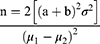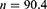Back to Journals » Open Access Journal of Clinical Trials » Volume 17
Effect of Consumption of Cape Gooseberries on Blood Glucose Control Among Patients with Type 2 Diabetes Mellitus in Kampala, Uganda: A Protocol for a Randomized Controlled Trial
Authors Ndahura NB , Nambooze J, Mangusho G , Najjuuko R
Received 4 June 2025
Accepted for publication 17 July 2025
Published 19 July 2025 Volume 2025:17 Pages 63—70
DOI https://doi.org/10.2147/OAJCT.S525661
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Arthur E. Frankel
Nicholas Bari Ndahura, Joweria Nambooze, Gilbert Mangusho, Rhona Najjuuko
Department of Nutritional Science and Dietetics, Kyambogo University, Kampala, Uganda
Correspondence: Nicholas Bari Ndahura, Department of Nutritional Science and Dietetics, Kyambogo University, P.O Box, 1, Kyambogo, Kampala, Uganda, Tel +256772636271, Email [email protected]
Purpose: This study seeks to examine the efficacy of Cape gooseberries (Physalis peruviana) in regulating blood glucose levels, contributing to diabetes management. By exploring this cost-effective treatment option, this study could inform public health policies, empower communities to use local resources for managing chronic diseases, and encourage further studies on indigenous foods, ultimately enhancing the understanding of their potential to prevent diseases and promote health.
Patients and Methods: A 12-week randomized controlled trial will be conducted with 200 diabetic patients recruited from St. Francis Nsambya and Mulago hospital diabetes clinics. The intervention group will consume 80 grams of fresh gooseberry per day in addition to regular diet and the control group will only consume their regular diet. Fasting blood glucose (FBG) will be assessed at baseline and bi-weekly, while the glycated haemoglobin (HbA1c) levels will be assessed at baseline, 6 weeks and 12 weeks. Adherence will be assessed through food intake diaries, bi-weekly group meetings, and Short Message Service (SMS) reminders. Statistical analysis will be conducted using SPSS. Descriptive statistics will summarize baseline characteristics for both the intervention and control arms. Independent t-tests will compare differences between the intervention and control arms. A p-value of < 0.05 will be considered statistically significant. The primary outcomes are change in the levels of FBG and HbA1c levels. The secondary outcomes are rates of adherence and reported side effects.
Discussion: The study is expected to provide evidence that daily consumption of Cape gooseberries improves FBG and HbA1c in patients with T2DM. Positive results could support the integration of indigenous fruit into the dietary recommendations, offering a potentially less expensive strategy for T2DM management and inform future research and public health interventions.
Trial Registration Number: The study was registered with The Pan African Clinical Trials Registry (PACTR202503652641300).
Keywords: glycated hemoglobin, diabetes, glycemic control, indigenous fruits
Introduction
Type 2 diabetes mellitus (T2DM) also known as insulin-resistant diabetes, is a significant public health challenge in Uganda, particularly in urban areas like Kampala. The rise in the prevalence of T2DM is largely attributed to increased consumption of highly processed, energy-dense foods and more sedentary lifestyles, which contribute to overweight and obesity among urban dwellers.1 Overweight and obesity have been identified as major contributors to insulin resistance and T2DM. For instance, a study reported that about 32% metabolically healthy obese individuals transitioned to metabolic unhealthiness after a five-year follow-up, such transitions are driven by underlying disturbances in insulin metabolism, which arise from excessive adipose tissue.2,3 The increasing prevalence of T2DM leads to more demand for healthcare services, causing financial strain on the healthcare system as more resources must be allocated toward T2DM management and its complications. At an individual level, a patient with T2DM faces direct expenses related to medications and hospitalizations and indirect costs related to productivity losses due to illness.4–6
T2DM places a substantial economic strain on Uganda’s healthcare system, costing an estimated UGX 2.2 trillion as of 2022, approximately 13% of the national health budget. These costs are incurred primarily for diabetes medications, outpatient care, and managing complications, such as kidney failure and eye diseases. Given the economic burden of T2DM, finding cost-effective management strategies and enhancing prevention measures is therefore critical.7 In response to the rising prevalence of T2DM and the high costs of conventional diabetes treatments, researchers are investigating more affordable traditional remedies, such as indigenous fruits and vegetables known for their therapeutic effects. For example, a recent study observed that intake of water infused from Abelmoschus esculentus (okra) significantly reduced blood sugar levels.8 Another study, that examined the relationship between fruit and vegetable consumption and blood glucose levels among local farmers in Indonesia, reported higher blood glucose levels among those who consumed fewer portions of vegetables and fruits.9 Additionally, a study by Gul et al indicated that the fiber content in grapefruit, particularly when consumed in whole fruit form rather than juice, contributed to better glycemic control.10 The dietary patterns observed by Burgess and McGrath further reinforce these findings as their results indicated that a diet high in fruits and vegetables significantly reduced FBG and HbA1c levels. Therefore, locally sourced dietary options could offer not only health benefits but also economic advantages by reducing dependency on high-cost pharmaceuticals.11
Cape gooseberries (Physalis peruviana), traditionally known as “entuntunu”, are a common indigenous fruit growing in many African countries, including Uganda. Cape gooseberries were selected for this study due to their widespread availability, affordability, and cultural acceptance across Ugandan communities.12 Animal studies have suggested that gooseberries may have potential anti-diabetic effects; however, there is limited research regarding their efficacy in humans.13 However, an animal trial, reported that cape gooseberries juice dosage of 1 mL/200 grams (g) body weight (BW) /day and 5 mL/200 g BW/day lowered the blood glucose level and improved insulin resistance in rats with T2DM. Furthermore, a meta-analysis found that gooseberry supplementation demonstrated some beneficial effects on blood sugar levels in patients with diabetes, but the researchers emphasized the need for larger and well-designed studies to confirm the findings.14,15 Another study conducted in India reported a significant decrease in fasting and post-meal blood glucose levels in the intervention group, compared with a control group, after consuming 1–3 grams of gooseberry powder daily for 21 days.16 Therefore, this study aims to assess the effectiveness of cape gooseberry as a potential traditional and cost-effective remedy for managing blood glucose levels in individuals with T2DM.
Materials and Methods
Study Design
This study will be a randomized controlled trial with two study arms: an intervention arm consuming gooseberries daily as part of their regular diet, and a control arm following a regular/usual diet without gooseberries as indicated in the study flow diagram (Figure 1). All study participants will continue to receive their routine medical care.
 |
Figure 1 Flow of the study. Notes: Adapted from Schulz KF, Altman DG, Moher D, CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Annals of Internal Medicine. 2010;152(11):726–732. Creative Commons.17 |
Study Site and Population
The study will include adult patients with T2DM who attend the Saint (St) Francis Nsambya hospital diabetes clinic and the Mulago hospital diabetes clinic in Kampala. Convenience sampling will be used to select patients from these clinics who meet specific study criteria and are readily accessible. The two study sites will be purposively selected due to having established T2DM clinics, high patient volume, diverse population and proper patient records. However, the study participants will be randomly allocated to the study arms.
Randomization
The study will comprise two study arms: a control arm and an intervention arm. A formula created in Microsoft Office Excel 2021 will be used to randomly assign study participants to either the intervention or control group. To account for bias when analyzing the data, the biostatistician will be blinded to the treatment allocation to ensure unbiased analysis of the study outcomes. However, the study participants will not be blinded because of the nature of the intervention.
Sample Size Determination
The sample size (n) will be determined using the formula below at 80% power to detect a 1.0% difference in HbA1c.18,19
n = the sample size in each of the study arms
μ1= mean in intervention arm (HbA1c = 8.6)
μ2 = mean in the control arm (HbA1c = 9.6).20
μ1-μ2 = the difference to be detected
σ2 = standard deviation 2.4.20 Assumed to be the same in both arms
(significance set at 5% and power at 80%).
a = 1.96
The sample size will be increased by a factor of 10% to 99.44 study participants per study arm to accommodate for any potential loss to follow-up. Thus, rounded off to 100 study respondents in each study arm, hence the total sample size will be 200 study participants.
Inclusion Criteria
- Diagnosed with T2DM (HbA1c ≥ 6.5% (48 mmol/mol) and Fasting Plasma Glucose ≥ 7.0 mmol/L (126 mg/dL).
- Aged ≥ 18 years.
- Stable on oral hypoglycemic agents or insulin for at least six months.
- Willing to adhere to the intervention for the duration of the study.
- Consent to taking part in the study.
Exclusion Criteria
- Pregnant or lactating women.
- Patients with severe comorbidities, such as renal or hepatic failure.
- Patients with known allergies to gooseberries.
- Patients taking any herbal supplements/medicines.
Data Collection
- Baseline assessment: Demographic data, medical history, and baseline fasting blood glucose (FBG) and HbA1c levels will be recorded for all participants.
- Follow-up: FBG will be measured biweekly and HbA1c levels will be measured at 6 weeks and 12 weeks. Participants in the intervention arm will also maintain food intake diaries to monitor adherence.
- Final assessment: At the end of the 12 weeks, final FBG and HbA1c levels will be compared between the two study arms.
Outcome Measures
- Primary outcomes: Change in HbA1c levels and fasting blood glucose levels from baseline at 6 weeks and 12 weeks.
- Secondary outcomes: Change in patient adherence, and any reported side effects.
Statistical Analysis
Statistical analysis will be conducted using Statistical Package for Social Sciences (SPSS) version 26. Descriptive statistics (means, medians, standard deviations, and proportions) will summarize baseline characteristics for both the intervention and control arms. Independent t-tests will be used to compare differences in continuous variables between the intervention and control groups if the data are normally distributed; otherwise, suitable non-parametric tests will be applied. Categorical data will be analyzed using chi-square tests or Fisher’s exact tests, as appropriate. A p-value of <0.05 will be considered statistically significant.
Within-group analysis using paired t-tests will be performed to examine the changes in HbA1c and FBG within each study arm to account for individual variability. This analysis will provide insight into the intervention’s impact on the study participants within each study arm and complement the between-group comparisons. Analysis of Covariance (ANCOVA) will be used to adjust for covariates such as sex, duration of diabetes, and medication dose/type, which may influence treatment effects.
Intention-to-Treat Analysis
All participants randomized into the intervention and control arms will be included in the analysis, regardless of adherence to the intervention, to maintain the randomization benefits and minimize bias.
Per-Protocol Analysis
A secondary analysis will include only those participants who adhered strictly to the intervention protocol, providing insights into the intervention’s effectiveness under optimal conditions.
Adherence Monitoring and Analysis
Study participants will each maintain a food intake diary and will receive daily SMS reminders to increase adherence. Furthermore, bi-weekly meetings will be conducted with members of the study team to discuss any issues regarding adherence. Adherence will be quantified as a percentage of days the gooseberries were consumed. A participant will be categorized as adherent if they consume gooseberries on at least 80% of the days during the 12 weeks period. Adherence rates will be compared using chi-square or Fisher’s exact tests, as appropriate, for categorical data. Logistic regression analysis will also be applied to explore predictors of adherence.
Study Intervention Duration
The intervention will last for 12 weeks.
Study Intervention Details (Form, Preparation and Consumption of the Cape Gooseberries)
- Each of the study participants in the intervention arm will take an equivalent amount measured at 80 grams per day, this will be consumed as part of their regular meals. The measurement is based on one fruit portion size.
- The intervention arm study participants will take fresh Cape gooseberries to retain their nutrients in their natural state.
- The gooseberries will be washed and given uncooked to avoid destruction or loss of nutrients because of cooking or preservation processes.
- The study participants will be supplied with fresh gooseberries weekly upon visits to the clinics.
- Study participants will be informed to eat their gooseberries according to the major meals such lunch or dinner or as snack, so they embed them in the ordinary regular food regimen more easily.
- Participants will be advised on the frequency of consumption of the gooseberries and how they can be incorporated into meals, for instance, with salads or with plain low-fat yoghurt.
Implementation Plan
Study Preparation
- Ethical approval: Ethical approval was granted by the Research Ethical Committee (REC) of Clarke International University (CLARKE-2025-1657).
- Training of the research team: The data collectors will be trained on data collection and ethical considerations.
Study Participant Recruitment
- 200 adult patients with T2DM will be recruited from St. Francis Nsambya Hospital and Mulago Hospital diabetes clinics as per the inclusion criteria.
Baseline Assessment
- Base line data (socio-demographic data, dietary intake, medical history, and baseline FBG and HbA1c levels) will be collected.
Intervention Implementation
- Study participants will be randomly allocated to the intervention arm (gooseberries and regular diet) or control arm (regular diet).
- A weekly supply of 560 grams of fresh Cape gooseberries (80 grams per day) will be provided to the intervention arm on a weekly basis for 12 weeks.
Follow-Up
- Bi-weekly follow-up meetings will be conducted to discuss adherence and challenges.
- FBG will be measured bi-weekly and HbA1c levels will be measured at 6 weeks and at the end of the 12-week intervention.
Reporting, Dissemination and Recommendations
- A final comprehensive report will be compiled and shared with Kyambogo University, the participating hospitals and study participants. Furthermore, the study findings will be shared with other stakeholders through workshops and publications as per the CONSORT guidelines.17
- Recommendations for future research and potential public health interventions based on the findings will be shared with the relevant stakeholders.
Post-Study Evaluation
Feedback will be gathered from all study participants and the research team on the study process to help improve future studies.
Adverse Events
Study participants will be requested to inform the principal investigator (PI) if they are allergic to gooseberries. Those that self-report prior allergic reactions to gooseberries will be excluded from the study. Furthermore, any adverse reactions or discomfort participants experience from consuming gooseberries (eg, gastrointestinal issues) will be tracked through the participant reports during the bi-weekly meetings, this will provide insight into the intervention’s tolerability and safety. The contacts of the PI will be provided to all participants, and they will be informed to immediately stop consuming the gooseberries and contact the PI in case of an adverse event. Furthermore, the intervention will not alter the study participant’s medication routine.
Consent and Participation
Informed consent will be obtained from all study participants, furthermore the study will compile with the Helsinki Declaration.
Ethical Considerations
Ethical approval was granted by the Research Ethical Committee (REC) of Clarke International University (CLARKE-2025-1657). In addition, the study is registered with The Pan African Clinical Trials Registry (PACTR202503652641300). Furthermore, the researchers will ensure that the study adheres to the Declaration of Helsinki. Informed consent will be obtained from all participants. Data confidentiality will be maintained, and participants will be free to withdraw from the study at any time.
Discussion
Finding effective and affordable methods to manage T2DM in Uganda is increasingly important due to the rising prevalence of the disease and the financial burden it places on families and the healthcare system.7 While traditional plants like gooseberries (Physalis peruviana) are commonly believed to have curative properties. However, there is still insufficient research to substantiate these claims. Due to the high costs associated with conventional treatments, many individuals seek traditional medicines, as they are often viewed as safer and more affordable than conventional treatments. Gooseberries and other indigenous plants have historically held significance in African cultures for managing various health issues, including diabetes, yet scientific investigations into their effectiveness are still limited.21
This study therefore seeks to examine the efficacy of gooseberries in regulating blood glucose levels, contributing to diabetes management. By exploring these cost-effective treatment options, this study could inform public health policies, empower communities to use local resources for managing chronic diseases, and encourage further studies on indigenous foods, ultimately enhancing the understanding of their potential to prevent diseases and promote health.
The study’s major strengths are that it’s a randomized controlled trial and that both intention-to-treat and per-protocol analyses will be conducted, which will effectively assess real-world adherence issues while also evaluate the intervention’s efficacy under ideal conditions. However, limitations like convenience sampling and the short intervention duration may reduce the findings generalizability. Though, stratifying participants by age, sex and socioeconomic status will help mitigate sampling biases, but a post-intervention follow-up would further enhance the study’s generalizability by assessing the sustainability of cape gooseberry consumption and its long-term impact on glycemic control.
In conclusion, the study will provide results that will support the integration of indigenous fruit into the dietary recommendations, offering an affordable strategy for T2DM management and inform future research and public health interventions.
Acknowledgments
The authors would like to thank all the staff in the Department of Nutritional Science and Dietetics and the School of Vocational Studies at Kyambogo University.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The trial will be supported by a grant from the Kyambogo University competitive research grants scheme (KYU10-25/26). The design, analysis, and reporting of this study are independent of the funder.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Arinda IK, Sserwanja Q, Nansubuga S, Mukunya D, Akampereza P. Factors associated with over-nutrition among men 15-54 years in Uganda: a national survey. Nutr Metab Insights. 2021;14:11786388211016832. doi:10.1177/11786388211016833
2. Cao Z, Zheng X, Yang H, et al. Association of obesity status and metabolic syndrome with site-specific cancers: a population-based cohort study. Br J Cancer. 2020;123(8):1336–1344. doi:10.1038/s41416-020-1012-6
3. Schmiegelow M, Hedlin H, Mackey RH, et al. Race and ethnicity, obesity, metabolic health, and risk of cardiovascular disease in postmenopausal women. J Am Heart Assoc. 2015;4(5). doi:10.1161/jaha.114.001695
4. Butt MD, Ong SC, Wahab MU, et al. Cost of illness analysis of type 2 diabetes mellitus: the findings from a lower-middle income country. Int J Environ Res Public Health. 2022;19(19):12611. doi:10.3390/ijerph191912611
5. Bao X, Yang C, Fang K, Shi M, Yu G, Hu Y. Hospitalization costs and complications in hospitalized patients with type 2 diabetes mellitus in Beijing, China. J Diabetes. 2016;9(4):405–411. doi:10.1111/1753-0407.12428
6. Bashier A, Farghaly M, Alali J, et al. Real-world evaluation of demographics, treatment pattern, and economic burden of heart failure and kidney disease in type 2 diabetes mellitus patient population in Dubai, United Arab Emirates. Dubai Diabetes Endocrinol J. 2023;29(1):42–54. doi:10.1159/000530467
7. Guloba MM, Atwine B, Nakitende P. Economic burden of type 2 diabetes mellitus (T2DM) in Uganda: a cost-of-illness analysis. 2023. Available from: https://eprcug.org/publication/economic-burden-of-type-2-diabetes-mellitus-t2dm-in-uganda-a-cost-of-illness-analysis/?wpdmdl=16260&refresh=67c0733476f0e1740665652.
8. Zaenab S. Effects of abelmoschus esculentus infused water on blood glucose levels of rattus norvegicus in hyperglycemia conditions. Prisma Sains Jurnal Pengkajian Ilmu Dan Pembelajaran Matematika Dan Ipa Ikip Mataram. 2024;12(1):179. v12i1.10690. doi:10.33394/j-ps
9. Fatimah AP, Susanto T, Susumaningrum LA, Kholidi M. Relationship of vegetable and fruit consumption with farmers’ blood sugar levels in pakusari health center, jember. J Community Empowerm Health. 2022;5(1):24. doi:10.22146/jcoemph.66469
10. Gul S, Khatoon H, Ahmed N, Rashid H, Mirza AZ. Possible role of grapefruit in controlling hyperglycemia and associated complications: better glycemic control in healthy subjects through fruits fibers as compared to fruit juices. Bangladesh J Med Sci. 2020;19(3):480–485. doi:10.3329/bjms.v19i3.45866
11. Burgess M, McGrath N. Investigating dietary quality among individuals aged 15 years and over by diabetes status in South Africa. medRxiv. 2024:2024–2110. doi:10.1101/2024.10.25.24316103
12. Tumuhe CL, Ategeka K, Sunday C, Tibaijuka D, Muhindo CB. The utilization of traditional and indigenous foods and seeds in Uganda. J Food Security. 2020;8(1):11–21.
13. Ángel-Martín A, Vaillant F, Moreno-Castellanos N. Daily consumption of golden berry (Physalis peruviana) has been shown to halt the progression of insulin resistance and obesity in obese rats with metabolic syndrome. Nutrients. 2024;16(3):365. doi:10.3390/nu16030365
14. Muzaffar K, Sofi SA, Makroo HA, Majid D, Dar BN. Insight about the biochemical composition, postharvest processing, therapeutic potential of Indian gooseberry (amla), and its utilization in development of functional foods—A comprehensive review. J Food Biochem. 2022;46(11):e14132. doi:10.1111/jfbc.14132
15. Singh A. Herbal-based nutraceuticals in management of lifestyle diseases: experience from Indian population. Future Integr Med. 2024;3(2):106–115. doi:10.14218/fim.2023.00055
16. Akhtar MS, Ramzan A, Ali A, Ahmad M. Effect of Amla fruit (Emblica officinalis Gaertn.) on blood glucose and lipid profile of normal subjects and type 2 diabetic patients. Int J Food Sci Nutr. 2011;62(6):609–616. doi:10.3109/09637486.2011.560565
17. Schulz KF, Altman DG, Moher D. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. doi:10.1136/bmj.c332
18. Noorani M, Ramaiya K, Manji K. Glycaemic control in type 1 diabetes mellitus among children and adolescents in a resource limited setting in Dar es Salaam - Tanzania. BMC Endocr Disord. 2016;16(1):29. doi:10.1186/s12902-016-0113-y
19. Florey CD. Sample size for beginners. BMJ. 1993;306(6886):1181–1184. doi:10.1136/bmj.306.6886.1181
20. Guwatudde D, Delobelle P, Absetz P, et al. Correction: prevention and management of type 2 diabetes mellitus in Uganda and South Africa: findings from the SMART2D pragmatic implementation trial. PLOS Global Public Health. 2024;4(6):e0003395. doi:10.1371/journal.pgph.0003395
21. Nakaziba R, Anyolitho MK, Amanya SB, et al. Traditional medicinal vegetables in Northern Uganda: an ethnobotanical survey. Int J Food Sci. 2021;2021(1):5588196. doi:10.1155/2021/5588196
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.