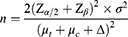Back to Journals » Journal of Inflammation Research » Volume 17
Efficacy of Self-Formulated Ergan Tang on Paroxysmal Atrial Fibrillation in Elderly Patients with Qi-Yin Deficiency: Impact on Inflammatory Marker Levels
Authors Wang XD, Wang Y, Zhang YC, Guo X, Xu WW, Zhang J, Li HY, Yuan LC
Received 17 May 2024
Accepted for publication 28 November 2024
Published 9 December 2024 Volume 2024:17 Pages 10729—10738
DOI https://doi.org/10.2147/JIR.S478734
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tara Strutt
Xue-Dong Wang,1 Yu Wang,1 Yu-Chen Zhang,2 Xi Guo,3 Wei-Wei Xu,1 Jing Zhang,1 Hai-Yan Li,1 Li-Chuang Yuan1
1Department of Cardiology, Beijing Hepingli Hospital, Beijing, 100013, People’s Republic of China; 2Department of Cardiology, Beijing Anzhen Hospital Affiliated to Capital Medical University, Beijing, 100029, People’s Republic of China; 3Aortic Surgery Center, Beijing Anzhen Hospital Affiliated to Capital Medical University, Beijing, 100029, People’s Republic of China
Correspondence: Xue-Dong Wang, Department of Cardiology, Beijing Hepingli Hospital, No. 18 of Hepingli North Street, Dongcheng District, Beijing, 100013, People’s Republic of China, Tel +86 10 5804 3212, Fax +86 10 6429 5714, Email [email protected]
Background: The aim of this study is to assess the clinical efficacy of the self-formulated Ergan Tang on managing paroxysmal atrial fibrillation (AF) characterized by qi-yin deficiency among elderly patients.
Methods: A cohort of 40 elderly patients diagnosed with paroxysmal AF who spontaneously reverted to sinus rhythm were enrolled based on predetermined inclusion and exclusion criteria. These participants were randomly divided into two groups: self-formulated Ergan Tang group and amiodarone group. Following an 8-week treatment regimen, alterations in traditional Chinese medicine (TCM) symptom scores, AF burden, and inflammatory factor levels were monitored in both groups. Furthermore, changes in these parameters were specifically analyzed before and after treatment with the self-formulated Ergan Tang.
Results: Baseline clinical and demographic characteristics demonstrated no statistically significant differences between the self-formulated Ergan Tang group and the amiodarone group. Following an 8-week treatment period no significant disparities were observed in TCM symptom scores, AF burden, or AF recurrence rates at the 6-month follow-up between the two groups (P> 0.05). In the self-formulated Ergan Tang group, a notable reduction in TCM symptom scores was observed from 18.30 ± 2.96 to 9.45 ± 2.84 (P< 0.001) compared to the pre-treatment levels. Moreover, there was a significant decline in AF burden by 171.8% (P = 0.043). Inflammatory markers including interleukin 6, tumor necrosis factor α, and high-sensitivity C-reactive protein exhibited substantial decreases (P< 0.05). Additionally, patients experienced a significant reduction in heart rate by 15.8% (P = 0.002) following treatment.
Conclusion: The self-formulated Ergan Tang demonstrates efficacy in alleviating AF burden, reducing inflammatory factor levels, and improving TCM symptom scores among elderly patients with paroxysmal AF characterized by qi-yin deficiency. Notably, its efficacy is comparable to that of amiodarone.
Keywords: elderly, inflammatory factors, paroxysmal atrial fibrillation, self-formulated Ergan Tang, Qi-yin deficiency
Introduction
Atrial fibrillation (AF) is the most prevalent non-benign cardiac arrhythmia encountered in clinical practice with its incidence escalating along with age. Recent epidemiological studies indicate that among adults aged over 45 years in China, AF affects approximately 1.8% of individuals, a prevalence that surges to nearly 5% among individuals aged 75 and above.1 As per the 2021 China Cardiovascular Health and Disease Report published in 2022, the estimated AF population in China stands at approximately 4.87 million individuals. Given its substantial associated mortality and morbidity rates, AF poses a formidable public health challenge.
The comprehensive management of AF adheres to the “ABC” approach as delineated in the 2020 ESC/EACTS Atrial Fibrillation Diagnosis and Management Guidelines, with “B” (better symptom management) chiefly encompassing two treatment strategies: rate control and rhythm control.2 Findings from the EAST-AF NET4 study published in the same year indicate that early rhythm control surpasses rate control in mitigating cardiovascular event risks among patients with AF within a year.3 This discovery, in contrast to earlier studies such as AFFIRM, underscores the contemporary paradigm shift towards “rhythm control” in AF treatment.4 Catheter ablation, pharmacological cardioversion, and direct current cardioversion have emerged as primary interventions for patients with AF. However, a critical and pragmatic concern arises post-intervention or spontaneous cardioversion: the risk of recurrence.
AF presents a considerable challenge due to its propensity for recurrence. Prior studies suggest that even with the administration of antiarrhythmic drugs (AADs), the recurrence rate (comprising AF, atrial flutter, and sustained atrial arrhythmias lasting over 30 seconds) may soar as high as 77.2% over a 3-year follow-up period.5 AF recurrence correlates with various factors including the subtype of AF, age, medical history, left atrial remodeling status, and the systemic inflammatory milieu. Inflammation processes foster atrial electrical and structural remodeling, thereby predisposing individuals to AF recurrence.6
Despite catheter ablation emerging as the primary choice for rhythm control in AF, numerous patients with AF opt for AADs due to the invasiveness of ablation procedures and associated patient discomfort.7 This is especially evident among elderly patients who exhibit reduced tolerance to ablative interventions, lower willingness to undergo such procedures, and a higher burden of comorbidities. Nonetheless, AADs are accompanied by drawbacks such as multiple side effects, poor tolerability, heightened risk of syncope with prolonged use, and the potential requirement for pacemaker implantation.8
In contrast to AADs, Chinese medicinal formulas offer several advantages in the treatment of arrhythmias, including fewer side effects, mild effects, and suitability for long-term use, which warrant further investigation.9 Drawing upon insights from traditional Chinese medicine (TCM) literature and modern pharmacological research, the classic formula of Zhigancao Tang has been modified with the addition of Chinese medicine Gansong (Nardostachyos Radix et Rhizoma) to create the self-formulated Ergan Tang. Tailored adjustments based on specific patient conditions render it a promising alternative to AADs for preventing AF recurrence.
Qi-yin deficiency syndrome represents a prevalent clinical syndrome associated with inflammation, particularly notable in cases of AF, encompassing approximately 40% of patients with AF.10 This study adopts a non-inferiority randomized controlled trial (RCT) design, focusing on elderly patients with paroxysmal AF characterized by qi-yin deficiency. The primary objective of this study is to examine the efficacy of self-formulated Ergan Tang in the management of paroxysmal AF in this elderly demographic, alongside its impact on inflammatory factor levels.
Materials and Methods
Study Design
In this study we employed a single-center, prospective, non-inferiority RCT design utilizing a parallel-group design with a 1:1 allocation ratio. The study protocol underwent thorough review and received approval from the Ethics Review Committee of Beijing Hepingli Hospital (Approval No. BJSHPLYY-IRB-KYXM-2022-03). Furthermore, the trial adhered to the ethical principles outlined in the Declaration of Helsinki.
Study Participants
The study cohort comprised elderly patients diagnosed with paroxysmal AF characterized by qi-yin deficiency who experienced spontaneous conversion to sinus rhythm. These patients either visited the Department of Internal Medicine for a consultation or were admitted to Beijing Hepingli Hospital during the period spanning from November 2020 to December 2022.
The diagnosis of AF adhered to the criteria delineated in the 2020 ESC Atrial Fibrillation Diagnosis and Management Guidelines,2 encompassing the following criteria: (1) evidence of AF episodes observed on a 12-lead electrocardiogram or though Holter monitoring; (2) presence of AF confirmed by a single-lead electrocardiogram, characterized by the absence of clearly discernible P waves lasting for a minimum of ≥ 30 seconds alongside irregular QRS waveforms at the R-R interval. Paroxysmal AF is defined as AF that spontaneously terminates within 7 days, either spontaneously or with intervention.
The diagnosis of qi-yin deficiency syndrome in TCM includes symptoms such as palpitations, restlessness, insomnia, vexing heat in chest, palms, and soles, dry mouth, night sweats aggravated by mental strain, accompanied by tinnitus, lumbosacral soreness, dizziness, irritability, a red tongue with scanty or absent coating, and a thin and rapid pulse.11
Exclusion criteria included: (1) acute ST-segment elevation myocardial infarction; (2) rheumatic heart valve disease, hyperthyroidism, sick sinus syndrome, high-degree atrioventricular block, or patients classified as New York Heart Association class IV heart failure; (3) severe hepatic or renal dysfunction; (4) pregnancy or lactation; (5) coexisting hematological disorders, severe cerebrovascular diseases, or mental disorders; (6) poor medication compliance or inability to accurately assess efficacy.
Following the diagnostic criteria outlined in both western medicine and TCM along with the specified exclusion criteria, syndrome differentiation was conducted by two senior TCM practitioners of associate chief physician level or above. Subsequently, a total of 40 patients diagnosed with paroxysmal AF characterized by qi-yin deficiency were identified for inclusion in this study. These patients, with a mean age of 76.40 ± 6.54 years (ages ranging from 61 to 89 years), had experienced spontaneous conversion to sinus rhythm.
Block randomization will be applied in this study. Randomization was conducted using randomly permuted blocks of size four to ensure a balanced allocation across treatment groups and the enrolled patients have be randomly allocated into the amiodarone group (n = 20) and self-formulated Ergan Tang group (n = 20) (Figure 1).
 |
Figure 1 Schematic diagram of clinical trial. |
Sample Size
A 5% significance level was considered; thus, the α value was determined at 0.05 and the β value was 0.10. The Zα/2 value was 1.96 while Uβ = 1.28. According to previous study, TCM syndrome score of amiodarone group and self-formulated Ergan Tang group were 8 (μt) and 9 (μc), respectively, while the difference between both groups was 3.2 (Δ). Thus, the minimum necessary sample size was determined by the following formula:
Considering a drop-out rate of 10%, the total sample size required is determined to be 20 in each group.
Data Collection
The general clinical characteristics, blood biochemical indicators, echocardiography findings, and results from Holter monitoring were recoded for all enrolled participants at baseline (the day of patient enrollment) and following an 8-week treatment period (the day 56 post-intervention treatment). Whole blood cell analysis was conducted utilizing the Sysmex XT-1800 automated hematology analyzer (Sysmex, Japan), while blood biochemical parameters were assessed using the BECKMAN AU5800 automated biochemical analyzer (Beckman Coulter, Inc., USA). Enzyme-linked immunosorbent assay (ELISA) was employed to qualify the levels of inflammatory cytokines, specifically tumor necrosis factor α (TNF-α) and interleukin 6 (IL-6), in the blood samples of the patients.
Echocardiographic parameters included measurements of left ventricular ejection fraction (LVEF), left atrial diameter (LAD), left ventricular end-diastolic dimension (LVEDD), and the ratio of early diastolic mitral inflow velocity to late diastolic mitral inflow velocity (E/A ratio). These measurements were performed utilizing the PHILIP Elite transthoracic Doppler ultrasound system (Philips, UK).
Treatment Protocol
In the self-formulated Ergan Tang group, the drug composition comprised the following ingredients: Gansong 15 g, Zhigancao (Glycyrrhizae Radix et RhizomaPraeparata cum Melle) 12 g, Dangshen (Codonopsis Radix) 6 g, Dihuang (Rehmanniae Radix) 30 g, Ejiao (AsiniCoriiColla) 6 g, Maidong (Ophiopogonis Radix) 10 g, Guizhi (CinnamomiRamulus) 9 g, Maren (Cannabis Fructus) 10 g, Shengjiang (ZingiberisRhizomaRecens) 10 g, and Dazao (Jujubae Fructus) 10 pieces. The medication method involved decocting the herbs in water and orally administering the solution twice daily. Adjustments were made based on the individual clinical conditions of the patients with the treatment duration spanning 8 weeks. Detailed records of the name and dosage of the medication for each patient were meticulously maintained.
In the amiodarone group patients received amiodarone hydrochloride tablets (Sanofi, China, Batch No. BHG0175). The dosage regimen entailed: during week 1, 0.2 g taken once, three times daily; in week 2, 0.2 g taken once, twice daily; from week 3 onwards, 0.2 g taken once daily. The treatment duration mirrored that of the self-formulated Ergan Tang group, spanning 8 weeks.
Efficacy Assessment
The primary outcomes composed of AF burden and duration of sinus rhythm maintenance, while the secondary outcome was TCM symptom. Additionally, the evaluation of TCM symptom efficacy adhered to the Clinical Research Guidelines for the Treatment of Palpitations with New Traditional Chinese Medicine, utilizing a semi-quantitative scoring method.
Subjective indicators for assessing the safety of patient medication encompassed complaints of discomfort reported by the patients themselves. Objective indicators included reports of abnormal liver and kidney function as well as any observed changes in liver and kidney function parameters.
Statistical Analysis
Count data are presented as percentages, while measurement data are expressed as mean ± standard deviation  . The comparison of means between two samples was conducted using the t-test, while the comparison of proportions between two samples was executed employing the chi-squared (χ²) test. Statistical analysis was performed utilizing SPSS 19.0 software, with statistical significance denoted by P< 0.05.
. The comparison of means between two samples was conducted using the t-test, while the comparison of proportions between two samples was executed employing the chi-squared (χ²) test. Statistical analysis was performed utilizing SPSS 19.0 software, with statistical significance denoted by P< 0.05.
Results
General Clinical Data and Demographic Characteristics of Enrolled Participants (Table 1)
The comparison of general clinical data and demographic characteristics between the two groups, encompassing variables such as age, gender, body mass index (BMI), duration of AF, blood pressure levels, heart rate, smoking history, history of diabetes, cardiac ultrasound results, and proportions of ACEI/ARB, β-blockers, spironolactone, and diuretics usage, revealed no significant differences (P> 0.05). Similarly, there were no significant differences observed in biochemical indicators including lipid levels, blood glucose levels, high-sensitivity C-reactive protein (hsCRP), and renal function (P> 0.05).
 |
Table 1 Baseline Clinical Data and Demographic Characteristics of the Two Groups |
Changes in AF Burden and TCM Symptom Scores After 8 weeks of Treatment in Both Groups
As shown in Table 2, following an 8-week treatment period with self-formulated Ergan Tang and amiodarone, there were no significant differences in AF burden (total duration of AF episodes on 24-hour Holter monitoring), duration of sinus rhythm maintenance, and TCM symptom scores12 between the two groups (P> 0.05). At the 6-month follow-up, there were 5 cases of AF recurrence in the self-formulated Ergan Tang group, yielding a recurrence rate of 25%, while the recurrence rate in the amiodarone group was 20%, with no statistically significant difference detected between the two groups (P = 0.705).
 |
Table 2 Comparison of TCM Symptom Score and AF Burden Changes Following 8-Week Treatment and 6-Month Recurrence Rate Between the Two Groups |
Changes in AF Burden, Inflammatory Factors, TCM Symptom Cores, and Liver and Kidney Function Before and After 8 weeks of Treatment in The Self-Formulated Ergan Tang Group
Comparison within the self-formulated Ergan Tang group before and after 8 weeks of treatment revealed noteworthy findings (Table 3): the AF burden decreased significantly from 0.318 ± 0.348 to 0.117 ± 0.255 (a decrease of 171.8%, P = 0.043); IL-6 levels decreased from 16.53 ± 11.16 pg/mL before treatment to 10.16 ± 6.89 pg/mL after treatment (a decrease of 62.70%, P = 0.036); TNF-α levels decreased from 15.69 ± 3.27 pg/mL before treatment to 13.75 ± 2.67 pg/mL after treatment (a decrease of 12.36%, P = 0.047); TCM symptom scores decreased from 18.30 ± 2.96 before treatment to 9.45 ± 2.84 after treatment (a decrease of 93.7%, P< 0.001).
 |
Table 3 Changes in TCM Symptom Scores, Inflammatory Factors, Heart Rate, and Liver and Kidney Function of Patients in the Self-Formulated Ergan Tang Group Before and After 8-Week Treatment |
Moreover, a significant decrease in heart rate compared to pre-treatment levels was observed with a decrease of 15.8% (P = 0.002). Notably, among the 20 patients in the self-formulated Ergan Tang group, no complaints were reported, and there were no significant changes in liver and kidney function before and after treatment, with no instances of abnormal liver and kidney function noted.
Discussion
TCM holds widespread acceptance in China for the treatment of various cardiovascular diseases owing to its mild effects, natural ingredients, and dual edible and medicinal properties. For instance, Gansong and Zhigancao Tang have long been utilized to address heart palpitations, boasting a rich historical pedigree of efficacy coupled with minimal side effects. Additionally, there exists a considerable body of research elucidating the pharmacological mechanisms underlying these two types of Chinese medicinal formulas.
Drawing upon insights gleaned from TCM literature and contemporary understanding of the pharmacological mechanisms governing Gansong13 and Zhigancao Tang,14 we combined these components to formulate a novel remedy termed as self-formulated Ergan Tang. Furthermore, we aim to evaluate its effectiveness in forestalling the recurrence of paroxysmal AF characterized by qi-yin deficiency, subsequent to the conversion to sinus rhythm, along with its potential impact on inflammatory factors.
In this study, in-depth statistical analysis revealed no significant disparities in basic clinical data and demographic characteristics between the two groups post-randomization, indicating excellent comparability among the selected patients. Following an 8-week treatment regimen with self-formulated Ergan Tang and amiodarone, no statistically significant differences were observed in TCM symptom scores, AF burden, and duration of sinus rhythm maintenance between the two groups. Furthermore, at the 6-month follow-up, the AF recurrence rate exhibited no statistically significant difference between the two groups, suggesting that self-formulated Ergan Tang is non-inferior to amiodarone in preventing the recurrence of paroxysmal AF.
The mechanism underlying the non-inferiority of self-formulated Ergan Tang to amiodarone may be attributed to its effects on ion channels. Previous studies have highlighted the stabilizing effect of Gansong extract, specifically jatamansone on cell membranes along with its concentration-dependent blocking effects on sodium channels (INa+), L-type calcium channels (ICa2+-L), delayed rectifier potassium channels (IK+), and transient outward potassium channels (Ito), akin to the effects exerted by Class I, Class III, and Class IV antiarrhythmic drug (AADs).15 Moreover, Gansong inhibits angiotensin II–induced Ca2+ overload in atrial myocytes, prolongs the action potential duration of atrial myocytes, and shortens the effective refractory period of atrial myocytes.16 Additionally, it demonstrates anti-cytotoxic and anti-inflammatory effects, mitigates oxidative stress, and improves cardiac sympathetic nerve remodeling.17
Recent investigations have indicated that core components of the self-formulated Ergan Tang, such as quercetin and luteolin, can upregulate the expression of silent information regulator 6 (SIRT6) by binding specifically to SIRT6. This process may promote histone mH2A1 accumulation and regulate the expression of cyclic adenosine monophosphate (cAMP) and related factors through their own ADP-ribosylation modification, thereby participating in various pathological and physiological processes including Ca2+ homeostasis regulation, cell permeability, and gene expression.18 Furthermore, quercetin can suppress the expression of inflammatory factors IL-12 and IL-8, while luteolin inhibits cyclooxygenase-2 and reduces NF-κB activity.19,20 The combined action mechanisms of these core components significantly alleviates atrial electrical and structural remodeling and uniformly prolongs repolarization of the endocardium and epicardium, thereby preventing AF episodes.21
The analysis of alterations in inflammatory factors following the pre and post treatment of self-formulated Ergan Tang exhibited significant reductions in both IL-6 and TNF-α levels following an 8-week treatment period. Furthermore, significant reductions in hsCRP levels were observed pre- and post-treatment. These outcomes suggest that administration of self-formulated Ergan Tang over the specified duration effectively suppresses the expression of inflammatory factors, aligning with previous studies. As previously indicated, both Gansong and self-formulated Ergan Tang possess anti-cytotoxic and anti-inflammatory properties contributing to the mitigation of oxidative stress within the body.22 Oxidative stress emerges as a pivotal factor implicated in atrial remodeling. Consequently, the inhibition of oxidative stress facilitates diminished atrial remodeling, thereby mitigating the burden of AF episodes.
Another noteworthy finding in this study is the significant decrease in heart rate observed among patients in the self-formulated Ergan Tang group following 8 weeks of treatment, suggesting that self-formulated Ergan Tang, similar to clinically utilized AADs, exerts a mild bradycardic effect. This effect can be partially ascribed to the electrophysiological properties of Gansong, which demonstrates the capability to block calcium channels, elevate the threshold potential, and diminish delayed afterdepolarizations in sinoatrial node cells.23,24 Additionally, as previously mentioned, the core components of self-formulated Ergan Tang, such as quercetin and luteolin, may modulate intracellular cAMP levels through the SIRT6/7 pathway, thereby attenuating the If current in sinoatrial node cells.25 The combination of these effects contributes to the deceleration of the automatic depolarization rate during Phase 4 diastole of sinoatrial node cells, resulting in a reduction in automaticity and subsequent heart rate.26
Following an 8-week treatment regimen with self-formulated Ergan Tang all 20 patients in the self-formulated Ergan Tang group reported no clinically significant complaints. Additionally, key blood tests assessing liver and kidney function, exhibited no significant alterations pre- and post-treatment, with no instances of abnormal liver and kidney function reported. These findings suggest that the utilization of self-formulated Ergan Tang in elderly patients with paroxysmal AF characterized by qi-yin deficiency is well-tolerated and safe.
However, it is imperative to acknowledge certain limitations of this study, including the small sample size, underscoring the necessity for larger RCTs to corroborate the obtained results. Furthermore, additional fundamental research is warranted to confirm the mechanism by which the core components of self-formulated Ergan Tang, namely quercetin, and luteolin, facilitate histone mH2A1 accumulation and modulate the expression of cAMP and related factors through their own ADP-ribosylation modification. Such endeavors are crucial for establishing a robust foundation for the widespread implementation of self-formulated Ergan Tang in patients with paroxysmal AF characterized by qi-yin deficiency.
Conclusion
This study substantiates the efficacy and safety of employing self-formulated Ergan Tang in elderly patients with paroxysmal AF characterized by qi-yin deficiency subsequent to cardioversion of sinus rhythm. Notably, self-formulated Ergan Tang exhibits non-inferiority to amiodarone in terms of sustaining sinus rhythm. Moving forward, future research endeavors should aim to further validate the findings of this study through larger RCTs. Additionally, in-depth investigations into the therapeutic mechanisms of self-formulated Ergan Tang, particularly concerning the molecular mechanisms underlying its core components, are warranted. It is anticipated that the outcomes of this study will furnish compelling scientific evidence supporting the widespread clinical utilization of self-formulated Ergan Tang.
Abbreviation
AF, Atrial Fibrillation; IL-6, Interleukin 6; TNF-α, Tumour Necrosis Factor α; HsCRP, High Sensitive C Reactive Protein; AADs, Antiarrhythmic Drugs; LAD, left atrial diameter; LVEDD, left ventricular ejection fraction; LVEF, left ventricular ejection fraction; ACEI/ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; SIRT6, silent information regulator 6; ADP, adenosine diphosphate; cAMP, Cyclic Adenosine monophosphate.
Data Sharing Statement
All data generated or analysed during this study are included in this article. Further enquiries can be directed to the corresponding author.
Ethics Approval and Consent to Participate
This study was conducted with approval from the Ethics Committee of Beijing Hepingli Hospital (Approval number: BJSHPLYY-IRB-KYXM-2022-03). This study was conducted in accordance with the declaration of Helsinki. Written informed consent was obtained from all participants.
Acknowledgments
We would like to acknowledge the hard and dedicated work of all the staff that implemented the intervention and evaluation components of the study.
Funding
This research was funded by the Dongcheng District Health Science and Technology Project grant number [2022]-2.
Disclosure
The authors declare that they have no competing interests.
References
1. Du X, Guo L, Xia S, et al. Atrial fibrillation prevalence, awareness and management in a nationwide survey of adults in China. Heart. 2021;107(7):535–541. PMID: 33509976; PMCID: PMC7958113. doi:10.1136/heartjnl-2020-317915
2. Hindricks G, Potpara T, Dagres N, et al.; ESC Scientific Document Group. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42(5):373–498. Erratum in: Eur Heart J. 2021 Feb 1;42(5):507. Erratum in: Eur Heart J. 2021 Feb 1;42(5):546–547. Erratum in: Eur Heart J. 2021 Oct 21;42(40):4194. PMID: 32860505. doi:10.1093/eurheartj/ehaa612
3. Kirchhof P, Camm AJ, Goette A, et al. EAST-AFNET 4 trial investigators. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med. 2020;383(14):1305–1316. PMID: 32865375. doi:10.1056/NEJMoa2019422
4. Wyse DG, Waldo AL, DiMarco JP, et al. Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) Investigators. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347(23):1825–1833. PMID: 12466506. doi:10.1056/NEJMoa021328
5. Andrade JG, Deyell MW, Macle L, et al. EARLY-AF investigators. Progression of atrial fibrillation after cryoablation or drug therapy. N Engl J Med. 2023;388(2):105–116. PMID: 36342178. doi:10.1056/NEJMoa2212540
6. Hatzinikolaou-Kotsakou E, Tziakas D, Hotidis A, et al. Relation of C-reactive protein to the first onset and the recurrence rate in lone atrial fibrillation. Am J Cardiol. 2006;97(5):659–661. PMID: 16490433. doi:10.1016/j.amjcard.2005.09.104
7. Turagam MK, Musikantow D, Whang W, et al. Assessment of catheter ablation or antiarrhythmic drugs for first-line therapy of atrial fibrillation: a meta-analysis of randomized clinical trials. JAMA Cardiol. 2021;6(6):697–705. PMID: 33909022; PMCID: PMC8082432. doi:10.1001/jamacardio.2021.0852
8. Kim YG, Lee HS, Kim H, et al. Association of antiarrhythmic drug therapy with syncope and pacemaker implantation in patients with atrial fibrillation. J Am Coll Cardiol. 2024;83(11):1027–1038. PMID: 38479951. doi:10.1016/j.jacc.2024.01.013
9. Jiang X, Luo Y, Wang X, et al. Investigating the efficiency and tolerability of traditional Chinese formulas combined with antiarrhythmic agents for paroxysmal atrial fibrillation: a systematic review and Bayesian network meta-analysis. Phytomedicine. 2022;94:153832. PMID: 34781230. doi:10.1016/j.phymed.2021.153832
10. Jiang RY, Wang T, Lan QY, et al. BuFeiXiaoJiYin ameliorates the NLRP3 inflammation response and gut microbiota in mice with lung cancer companied with Qi-yin deficiency. Cancer Cell Int. 2022;22(1):121. PMID: 35292015; PMCID: PMC8922837. doi:10.1186/s12935-022-02543-9
11. Chen J, Wang S, Shen J, et al. Analysis of gut microbiota composition in lung adenocarcinoma patients with TCM Qi-Yin deficiency. Am J Chin Med. 2021;49(7):1667–1682. PMID: 34488552. doi:10.1142/S0192415X21500786
12. Zeng C, Yuan Z, Pan X, et al. Efficacy of Traditional Chinese Medicine, Maxingshigan-Weijing in the management of COVID-19 patients with severe acute respiratory syndrome: a structured summary of a study protocol for a randomized controlled trial. Trials. 2020;21(1):1029. PMID: 33357239; PMCID: PMC7755980. doi:10.1186/s13063-020-04970-3
13. Fang JM, Li R, Zhang Y, et al. Aristolone in Nardostachys jatamansi DC. induces mesenteric vasodilation and ameliorates hypertension via activation of the KATP channel and PDK1-Akt-eNOS pathway. Phytomedicine. 2022;104:154257. PMID: 35738117. doi:10.1016/j.phymed.2022.154257
14. Yang Y, Ge FL, Huang Q, et al. Randomized controlled trials of Zhigancao Decoction combined with metoprolol in the treatment of arrhythmia: a systematic review and meta-analysis. Front Cardiovasc Med. 2022;9:795903. PMID: 35282353. doi:10.3389/fcvm.2022.795903.eCollection2022
15. Pathak S, Godela R. Nardostachys jatamansi: phytochemistry, ethnomedicinal uses, and pharmacological activities: a comprehensive review. Fitoterapia. 2024;172:105764. PMID: 38042505. doi:10.1016/j.fitote.2023.105764
16. Bose B, Tripathy D, Chatterjee A, Tandon P, Kumaria S. Secondary metabolite profiling, cytotoxicity, anti-inflammatory potential and in vitro inhibitory activities of Nardostachys jatamansi on key enzymes linked to hyperglycemia, hypertension and cognitive disorders. Phytomedicine. 2019;55:58–69. PMID: 30668444. doi:10.1016/j.phymed.2018.08.010
17. He J, Deng Y, Ren L, et al. Isoliquiritigenin from licorice flavonoids attenuates NLRP3-mediated pyroptosis by SIRT6 in vascular endothelial cells. J Ethnopharmacol. 2023;303:115952. PMID: 36442759. doi:10.1016/j.jep.2022.115952
18. Simonet NG, Thackray JK, Vazquez BN, et al. SirT7 auto-ADP-ribosylation regulates glucose starvation response through mH2A1. Sci Adv. 2020;6(30):eaaz2590. PMID: 32832656; PMCID: PMC7439345. doi:10.1126/sciadv.aaz2590
19. Akter R, Afrose A, Rahman MR, et al. A comprehensive analysis into the therapeutic application of natural products as SIRT6 modulators in Alzheimer’s disease, aging, cancer, inflammation, and diabetes. Int J Mol Sci. 2021;22(8):4180. PMID: 33920726; PMCID: PMC8073883. doi:10.3390/ijms22084180
20. Xia LM, Zhang AP, Zheng Q, et al. Quercetin-3-O-β-D-glucuronide inhibits mitochondria pathway-mediated platelet apoptosis via the phosphatidylinositol-3-kinase/AKT pathway in immunological bone marrow failure. World J Tradit Chin Med. 2022;8:115–122. doi:10.4103/wjtcm.wjtcm_44_21
21. Gao L, Kan C, Chen X, Xu S, Shi K. Mechanism of action of Zhi Gan Cao decoction for atrial fibrillation and myocardial fibrosis in a mouse model of atrial fibrillation: a network pharmacology-based study. Comput Math Methods Med. 2022;2022:4525873. Retraction in: Comput Math Methods Med. 2023 Jun 28;2023:9796483. doi: 10.1155/2023/9796483. PMID: 35720023; PMCID: PMC9203184. doi:10.1155/2022/4525873
22. Maiwulanjiang M, Chen J, Xin G, et al. The volatile oil of Nardostachyos Radix et Rhizoma inhibits the oxidative stress-induced cell injury via reactive oxygen species scavenging and Akt activation in H9c2 cardiomyocyte. J Ethnopharmacol. 2014;153(2):491–498. PMID: 24632018. doi:10.1016/j.jep.2014.03.010
23. Yoon CS, Kim KW, Lee SC, Kim YC, Oh H. Anti-neuroinflammatory effects of sesquiterpenoids isolated from Nardostachys jatamansi. Bioorg Med Chem Lett. 2018;28(2):140–144. PMID: 29221883. doi:10.1016/j.bmcl.2017.11.041
24. Maiwulanjiang M, Bi CW, Lee PS, et al. The volatile oil of Nardostachyos Radix et Rhizoma induces endothelial nitric oxide synthase activity in HUVEC cells. PLoS One. 2015;10(2):e0116761. PMID: 25643147; PMCID: PMC4359165. doi:10.1371/journal.pone.0116761
25. Grammatika Pavlidou N, Dobrev S, Beneke K, et al. Phosphodiesterase 8 governs cAMP/PKA-dependent reduction of L-type calcium current in human atrial fibrillation: a novel arrhythmogenic mechanism. Eur Heart J. 2023;44(27):2483–2494. PMID: 36810794; PMCID: PMC10344654. doi:10.1093/eurheartj/ehad086
26. Lerebours G. Le rythmesinusal. mécanisme et fonction [Sinus rhythm: mechanisms and function]. Med Sci. 2007;23(6–7):657–662. French. PMID: 17631846. doi:10.1051/medsci/20072367657
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.