believe that a strength of our program was attention placed towards overcoming language barriers as described earlier.
Our uniquely designed vision self-test kit showed high reproducibility for near VA with that obtained in a clinical setting in a primarily Asian, non-English speaking, socially disadvantaged population. The successful use of the at-home screening tool adds to an already robust literature in which at-home vision screening correlated well with in-person testing in English-speaking populations in the United States.8,10 One prior study demonstrated that the VA measured using a home acuity test with Sloan letters in an English-speaking population had excellent repeatability and good agreement of last recorded in-clinic VA.12 Another study investigated 3 different configurations of VA charts that were either printed or accessible on a technological device, and they found that all 3 at-home VA test results were comparable with in-office VA measurements.14 Our paper adds to existing knowledge by showing that at-home vision testing can be performed with relative accuracy when deployed in a thoughtful manner, especially in our unique population.
Given our excellent concordance between at-home measurements and in-person measurements, our vision self-test kit could be useful for triage in our non-English speaking, socially disadvantaged community. For example, the Amsler grids have been found to be good at detecting changes within the macula and progression of various retinal diseases.40 Prior studies have found that late intervention for patients with newly developed age-related macular degeneration often results in worse visual outcomes.41 By pairing visual acuity screening with Amsler grid testing, this tool can help better detect and monitor progression in ophthalmic conditions while allowing for timelier intervention if worsening vision is noted with the screening kit. Vision screening kits have also been shown to be useful as a triage tool to determine whether patients need to be seen urgently in clinic.13 This is especially useful in a community health clinic setting that has limited eye care appointments to help ensure that resource utilization is being maximized. Thus, our outcomes underscore the potential to use a simple vision kit to monitor progression of various eye diseases, triage eye complaints, and to improve accessibility to vision care.
Despite the promising outcomes, it is essential to acknowledge certain limitations. Ease of use comes at the cost of testing comprehensiveness. One such limitation is the inability of the tool to diagnose complex eye conditions that may require specialized examination. In addition, participants in this study had predominantly good vision and the correlation of in-person and at-home testing may be affected by severe visual loss.
A notable concern also revolves around the potential challenges associated with generalizability. The study was conducted with a specific population, primarily comprised of Asian patients. There is limited representation of various age groups, socioeconomic statuses, geographic locations, and patients who speak other languages. To enhance the generalizability of future research, it is crucial to include a more diverse participant pool, encompassing a broader range of demographic factors and ophthalmic conditions.
Conclusion
In conclusion, our study provides valuable insights into the potential of at-home vision screening tools to enhance accessibility to eye care. These findings suggest that the simple at-home vision screening kit is easy to use and generally well accepted by an underserved population in which English is not the primary spoken language. The success of our vision screening protocol in this population again exemplifies the importance of considering language and social factors when designing a self-test kit. Additionally, the near VA measurements tested in-clinic can be accurately reproduced in an at-home setting and with high correlation. The Amsler grid also showed highly reproducible results in both settings. While the outcomes of the study are positive, it is imperative to remain vigilant in addressing challenges and refining these tools for widespread and equitable use. The next steps are to use the at-home vision screening kit as a triage tool to improve access to vision care for those who need it most. Future directions may also include correlating vision measured with the at-home kit with specific ocular co-morbidities.
Abbreviations
ADI, Area Deprivation Index; VA, visual acuity; BCVA, best corrected visual acuity; SD, standard deviation.
Acknowledgments
We wanted to thank the ophthalmology committee and interpreters at Paul Hom Asian Clinic, Sacramento, CA, for their help in recruiting patients in this study. This work was presented in part at The Association for Research in Vision and Ophthalmology Annual Meeting, 2024 in Seattle, WA (May 5–9) and The American Ophthalmological Society 160th Annual Meeting, 2024 in San Diego, CA (May 16–18).
Funding
Herman & Helen Schalk Fund at UC Davis Health Eye Center. J. William Kohl Summer Scholarship for Medical Students (TD). The sponsor or funding organization had no role in the design or conduct of this research.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Frick KD, Gower EW, Kempen JH, Wolff JL. Economic impact of visual impairment and blindness in the United States. Arch Ophthalmol. 2007;125(4):544–550. doi:10.1001/archopht.125.4.544
2. Chan T, Friedman DS, Bradley C, Massof R. Estimates of incidence and prevalence of visual impairment, Low Vision, and Blindness in the United States. JAMA Ophthalmol. 136(1):12–19. doi:10.1001/jamaophthalmol.2017.4655
3. Zhang X, Beckles GL, Chou CF, et al. Socioeconomic disparity in use of eye care services among US adults with age-related eye diseases: national Health Interview Survey, 2002 and 2008. JAMA Ophthalmol. 2013;131(9):1198–1206. doi:10.1001/jamaophthalmol.2013.4694
4. Zou M, Guo D, Chen A, et al. Prevalence of visual impairment among older Chinese population: a systematic review and meta-analysis. J Glob Health. 2021;11:08004. doi:10.7189/jogh.11.08004
5. Jiang X, Varma R, Torres M, Hsu C, McKean-Cowdin R. Self-reported Use of Eye Care Among Adult Chinese Americans: the Chinese American Eye Study. Am J Ophthalmol. 2017;176:183–193. doi:10.1016/j.ajo.2017.01.018
6. Phillips S, Wyatt LC, Turner MM, Trinh-Shevrin C, Kwon SC. Patient-provider communication patterns among Asian American immigrant subgroups in New York City. Patient Educ Couns. 2021;104(5):1049–1058. doi:10.1016/j.pec.2020.10.002
7. Lee S, Martinez G, Ma GX, et al. Barriers to health care access in 13 Asian American communities. Am J Health Behav. 2010;34(1):21–30. doi:10.5993/ajhb.34.1.3
8. Al Shamsi H, Almutairi AG, Al Mashrafi S, Al Kalbani T. Implications of language barriers for healthcare: a systematic review. Oman Med J. 2020;35(2):e122. doi:10.5001/omj.2020.40
9. Foot B, MacEwen C. Surveillance of sight loss due to delay in ophthalmic treatment or review: frequency, cause and outcome. Eye. 2017;31(5):771–775. doi:10.1038/eye.2017.1
10. Ting DSJ, Krause S, Said DG, Dua HS. Psychosocial impact of COVID-19 pandemic lockdown on people living with eye diseases in the UK. Eye. 2021;35(7):2064–2066. doi:10.1038/s41433-020-01130-4
11. Kantamneni N. The impact of the COVID-19 pandemic on marginalized populations in the United States: a research agenda. J Vocat Behav. 2020;119:103439. doi:10.1016/j.jvb.2020.103439
12. Crossland MD, Dekker TM, Hancox J, Lisi M, Wemyss TA, Thomas PBM. Evaluation of a home-printable vision screening test for telemedicine. JAMA Ophthalmol. 2021;139(3):271–277. doi:10.1001/jamaophthalmol.2020.5972
13. Chen TA, Li J, Schallhorn JM, Sun CQ. Comparing a home vision self-assessment test to office-based Snellen visual acuity. Clin Ophthalmol. 2021;15:3205–3211. doi:10.2147/opth.S309727
14. Bellsmith KN, Gale MJ, Yang S, et al. Validation of home visual acuity tests for telehealth in the COVID-19 Era. JAMA Ophthalmol. 2022;140(5):465–471. doi:10.1001/jamaophthalmol.2022.0396
15. Kind AJH, Buckingham WR. Making neighborhood-disadvantage metrics accessible - the neighborhood atlas. N Engl J Med. 2018;378(26):2456–2458. doi:10.1056/NEJMp1802313
16. Kind AJ, Jencks S, Brock J, et al. Neighborhood socioeconomic disadvantage and 30-day rehospitalization: a retrospective cohort study. Ann Intern Med. 2014;161(11):765–774. doi:10.7326/m13-2946
17. Index AD. Area deprivation index. University of Wisconsin School of Medicine Public Health. Available from: https://www.neighborhoodatlas.medicine.wisc.edu.
18. Google Maps. Available from: https://www.google.com/maps.
19. Siderov J, Tiu AL. Variability of measurements of visual acuity in a large eye clinic. Acta Ophthalmol Scand. 1999;77(6):673–676. doi:10.1034/j.1600-0420.1999.770613.x
20. Rosser DA, Cousens SN, Murdoch IE, Fitzke FW, Laidlaw DA. How sensitive to clinical change are ETDRS logMAR visual acuity measurements? Invest Ophthalmol Vis Sci. 2003;44(8):3278–3281. doi:10.1167/iovs.02-1100
21. Schubert TM. Why are digits easier to identify than letters? Neuropsychologia. 2017;95:136–155. doi:10.1016/j.neuropsychologia.2016.12.016
22. Chaikitmongkol V, Nanegrungsunk O, Patikulsila D, Ruamviboonsuk P, Bressler NM. Repeatability and agreement of visual acuity using the ETDRS number chart, Landolt C chart, or ETDRS alphabet chart in eyes with or without sight-threatening diseases. JAMA Ophthalmol. 2018;136(3):286–290. doi:10.1001/jamaophthalmol.2017.6290
23. Kang EY, Tai WC, Lin JY, et al. Eye-related emergency department visits with ophthalmology consultation in Taiwan: visual acuity as an indicator of ocular emergency. Sci Rep. 2020;10(1):982. doi:10.1038/s41598-020-57804-2
24. Su D, Greenberg A, Simonson JL, et al. Efficacy of the Amsler grid test in evaluating glaucomatous central visual field defects. Ophthalmology. 2016;123(4):737–743. doi:10.1016/j.ophtha.2015.12.003
25. Bjerager J, Schneider M, Potapenko I, et al. Diagnostic accuracy of the Amsler grid test for detecting neovascular age-related macular degeneration: a systematic review and meta-analysis. JAMA Ophthalmol. 2023;141(4):315–323. doi:10.1001/jamaophthalmol.2022.6396
26. Baummer-Carr A, Nicolau DP. The challenges of patient satisfaction: influencing factors and the patient - provider relationship in the United States. Expert Rev Anti Infect Ther. 2017;15(10):955–962. doi:10.1080/14787210.2017.1378097
27. Moxley J, Sharit J, Czaja SJ. The factors influencing older adults’ decisions surrounding adoption of technology: quantitative experimental study. JMIR Aging. 2022;5(4):e39890. doi:10.2196/39890
28. Thies K, Anderson D, Cramer B. Lack of adoption of a mobile app to support patient self-management of diabetes and hypertension in a federally qualified health center: interview analysis of staff and patients in a failed randomized trial. JMIR Hum Factors. 2017;4(4):e24. doi:10.2196/humanfactors.7709
29. Atta S, Zaheer HA, Clinger O, et al. Characteristics associated with barriers to eye care: a cross-sectional survey at a free vision screening event. Ophthalmic Res. 2023;66(1):170–178. doi:10.1159/000526875
30. Derose KP, Bahney BW, Lurie N, Escarce JJ. Review: immigrants and health care access, quality, and cost. Med Care Res Rev. 2009;66(4):355–408. doi:10.1177/1077558708330425
31. Kang YJ, McCormick D, Zallman L. Affordability of and access to information about health insurance among immigrant and non-immigrant residents after Massachusetts health reform. J Immigr Minor Health. 2017;19(4):929–938. doi:10.1007/s10903-016-0479-y
32. Goyal A, Richards C, Patel V, et al. The Vision Detroit project: visual burden, barriers, and access to eye care in an urban setting. Ophthalmic Epidemiol. 2022;29(1):13–24. doi:10.1080/09286586.2021.1884264
33. Chheda K, Wu R, Zaback T, Brinks MV. Barriers to eye care among participants of a mobile eye clinic. Cogent Med. 2019;6(1). doi:10.1080/2331205x.2019.1650693
34. Kang JM, Chatterjee A, Rosdahl JA, et al. Health literacy and success with glaucoma drop administration. Ophthalmol Glaucoma. 2022;5(1):26–31. doi:10.1016/j.ogla.2021.05.004
35. Hark LA, Radakrishnan A, Madhava M, et al. Awareness of ocular diagnosis, transportation means, and barriers to ophthalmology follow-up in the Philadelphia telemedicine glaucoma detection and follow-up study. Soc Work Health Care. 2019;58(7):651–664. doi:10.1080/00981389.2019.1614711
36. Cochran AL, McDonald NC, Prunkl L, et al. Transportation barriers to care among frequent health care users during the COVID pandemic. BMC Public Health. 2022;22(1):1783. doi:10.1186/s12889-022-14149-x
37. Elam AR, Lee PP. Barriers to and suggestions on improving utilization of eye care in high-risk individuals: focus group results. Int Sch Res Notices. 2014;2014:527831. doi:10.1155/2014/527831
38. Lee BW, Murakami Y, Duncan MT, et al. Patient-related and system-related barriers to glaucoma follow-up in a county hospital population. Invest Ophthalmol Vis Sci. 2013;54(10):6542–6548. doi:10.1167/iovs.13-12108
39. Andrulis DP, Brach C. Integrating literacy, culture, and language to improve health care quality for diverse populations. Am J Health Behav. 2007;31(Suppl 1):S122–33. doi:10.5555/ajhb.2007.31.supp.S122
40. Faes L, Bodmer NS, Bachmann LM, Thiel MA, Schmid MK. Diagnostic accuracy of the Amsler grid and the preferential hyperacuity perimetry in the screening of patients with age-related macular degeneration: systematic review and meta-analysis. Eye. 2014;28(7):788–796. doi:10.1038/eye.2014.104
41. Lim JH, Wickremasinghe SS, Xie J, et al. Delay to treatment and visual outcomes in patients treated with anti-vascular endothelial growth factor for age-related macular degeneration. Am J Ophthalmol. 2012;153(4):678–686.e1–2. doi:10.1016/j.ajo.2011.09.013
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


