Back to Journals » Clinical Ophthalmology » Volume 18
Effect of Lipiflow (Thermal Pulsation) on Ocular Surface Disease Management After Cataract Surgery
Authors Vasudevan B, Helmuth K, Fintelmann RE
Received 23 February 2024
Accepted for publication 25 June 2024
Published 8 August 2024 Volume 2024:18 Pages 2239—2252
DOI https://doi.org/10.2147/OPTH.S459472
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Balamurali Vasudevan, Kevin Helmuth, Robert E Fintelmann
Arizona College of Optometry, Midwestern University, Glendale, AZ, USA
Correspondence: Balamurali Vasudevan, Email [email protected]
Purpose: The aim of the current study was to investigate the efficacy of Thermal pulsation treatment, completed one month prior to cataract surgery, as a means of eliminating or significantly mitigating the exacerbating effects of cataract surgery on dry eye patients.
Setting: Glendale, Arizona.
Design: Prospective, longitudinal, non-masked, randomized clinical investigation.
Methods: The treatment group received Thermal pulsation therapy approximately 1 month prior to undergoing immediate sequential, same-day bilateral cataract surgery. The control group did not receive pre-operative Thermal pulsation but had cataract surgery performed in the same way, approximately 1 month after their baseline visit. Subjective questionnaires and objective clinical findings were evaluated at baseline, 1, 3, and 6 months after cataract surgery in the treatment group and control group.
Results: A total of 62 patients were randomized into two groups of 31 representing 124 eyes. Subjective improvement was observed in the treatment group with OSDI and SPEED II scores. Mean (SD) of the OSDI improved significantly (p< 0.01) from 56.98 (18.30) from visit 1 to 14.73 (12.22) at visit 4, and the mean (SD) of the SPEED II scores improved significantly (p=0.01) from 13.84 (6.12) during visit 1 to 7.1 (5.00) at visit 4 in the treatment group.
Conclusion: Pre-operative Thermal pulsation treatment in patients with dry eye secondary to MGD appears to reduce dry eye symptoms after cataract surgery. Expectations should be moderated by the fact that the reduction in symptoms appears to reduce prior to 3 months post-op after cataract surgery.
Keywords: lipiflow (thermal pulsation), dry eye, meibomian gland, cataract surgery, inflammatory cytokines
Introduction
Ocular surface disease is a common condition characterized by abnormalities in the tear film and ocular surface, resulting in symptoms such as dryness, redness, and irritation. Ocular surface disease is very common in older individuals. Cataract surgery is one of the most commonly performed surgical procedures globally and has a high success rate in improving visual acuity. However, it has been noted that cataract surgery can have adverse effects on the ocular surface, leading to dry eye symptoms and signs.1 As these individuals approach the need for cataract surgery, dry eye should be a consideration. As many as two-thirds of patients undergoing cataract surgery have dry eye.2 Furthermore, phacoemulsification procedures performed for cataract surgery can exacerbate dry eye symptoms.3 It has been reported that the presence of dry eye post cataract surgery ranges from 10% to 42%.4 Following cataract surgery, ocular symptom scores degrade in the subsequent 1–3 months postoperatively.5 Cataract surgery is also associated with lid margin abnormalities.6 It may be beneficial for surgeons to treat the patients for signs and symptoms of ocular surface disease before the cataract surgery is performed. Traditionally, artificial tears, ointments, anti-inflammatory medications etc. have been used to address dry eye post-operatively which creates a greater treatment burden for the patient. Often such treatments are merely palliative.
Dry eye is a multifactorial condition characterized by inflammation of the ocular surface and tear film instability. Up to 86% of the patients with dry eye have an evaporative dry eye component type of dry eye primarily due to meibomian gland dysfunction.7 Inflammatory processes play a crucial role in the development and progression of dry eye. Cataract surgery also creates additional ocular inflammation during the post-operative period.8 Assessment of tear cytokine levels provides valuable insights into the inflammatory status of the ocular surface in dry eye patients. Elevated levels of pro-inflammatory cytokines, such as interleukin-1 (IL-1), interleukin-6 (IL-6), and matrix metalloproteinase-9 (MMP-9), have been detected in tears of patients experiencing post-operative dry eye.9 Measurement of tear cytokines can aid in identifying the specific inflammatory pathways involved in dry eye pathogenesis. Tear cytokine analysis can help evaluate the effectiveness of anti-inflammatory treatments in managing post-operative dry eye symptoms. Correlations between tear cytokine levels and clinical parameters, such as ocular surface disease index (OSDI) scores and tear film stability, can provide a comprehensive assessment of post-operative dry eye severity. Longitudinal monitoring of tear cytokine levels post-cataract surgery enables tracking of the inflammatory response over time and guides the optimization of treatment strategies.
Thermal pulsation is an FDA-approved therapeutic approach for MGD that combines controlled heat and pulsatile pressure to unclog blocked Meibomian glands.10 By restoring the normal function of these glands, Thermal pulsation aims to enhance tear film stability and alleviate dry eye symptoms associated with OSD. Thermal pulsation treatment has gained recognition as a non-invasive intervention to manage MGD and subsequent OSD symptoms.11 One single 12-min Thermal pulsation treatment has been reported to significantly improve the meibomian gland secretion score, tear film break-up time, and survey symptoms scores when measured at 1 month and 9 months post-treatment.12 In addition, significant improvement in meibomian gland secretion scores was observed from baseline measurements to 1-month post-treatment, and this was maintained at 1-year follow-up.13 Another study has reported on the benefit of maintaining these improvements over 3 years.14 Furthermore, studies have reported that Thermal pulsation treatment after cataract surgery can lead to better visual outcomes, reduced postoperative complications, and improved patient satisfaction.15 These benefits were attributed to the optimized ocular surface health resulting from improved MGD management.
The aim of the current study was to investigate the efficacy of Thermal pulsation treatment as a means of eliminating or significantly mitigating the exacerbating effects of cataract surgery on dry eye patients, by evaluating the comparative impact on post-operative signs, symptoms, and inflammatory cytokine levels within the tears.
Methods
To ensure a power of 0.90, a sample size estimate was conducted using the G-power, indicating that a sample size of 62 was required. The study was initiated at the beginning of the COVID-19 pandemic, which had a significant impact on the patient dropout rate. Due to this high dropout rate, we needed to enroll 87 patients to eventually acquire 62 subjects (per the original sample size estimate) that completed all 4 study visits. Subjects were randomly assigned to achieve a total of 31 in the treatment and control groups.
The study adhered to the principles outlined in the Declaration of Helsinki and received approval from the Midwestern University Investigational Review Board (IRB). Prior to participation, all subjects provided written informed consent. Screening of participants was conducted to identify the presence of MGD and evaporative dry eye. In order to meet the inclusion criteria for the study, individuals needed to demonstrate a NIKBUT (NIKBUT 1st-Oculus 5M) score of less than 10 seconds in both eyes secondary to MGD, an OSDI (Ocular Surface Disease Index) score of 20 or higher and demonstrate willingness to attend four scheduled study visits over a 7-month period. This was in addition to any post-op surgical visits that they incurred.
The following were exclusion criteria for all subjects:
- History of Sjogren’s disease, Glaucoma, Uveitis, Herpes Simplex, and/or Shingles.
- Any ocular or refractive surgeries in the three months prior to the baseline visit.
- Ocular infections, moderate to severe (grade 2–4) allergic, vernal, or giant papillary conjunctivitis, severe (grade 3 or 4) inflammation of the eyelid at baseline.
- Use of Restasis or any other prescription-based dry eye treatment drugs. To be considered for inclusion a 6-month washout period was required.
- Chronic use of systemic ocular medications that could cause dry eye, including estrogen, progesterone, loratadine, chlorpheniramine, diphenhydramine, cetirizine, desloratadine, and topical glaucoma medications.
- Any significant, prolonged post-cataract complication deemed well beyond the norm as determined by the surgeon.
The Lipiflow system uses vectored thermal pulsation technology to gently heat (105–108F) the inner eyelid surface and massage the outer eyelids.10 The proprietary Thermal pulsation Activators are designed to be placed under and over the eyelid and are specifically shaped to avoid contact with the ocular surface. With a single 12-minute Thermal pulsation procedure, maximum results are typically experienced up to 4 months weeks post-treatment.16 In another study by Blackie et al,17 gland function continued to improve through 6 months, and was maintained to up to 12 months post treatment.
During each visit, a comprehensive set of subjective and objective measurements were obtained to assess the subjects’ ocular surface health. Subjective measurements included the completion of the OSDI, SPEED II questionnaire, and IDEEL quality of life questionnaire. These questionnaires were filled out by the participants in the office setting, allowing them a brief interval to blink and replenish their tears between performing other clinical tests. See Figure 1. The IDEEL questionnaire (used with permission from Novartis) includes 57 items and 3 separate modules related to dry eye. The modules cover symptom bother, impact on daily life and treatment satisfaction. IDEEL utilizes a 5-point Likert scale in which IDEEL scores are measured on a scale of 0 to 100, where higher scores for the dimensions of the Dry Eye Impact on Daily Life module indicated less impact on daily activities, work and emotions; higher scores for the Symptom-bother dimension indicated greater bother due to symptoms; higher scores for Satisfaction with Treatment Effectiveness dimension indicated greater satisfaction with treatment effectiveness; higher scores with Treatment-related Bother/Inconvenience indicated less treatment-related bother or inconvenience.
 |
Figure 1 Flowchart of the study visits and randomization. All the subjects underwent bilateral cataract surgery with intracameral antibiotics and subconjunctival steroids. |
Following the baseline visit, the treatment group received bilateral Thermal pulsation therapy. Both treatment and control groups were instructed to utilize 4 drops/day of non-preserved artificial tears until they received cataract surgery. In the study, all participants received immediate sequential, same-day bilateral, standard cataract surgery with the extraction of the cataract and the implantation of a posterior chamber intraocular lens (PC-IOL). All surgeries were performed by the same surgeon, who was not aware of the randomization. All patients received post-operative injection of Moxifloxacin 0.3 to 0.4 cc (450 to 600 mcg) (Intracameral) and triamcinolone 0.6% (subconjunctival). This form of medication delivery was less likely to induce ocular surface interference secondary to topical medication use. After surgery, subjects were instructed to use non-preserved artificial tears as needed, and they were to record the frequency of use each day. Patients in both groups returned for standard cataract surgery post-op care and thereafter received study visits at one-month post-op, three-months post-op, and six-months post-op intervals. Following the three-months post-op study visit, the control group also received Thermal pulsation therapy.
Objective clinical assessments had the potential to impact one another. Therefore, they were completed in a consistent and systematic sequence. First, the OCULUS 5M was utilized to evaluate the NIKBUT, OD followed by OS. These measurements were repeated again, and an average was calculated for each eye. Tear meniscus height was calculated centrally along the lower lid. Thereafter, tears were extracted by placing a microcapillary tube (0.5 to 2 μL) alongside the eye within the inferior fornix. Tears were combined between OD and OS with the assumption that there would be a similar level of inflammation existing between the eyes. We needed to combine tear volumes to have sufficient tears for analysis (goal of 3 μL). After tear extraction, OSDI, SPEED II, and IDEEL surveys were completed, followed by meibomian gland imaging with the Lipiscan, which allowed time for the tears to replenish. Henceforth, tear osmolarity (Trukera Medical) and Schirmer’s test were performed (tear osmolarity was measured twice and averaged in each eye). Thereafter, we assessed the level of corneal followed by conjunctival staining. Five sectors on the cornea were evaluated in each eye (central, superior, inferior, nasal, and temporal) for fluorescein staining. The nasal, temporal and inferior conjunctiva were assessed for lissamine green staining. Lastly, the number of expressing glands using the meibomian gland evaluator was determined. A total of 5 glands were evaluated in each of the temporal, central, and nasal sectors of the lower lid in each eye using the Korb meibomian gland evaluator. The volume of expression (MGE) plus quality of the meibum (MGQ) expressed was graded for each gland on a 0–4-point scale (MGE: 0 = no expression, 4 = full expression, MGQ: 0 = no expression, 1 = toothpaste like, 2 = buttery, 3 = inspissated 4 = normal expression). By gathering both subjective and objective measurements, a comprehensive evaluation of the participants’ ocular surface health status was achieved during each visit.
Following the visit, tear cytokine levels were analyzed. Tear samples were centrifuged and stored frozen at −80°C until processed. Within the 96-well array, each individual cytokine was represented two times, which allowed for an analysis of standard deviation. To each well, 200 μL of 1X wash buffer was added. The plate was then shaken for 10 minutes at room temperature at 500 revolutions per minute (rpm), followed by decanting the buffer. The samples were thawed to room temperature and centrifuged at 10,000×g for 5 minutes at 4°C. Subsequently, the samples were diluted 10-fold in a final volume of 22 μL. The total volume of the diluted sample was adjusted to 22 μL to accommodate the need for 10 μL per well with 2 replicates for each sample, while an additional 2 μL was included as a precautionary measure to ensure sufficient diluted sample for the experiment. These procedures were followed as per the manufacturers’ guidelines.
Data Analysis
A comparative analysis was conducted between the treatment and control groups across all four visits, encompassing objective measurements, subjective assessments, and evaluations of cytokine levels. A change value was calculated for the analysis with baseline – 1 month, baseline – 3 months, and baseline – 6 months. An additional change value analysis was calculated for the control group between the 3-month post-op visit (after which they received Thermal pulsation) and the 6 months post-op visit. The statistical test used to compare the change values between control and treatment patients was conducted using the Welch’s Two Sample T-test. This t-test is more appropriate when the assumption of equal variances between the two groups is violated. In our preliminary analysis, we observed that the variances between the groups were significantly different. To address this, we opted for Welch’s test to provide a more accurate analysis. The mean and standard deviation of the change values (baseline – timepoint) for the Control and Treatment patients in each group was calculated. The p-values from the two-sample t-test, difference in change values between control – treatment groups, and the 95% confidence interval of that difference were calculated. Significance was set at p-value < 0.05. Furthermore, correlations were explored to assess the relationships between the objective measurements and subjective assessments.
Results
Subjects
A total of 62 subjects successfully completed all four visits, with 31 participants in both the treatment and control groups representing 124 eyes. In the treatment group, there were 19 females and 12 males, with a mean (SD) age of 72.67 (6.19) years. The control group consisted of 25 females and 6 males, with a mean (SD) age of 73.54 (8.02) years.
OSDI
The mean (SD) OSDI scores for the treatment group showed improvement from 56.98 (18.30) at visit 1 to 23.32 (17.52) at visit 2 to 21.92 (18.61) at visit 3. Similarly, the mean (SD) OSDI scores for the control group improved from 48.52 (22.85) at visit 1 to 23.32 (17.52) at visit 2 to 19.19 (15.21) at visit 3. This represented a 65% and 60% reduction of the OSDI for the treatment and control groups, respectively. This improvement in OSDI was significantly larger in the treatment vs the control (p=0.05) for visit 1 but did not demonstrate significance for visit 2 (p=0.11). At the 6-month post-op visit for the treatment group, there was a marginal further reduction in OSDI to 14.73 (12.22). After crossover, the control group experienced a similar marginal reduction in OSDI at this 6-month post-op visit to 17.47 (15.75). See Figure 2.
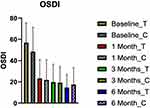 |
Figure 2 Plot of mean (SD) of OSDI scores at each visit for the treatment and control groups. |
SPEED II
The mean (SD) SPEED II scores for the treatment group showed improvement from 13.84 (6.12) at baseline to 5.93 (4.23) at the one-month post-op visit. The control group improved from 11.29 (7.13) to 7.07 (4.02) over this same period. This represented a 57% vs 37% improvement in SPEED II scores for the treatment versus control group, respectively, at visit 2. Decreases in these scores from the baseline were significantly different between the treatment and control groups (p=0.019) at visit 2. By visit 3, SPEED II scores decreased further in both groups, but the difference between them was no longer significant (p=0.15). The treatment group demonstrated an average SPEED II score of 7.03 (5.43), while the control group average was 6.87 (4.71). The control group received crossover Thermal pulsation treatment after visit 3 and thereafter experienced a 31% reduction in the mean SPEED II score to 4.72 (3.89) at visit 4. At this six-month post-op visit, the treatment group remained relatively unchanged versus visit 3 with mean SPEED II score of 7.10 (5.00). Its value decreased by 31% from visit 3. See Figure 3.
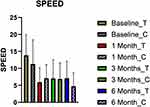 |
Figure 3 Plot of mean (SD) of SPEED II scores at each visit for the treatment and control groups. |
IDEEL
The treatment satisfaction component (10 survey items) of the IDEEL questionnaire showed no significant difference between baseline values and all visits in both the treatment and control groups. The average baseline scores in this category were 21.66 (10.61) for the treatment group and 21.48 (9.24) for the control group. The net changes vs baseline at all visits were negligible for both groups at all time intervals and were virtually unchanged throughout: (+1.58 vs +1.67) for visit 2 (−0.88 vs +4.10) for visit 3 and (−0.22 vs +4.26) for visit 4 (treatment vs control respectively). See Figure 4.
 |
Figure 4 Plot of mean (SD) of Treatment Satisfaction (IDEEL Survey) scores at each visit for the treatment and control groups. |
Looking at the impact on daily life portion of the survey, baseline values were 25.87 (20.41) and 19.00 (20.51) for treatment vs controls, respectively. At one month, these values were 9.43 (15.31) vs 8.00 (10.06). However, there was a significant difference (p=0.04) on the impact on daily life component of the IDEEL questionnaire between the treatment and control groups at the 1-month interval. This difference was not observed at the 3-months (16.9 vs 12.74, p=0.43) and 6-months (17.8 vs 12.46, p=0.42) intervals.
The baseline symptom bother score was 43.00 (16.58) and 33.41 (18.38) for the treatment and control groups, respectively. At visit 2, these values had decreased to 25.61 (16.09) versus 25.48 (12.31). The drop was greater in the treatment group, and this difference between groups did show statistical significance (p=0.016). However, this difference between the groups was not significant at visits 3:27.41 (16.76) vs 25.96 (10.82) (p=0.13) and visits 4 27.29 (15.62) vs 23.29 (11.47) (p=0.75) (treatment vs control respectively). So, at one-month post-op the treatment group was less bothered by symptoms of dryness. The control group did not experience much of a difference after crossover following visit 3.
Objective Tests
There were no significant changes in NIKBUT between the baseline and any of the subsequent visits for both the treatment and control groups. Please refer to Table 1 for more details. Additionally, central tear meniscus height (TMH) did not show significant variation between the baseline and the subsequent visits in either the treatment or control groups. Similarly, tear osmolarity and Schirmer’s test results did not demonstrate significant variations between the baseline and the subsequent visits in either the treatment or control groups. Refer to Table 1 for specific data. Corneal and conjunctival staining did not reveal any significant changes between baseline and any of the subsequent visits for both the treatment and control groups.
 |
Table 1 Summary Table with the Mean and Standard Deviation for All the Key Metrics at Each Visit |
The mean number of secreting glands in each section of 5 glands of the lower lid at baseline for the treatment group was 0.92. This improved to 1.34 (visit 2), 1.44 (visit 3) and 1.57 (visit 4). Between baseline and visit 3 this represents a 57% improvement in the number of secreting glands. By visit 4 that improvement had reached 73%. For the control group, these values were as follows: 1.01 (baseline), 1.07 (visit 2), 1.13 (visit 3) and 1.23 (visit 4). The change between baseline and visit 3 was 12%. This represents a value that is ~45% less of an improvement than shown by the treatment group. After the crossover treatment following visit 3, the number of secreting glands in the control group improved by ~9% at visit 4. Similarly, the quality of the meibomian individual gland secretions also demonstrated a greater improvement post Thermal pulsation. The mean (SD) quality of the secretions coming from the glands noted above for the treatment group were graded as follows at the specified visits: 0.86 (baseline), 1.26 (visit 2), 1.39 (visit 3), and 1.54 (visit 4). For the control group, these values were as follows: 0.95 (baseline), 1.12 (visit 2), 1.06 (visit 3), and 1.25 (visit 4-following crossover). For the treatment group, this represents ~61% improvement of the secretions at the 3-month post-op visit vs the control group, which experienced only a 10% improvement. Following the crossover, the control group experienced a 17% improvement over the next three months. However, despite these numeric improvements in both gland expression and meibum quality, the changes for neither group neither were found to be statistically significant at any time point.
Tear Cytokines
Inflammation, as measured by the change in the mean (SD) of all tear cytokines including MMP-9, IL-1a, IL-6, and IL-8, did not show a significant difference between the baseline and subsequent visits for both the treatment and control groups at each time interval. There were several potential numerical advantages to the treatment group, but these did not rise to a level that was statistically significant. Please refer to Table 1.
Artificial Tear Usage
Patients kept a log in which they recorded the number of artificial tear drops they used daily in the 6 months after cataract surgery. There was no significant difference between the treatment or control groups in how often artificial tears were used. Thermal pulsation therapy had no impact on tear usage including in the control group following crossover treatment.
Discussion
The purpose of this study was to compare the effects of Thermal pulsation on the signs and symptoms of dry eye patients following cataract surgery. Both groups received immediate-sequential, same-day, bilateral cataract surgery, with the same surgeon using injectable post-operative intracameral antibiotics and subconjunctival steroids. These unique study attributes combined to control for inflammatory differences between treatment and control groups secondary to surgery and/or post-operative care. The treatment group received Thermal pulsation approximately one month prior to cataract surgery in this fashion, with the control group receiving Thermal pulsation treatment at the 3-month post-op visit. This time interval allowed for the examination of the effects of Thermal pulsation and artificial tears on signs and symptoms at 1-month post cataract surgery when the control group was still using artificial tears only. At the 3-month time frame, the effects of Thermal pulsation on signs and symptoms were assessed in the treatment group compared to the control group. Lastly, at the 6-month time frame, the effects of Thermal pulsation before and after cataract surgery were compared. The 3-month data from the treatment group and the 6-month data from the control group, which included the effects of Thermal pulsation after 3 months of treatment, were analyzed. Since most subjects had minimal use of artificial tears by the 6-month visit, any improvements in signs and symptoms were primarily attributed to the Thermal pulsation treatment.
Bilateral, same-day cataract surgery was employed because dry eye questionnaires are not designed to consider unilateral conditions. Additionally, for accuracy, quantitative tear analysis requires a minimum sample volume, which is obviously complicated by the presence of dry eye in all subjects. We overcame the tear volume challenge by combining the tears from the right and left eyes, presuming that they would possess a similar range of inflammation having had their surgery performed on the same day. The goal of controlling for surgery-induced inflammation versus inflammation caused by dry eyes was further enhanced by utilizing the same surgeon, performing the same surgical technique, for those patients in the study. Furthermore, the use of injectable post-operative antibiotics and steroids was important in order to eliminate the variables of patient compliance and the influence of topical medications on the anterior ocular surface.
The 2 key findings of this study were as follows:
- Both groups demonstrated decreases in subjective symptoms following cataract surgery. However, one month post-operatively, the treatment group showed a statistically significant improvement in subjective symptoms across all three surveys (OSDI, SPEED II, and IDEEL) compared to the control group. The symptom difference between groups lost its significance at visits 3 and 4, although OSDI demonstrated a significant reduction again at the sixth-month follow-up visit despite the control group having received crossover therapy after visit 3.
- There were no statistically significant differences between the treatment group and the control group for all objective signs measured across all visits, but some interesting trends were observed.
In this study, the assessment of symptoms was conducted using various questionnaires including OSDI, SPEED II, and IDEEL. It is somewhat surprising that both treatment and control groups experienced a reduction in dry eye symptoms following cataract surgery. This is likely attributable to the use of injectable steroids following cataract surgery. Since compliance was eliminated as a variable, all patients benefitted similarly from the improved comfort brought on by the use of the steroid. With the OSDI, there was a 65% and 60% reduction in symptoms for the treatment and control groups, respectively. With the SPEED II, it was 57% and 37% reduction in symptoms for the treatment and control groups, respectively. This trend was also visible with the results of the two components of the IDEEL questionnaire, namely the impact on daily life and symptom bother. These findings align with a recent study by Li et al18 who demonstrated that the SPEED II scores decreased significantly at 4 weeks, 8 weeks, and 12 weeks post Thermal pulsation treatment. More recently, Mencucci et al19 demonstrated a similar trend in subjects who were followed up for 1 month after cataract surgery. The improvement in OSDI scores at the 6-month time point further supported the effectiveness of Thermal pulsation in reducing symptoms, and this trend was consistent with previous findings by Greiner,20 who studied OSDI improvement for 12 months following Thermal pulsation treatment. Additionally, a recent study by Park et al15 demonstrated a reduction in OSDI scores from 37.92 to 22.33 (a 41% drop) in the treatment group when Thermal pulsation was performed prior to cataract surgery.
OSDI and SPEED II are both dry eye validated surveys. However, for the purpose of dry eye assessment following cataract surgery, the SPEED II survey is the better choice due to the OSDI having questions that consider quality of vision. Since cataract surgery will impact the quality of vision, SPEED II isolates better those subjective symptoms that are directly related to dry eye without potential impact from improved quality of vision due to cataract removal.
While it is true that there were not any statistically significant differences in the signs studied, there were several observable numerical improvements in the treatment groups versus the control group. A deeper look at the signs studied provided a more nuanced interpretation of the data. One such area was in the number of expressing glands and meibum quality. Following Thermal pulsation therapy, at the 1-month, 3-months, and 6-months post-op mark, the treatment group demonstrated a 45%, 55%, and 69% greater improvement in expressible glands, respectively. In the control group, at the 1-month, 3-months and 6-months post-op mark, there was a 16%, 22%, and 33% improvement in expressible glands. Following the crossover, the control group did not realize as much of an improvement (10%) thereafter. However, even these measures, while trending towards significance, did not show a statistically significant improvement. These findings were different from those of Matossian et al21 who followed up subjects for 3 months post-op with Lipiflow performed prior to the cataract surgery. They noticed an improvement in the meibomian gland score.
The aim of vectored, thermal pulsation treatment is to enhance the function of the meibomian glands and alleviate dry eye symptoms. In Thermal pulsation, stagnated meibum within the glands is softened by the 108.5° (F) heat and then pulsatile pressure helps to evacuate the meibum out of the gland. Theoretically, this should result in a reduced evaporative dry eye. While we did observe a numerical improvement in the glandular secretions (both number of expressible glands and meibum quality) post Thermal pulsation, we were not able to demonstrate a statistically significant impact on the evaporation rate of the tears (NIKBUT). Our results were similar to the trend observed by Park et al,15 who demonstrated a mild, non-significant increase in TBUT 1-month post-op in which Thermal pulsation preceded cataract surgery by 3 weeks. However, unlike Park et al,15 this modest increase in NIKBUT did not reach significance at the 3-month post-op visit nor at any time thereafter.
We postulated at the beginning of the study that better quality tears due to the impact Thermal pulsation had on the meibomian glands would create a healthier environment for epithelial cells both on the cornea and conjunctiva. Using the Johnson & Johnson Vision Care Institute Clinical Grading Scale, we assessed 5 zones for fluorescein staining. Grading on a 0–4 scale we assessed the central/nasal/inferior/superior and temporal zones of each eye. Combining the data for all fluorescein corneal values we realized a 30.6% reduction in staining in the treatment group at visit 2. The control group saw only a 7.4% reduction over this same period. At visit 3, these values had dropped further in both cohorts to 41.1% for the treatment group and 27.8% in the control group. Both dry eye and cataract surgery cause inflammation. Corticosteroids can have a known positive effect on corneal staining in dry eye patients22 It’s possible that the post-surgical injectable steroid had a positive effect on the corneal staining in both groups. Still, it appears that Thermal pulsation may have contributed an additional positive impact on the condition of the corneal epithelial cells. At visit 2 (2 months post-baseline) there was 23.2% less staining than seen in the control group. This effect appeared to wane over time. By visit 3 (4 months post-baseline), the difference in reduced corneal staining between the two groups had dropped to 13.3%. Crossover treatment occurred in the control group following visit 3. By visit 4, the control group saw a further decrease in staining of 6.1%, while the treatment group increased staining by 13.0% (a difference of 19.1%). While all of the corneal staining values above demonstrated a numerical improvement, the results were not deemed to be statistically significant at any visit both for the reductions seen nor for the differences between the two groups. See Figure 5.
 |
Figure 5 Plot of comparison of corneal staining for all the zones in the treatment and control group. |
Lissamine green staining in both treatment and control groups saw an equal 30% reduction in overall conjunctival staining at the one-month visit versus baseline (29.3% treatment, 29.9% control). At the 3-month post-op visit, an additional and nearly equal 30% reduction was seen in both groups (34.2% treatment, 28% control). Like the reduced staining seen with the cornea, the injectable post-op steroid likely had an impact on the level of conjunctival staining seen in each group. At visit 4, the lissamine green staining increased by 19.9% in the treatment group but only 5.7% in the control group. While the level of staining increased marginally in both groups, it is possible that the crossover Thermal pulsation therapy (after visit 3) helped to mitigate this increase in conjunctival staining slightly in the control group. Similar to the corneal findings, none of the changes in lissamine green staining of the conjunctiva were deemed to be statistically significant at any visit, nor was there a significant difference between groups.
Tear osmolarity is an essential marker in diagnosing and monitoring dry eye disease.23 In this study, we found that Thermal pulsation treatment, 1-month prior to cataract surgery, had a negligible impact on tear osmolarity. The lack of significant effect was observed in both the treatment and control groups. There was also no visible change to tear osmolarity in the control group after crossover treatment with Thermal pulsation at the 3-months post-op visit. These findings suggest that although Thermal pulsation may improve meibum expression and quality, it may not be effective in reducing tear osmolarity. It is possible that the measurement of tear osmolarity may have been impacted by the study technique. Tear lab was completed post NIKBUT. We intentionally had patients complete study questionnaires after meibography in order to allow for approximately 10–15 minutes of down time so that the eyes could replenish their tears. However, it is possible that some reflex tearing occurred during this sequence, which could have impacted the measured tear osmolarity.
Non-anesthetized Schirmer’s test is a gold standard to measure tear production. In the present study, there was no significant change in tear volume in either group. The lack of improvement in tear production observed in the treatment group may be attributed to several factors. Firstly, it is important to acknowledge that tear production is a complex process influenced by various factors, including tear gland function and ocular surface health. While Thermal pulsation treatment targets the meibomian glands and aims to enhance their function, it may not directly impact tear production from the lacrimal glands, which are responsible for the majority of tear secretion.
The levels of IL-1, IL-1Ra, MMP-9, IL-6, and IL-8 have been reported to play a role in the pathogenesis of ocular surface inflammation and dry eye disease.24 While there were no statistically significant changes to the levels of tear cytokines noted above, a deeper look at the measured values is worth reviewing. Cataract surgery has a known impact on the expression of MMP-9. As expected, mean MMP-9 levels increased at the one-month post-op visit in both groups: 14% in the treatment group and 30% in the control group. By the 3-month post-op visit, the level of mean MMP-9 reduced to 53% of the pre-surgical level in the treatment group and was at 97% of pre-surgical levels in the control group. This represents a 44% relative difference in the presence of mean MMP-9 in the treatment group. After crossover treatment in the control group, the level of mean MMP-9 reduced by an additional 4% compared to visit 3. However, in the treatment group (now 7 months post-Thermal pulsation) mean MMP-9 levels increased by 15% compared to the levels seen after visit 3.
For IL-1Ra, both groups experienced an increase at the 1-month post-op visit, but the increase in the treatment group was lower than the treatment group (11%) versus the control group (35%). These values were close to normalizing just slightly below pre-surgical values at the 3-month post-op visit. Following crossover therapy after visit 3, the control group saw IL-1Ra levels fall to 42% below pre-surgical levels by visit 4. The treatment group (now 7 months post Thermal pulsation) had IL-1Ra values of 4% above pre-surgical levels at this same visit. For IL-1a, the 1-month post-op visit saw a substantial difference between the treatment group, which had a 26% reduction in this cytokine, while the control group saw a 38% increase. By the 3-month post-op visit, the control group demonstrated a lower concentration versus baseline than the treatment group (93%-treatment vs 64% control). At the 6-month post-op visit, the IL-1a levels had increased in the treatment group (now 7 months post Thermal pulsation) to 17% above baseline levels, while the control group, post-crossover treatment at visit 3, saw a reduction to 59% versus baseline levels.
For IL-8, the 1-month post-op visit saw a difference between the treatment group, which had a 6% increase of this cytokine, while the control group saw a 10% increase. By the 3-month post-op visit, the treatment group saw a substantial drop to 37% of pre-surgical values, while the control group increased by 5% versus those same values. At the 6-month post-op visit, the IL-8 levels were still 46% reduced vs pre-surgical levels, while the control group, post-crossover treatment at visit 3, saw a reduction of 9% versus pre-surgical levels.
There was very little impact in either the control or treatment groups on the presence of IL-6.
Although all of the values noted above failed to achieve statistical significance, it is interesting to note that there were numerical improvements in the treatment group versus the control group for MMP-9, IL-1Ra, IL-1a, and IL-8 within the first three months after cataract surgery. The impact tended to wane beyond 3 months. Crossover treatment of the control group seemed to confirm this positive impact on these cytokine concentrations three months later at visit 4.
The lack of statistically significant improvement in the levels of these inflammatory markers in the treatment group may be attributed to several factors. Firstly, it is important to consider that this study looked at 2 variables simultaneously (cataract surgery and dry eye), both of which can impact inflammation. Secondly, at least in the case of dry eye disease, the inflammatory cascade is complex and multifactorial, involving multiple cytokines, chemokines, and proteases. While cataract surgery and Thermal pulsation treatment may have targeted certain aspects of the inflammatory process, they might not have directly influenced the levels of IL-1, IL-1Ra, MMP-9, IL-6, and IL-8 in the ocular surface. While an improvement was noticed with some of the cytokines post Thermal pulsation, it is difficult to conclude with confidence that it made a significant difference in the expression of these inflammatory markers.
Conclusions
The findings of this study suggest that Thermal pulsation treatment leads to an improvement in symptoms of ocular surface disease, such as dryness, discomfort, and irritation. The positive outcomes in symptom relief following Thermal pulsation treatment align with previous research and support the effectiveness of this intervention in addressing patient-reported discomfort associated with ocular surface conditions. Patients experienced notable alleviation of dryness and improved overall comfort, which can greatly enhance their quality of life. However, no statistically significant improvements were observed in the objective signs of the disease, including tear film stability, ocular surface staining, and levels of inflammatory cytokines. There were numerical improvements not reaching levels of significance in meibomian gland expression, meibum quality, NIKBUT, corneal staining, and multiple tear cytokines. Most of these effects were seen in the first three months after baseline and waned thereafter. Despite the lack of conclusive significant improvements in signs, the observed positive effects on symptoms highlight the potential of Thermal pulsation as a therapeutic option for patients suffering from ocular surface disease. Its pre-surgical use in patients prior to receiving cataract surgery provides some benefit within the first few months after surgery. Its impact thereafter appears to be less conclusive. Future research should aim to explore the long-term benefits of Thermal pulsation, as well as its effects on a broader range of clinical parameters and inflammatory markers.
Synopsis
This study adds a concrete understanding of Lipiflow (thermal pulsation)’s clinical effectiveness for signs and symptoms over 6 months. Subjects who received thermal pulsation did show a slightly larger average reduction of symptoms only (not signs) versus controls.
Ethics Board/IRB
Midwestern University IRB application number: AZ 1306
Acknowledgments
The authors express their gratitude to the study coordinators, Ms. Tannaz Besharitian and Kelly Shim, as well as our statistician, Dr. Charlotte Bolch, for their invaluable assistance with the study.
Funding
This study was supported by an investigator-initiated trial sponsored by Johnson and Johnson Surgical Care awarded to the university.
Disclosure
None of the authors have any other financial conflicts.
References
1. Benito A, Perez GM, Mirabet S, et al. Objective optical assessment of tear-film quality dynamics in normal and mildly symptomatic dry eyes. J Cataract Refract Surg. 2011;37:1481–1487.
2. Miura M, Inomata T, Nakamura M, et al. Prevalence and characteristics of dry eye disease after cataract surgery: a systematic review and meta-analysis. Ophthalmol Ther. 2022;11(4):1309–1332. doi:10.1007/s40123-022-00513-y
3. Cetinkaya S, Mestan E, Acir NO, et al. The course of dry eye after phacoemulsification surgery. BMC Ophthalmol. 2015;15(68). doi:10.1186/s12886-015-0058-3
4. Trattler WB, Majmudar PA, Donnenfeld ED, et al. The prospective health assessment of cataract patients’ ocular surface (PHACO) study: the effect of dry eye. Clin Ophthalmol. 2017;11:1423–1430. doi:10.2147/OPTH.S120159
5. Han KE, Yoon SC, Ahn JM, et al. Evaluation of dry eye and meibomian gland dysfunction after cataract surgery. Am J Ophthalmol. 2014;157(6):1144–1150.e1. doi:10.1016/j.ajo.2014.02.036
6. El Ameen A, Majzoub S, Vandermeer G, et al. Influence of cataract surgery on Meibomian gland dysfunction. J Fr Ophtalmol. 2018;41(5):e173–e180. doi:10.1016/j.jfo.2018.03.001
7. Willcox MDP, Argüeso P, Georgiev GA, et al. TFOS DEWS II Tear Film Report. Ocul Surf. 2017;15(3):366–403. doi:10.1016/j.jtos.2017.03.006
8. Jung JW, Park SY, Kim JS, et al. Analysis of factors associated with the tear film lipid layer thickness in normal eyes and patients with dry eye syndrome. Invest Ophthalmol Vis Sci. 2016;57(10):4076–4083. doi:10.1167/iovs.16-19251
9. Chotikavanich S, de Paiva CS, Li de Q, et al. Production and activity of matrix metalloproteinase-9 on the ocular surface increase in dysfunctional tear syndrome. Invest Ophthalmol Vis Sci. 2009;50(7):3203–3209. doi:10.1167/iovs.08-2476
10. Lane SS, DuBiner HB, Epstein RJ, et al. A new system, the LipiFlow, for the treatment of meibomian gland dysfunction. Cornea. 2012;31(4):396–404. doi:10.1097/ICO.0b013e318239aaea
11. Blackie CA, Carlson AN, Korb DR. Treatment for meibomian gland dysfunction and dry eye symptoms with a single-dose vectored thermal pulsation: a review. Curr. Opin Ophthalmol. 2015;26(4):306–313. doi:10.1097/ICU.0000000000000165
12. Greiner JV. A single LipiFlow® thermal pulsation system treatment improves meibomian gland function and reduces dry eye symptoms for 9 months. Curr Eye Res. 2012;37:272–278. doi:10.3109/02713683.2011.631721
13. Greiner Jack V. Long-term (12-month) improvement in meibomian gland function and reduced dry eye symptoms with a single thermal pulsation treatment. Clin Exp Ophthalmol. 2013;41(6):524. doi:10.1111/ceo.12033
14. Greiner JV. Long-term (3 Year) effects of a single thermal pulsation system treatment on meibomian gland function and dry eye symptoms. Eye Contact Lens. 2016;42:99–107. doi:10.1097/ICL.0000000000000166
15. Park J, Yoo YS, Shin K, et al. Effects of lipiFlow™ treatment prior to cataract surgery: a prospective, randomized, controlled study. Am J Ophthalmol. 2021;230:264–275. doi:10.1016/j.ajo.2021.04.031
16. Marshall LL, Roach JM. Treatment of dry eye disease. Consult Pharm. 2016;31(2):96–106. doi:10.4140/TCP.n.2016.96
17. Blackie CA, Coleman CA, Holland EJ. The sustained effect (12 months) of a single-dose vectored thermal pulsation procedure for meibomian gland dysfunction and evaporative dry eye. Clin Ophthalmol. 2016;10:1385–1396. doi:10.2147/OPTH.S109663
18. Li B, Fu H, Liu T, et al. Comparison of the therapeutic effect of meibomian thermal pulsation lipiflow™on obstructive and hyposecretory meibomian gland dysfunction patients. Int Ophthalmol. 2020;40(12):3469–3479. doi:10.1007/s10792-020-01533-y
19. Mencucci R, Boccalini C, Caputo R, et al. Dry eye after cataract surgery: efficacy of topical therapy with 0.3% hyaluronic acid. J Ophthalmol. 2023;2023:8153694.
20. Greiner M. Comparison of dry eye disease symptoms in patients with and without dry eye: a case-control study. Clin Ophthalmol. 2013;7:391–396.
21. Matossian C. Impact of Thermal Pulsation Treatment on Astigmatism Management and Outcomes in Meibomian Gland Dysfunction Patients Undergoing Cataract Surgery. Clinical ophthalmology. 2020;14:2283–2289. doi:10.2147/OPTH.S263046
22. Prinz J, Maffulli N, Fuest M, et al. Efficacy of topical administration of corticosteroids for the management of dry eye disease: systematic review and meta-analysis. Life. 2022;12(11):1932. doi:10.3390/life12111932
23. Kim MJ, Stinnett SS, Gupta PK. Effect of thermal pulsation treatment on tear film parameters in dry eye disease patients. Clin Ophthalmol. 2017;11:883–886. doi:10.2147/OPTH.S136203
24. Hessen M, Akpek EK. Dry eye: an inflammatory ocular disease. J Ophthalmic Vis Res. 2014;9(2):240–250.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

