Back to Journals » Journal of Pain Research » Volume 17
Efficacy of Ultrasound-Guided Interscalene Brachial Plexus Block for Acute Post-Hepatectomy Shoulder Pain: A Randomized Controlled Trial
Authors Zhou G, Yang Y , Zhang Y, Pan C, Wu X , Zhang J
Received 16 July 2024
Accepted for publication 24 September 2024
Published 30 September 2024 Volume 2024:17 Pages 3177—3185
DOI https://doi.org/10.2147/JPR.S478735
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Karina Gritsenko
Guoxia Zhou,1,2,* Yuecheng Yang,1,2,* Yunkui Zhang,1,2,* Congxia Pan,1,2 Xing Wu,1,2 Jun Zhang1,2
1Department of Anesthesiology, Fudan University Shanghai Cancer Center, Shanghai, People’s Republic of China; 2Department of Oncology, Fudan University Shanghai Medical College, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jun Zhang, Department of Anesthesiology, Fudan University Shanghai Cancer Center, No. 270, Dong-An Road, Shanghai, 200032, People’s Republic of China, Tel +86-021-64175590, Fax +86-021-64174774, Email [email protected]
Objective: To investigate the efficacy of ultrasound-guided interscalene brachial plexus block in the treatment of shoulder pain following hepatectomy.
Design: A randomized controlled trial.
Methods: We conducted a single-center, randomized controlled trial. Forty-four patients with shoulder pain scores of at least 5 were randomly assigned to two groups: the treatment group, which received 0.5% ropivacaine (5mL) combined with dexamethasone (5 mg) (n=22), and the control group, which received normal saline (5mL) (n=22). The intervention was performed in the postanesthesia care unit after shoulder pain was identified by using the visual analogue scale. The shoulder pain was re-evaluated 15 minutes after intervention. The incidence of effective pain relief, defined as at least 75% reduction in pain intensity, was the primary outcome. Secondary outcomes included shoulder pain intensity within 2 days after surgery, the timing of the first rescue analgesia, total additional analgesic use, arterial oxygen saturation, intervention-related adverse reactions, and patient satisfaction regarding shoulder pain.
Results: The incidence of effective pain relief was significantly higher in the treatment group compared to the control group (15 (68.2%) vs 2 (9.1%), P< 0.001). The interscalene brachial plexus block not only prolonged the time to first analgesic request (P < 0.001), but also reduced the number of analgesic requests (P < 0.001). In the comparison between groups, arterial oxygen saturation was lower in the control group than that in the treatment group, attributed to the use of sufentanil for remedial analgesia (92.4% vs 94.5%, P=0.014).
Conclusion: Interscalene brachial plexus block can effectively relieve post-hepatectomy shoulder pain without clinically significant hypoxemia.
Keywords: interscalene brachial plexus block, hepatectomy, referred pain, phrenic nerve
A Letter to the Editor has been published for this article.
A Response to Letter by Dr Maqsood has been published for this article.
Introduction
Acute shoulder pain commonly occurs after hepatectomy, which greatly influences patients’ postoperative recovery. Our previous study has reported that the incidence of acute post-hepatectomy shoulder pain was approximately 40%.1 The primary etiology involves intraoperative surgical trauma irritating the diaphragm, which causes referred shoulder pain via the phrenic nerve. Surgical sites involving the right liver lobe increase the risk of postoperative shoulder pain, as the right liver lobe is closely adjacent to the diaphragm.2 The efficacy of phrenic nerve block after hepatectomy further confirms that shoulder pain is associated with diaphragmatic irritation via the phrenic nerve.3
To enhance the visual field, a padded cushion is often placed under the right waist of the patient, potentially resulting in right shoulder ligament strain. The effectiveness of parecoxib on shoulder pain after thoracic surgery suggests that postoperative shoulder pain may include musculoskeletal pain.4 Overall, shoulder ligament strain and referred pain might represent a dual etiology for postoperative shoulder pain.5 A previous study found no correlation between intraoperative analgesia use and postoperative shoulder pain.6 Additionally, neither pregabalin nor gabapentin has been shown to reduce the incidence or intensity of referred shoulder pain.7,8 Consequently, nerve block has become a mainstream treatment option, for example, phrenic nerve block for shoulder pain after non-shoulder surgeries.9,10 The phrenic nerve arises from the anterior roots of the C3-C5 nerves. The incidence of a phrenic nerve block is nearly 100% following an interscalene brachial plexus block (ISB) with high-volume local anesthetics.11 Compared to other approaches to brachial plexus nerve block, such as the axillary and supraclavicular approaches, the interscalene approach more effectively meets the needs of shoulder pain management. Theoretically, an interscalene brachial plexus block may target both mechanisms underlying shoulder pain. However, to the best of our knowledge, no clinical trial has been conducted to evaluate the efficacy of an ISB for post-hepatectomy shoulder pain.
To fill the gap, we designed this randomized controlled trial, aiming to examine our hypothesis that ISB could alleviate shoulder pain without significant complications.
Methods
Study Design
The study was a single-center, randomized controlled trial with a 1:1 allocation ratio for both groups. The protocol was registered with the Chinese Clinical Trial Registry (ChiCTR2300070378) on April 11, 2023. This trial was conducted at the Fudan University Shanghai Cancer Center from April 2023 to April 2024. The study protocol was approved by the Ethics Committee of Fudan University Shanghai Cancer Center (No: 2302270–7). Written informed consent was obtained from all participants. The study complies with the Declaration of Helsinki.
Inclusion Criteria
Inclusion criteria were as follows: (1) American Society of Anesthesiologists physical status I–III. (2) Aged between 18 and 75 years. (3) Scheduled for open hepatectomy. (4) Body Mass Index (BMI) < 35.
Exclusion Criteria
Exclusion criteria included: (1) Refuse to sign an informed consent. (2) A history of brachial plexus neuropathy. (3) Blood coagulation disorders. (4) Allergic to local anesthetics. (5) Diaphragmatic diseases. (6) Abnormal cardiopulmonary function. (7) Opioid dependence.
Perioperative Management
All patients were monitored routinely. Epidural punctures were performed at the T7-T8 intervertebral levels, with the epidural catheters inserted to a depth of 4 cm. Subsequently, a test dose of 1% lidocaine (3m) was administered. Epidural administration of 0.25% ropivacaine, totaling 8–9 mL in two divided doses, was performed before the incision. Additionally, 3–4 mL of ropivacaine was administered at one-hour intervals.
Anesthesia was induced using propofol (2–2.5 mg/kg), rocuronium (0.6 mg/kg), and sufentanil (0.03μ/kg). Anesthesia was maintained with 1.0–1.3% sevoflurane in 60% oxygen concentration, targeted controlled infusion of remifentanil and propofol. Flurbiprofen axetil 50 mg was administered after closing the abdomen. Sugammadex 4 mg/kg was administered to reverse muscle relaxation effect at the end of surgery. Postoperative epidural analgesia was administered to all patients, comprising 300 mg ropivacaine and 100μg sufentanil diluted in 200mL saline. The background dose was set at 4 mL/h, and the bolus dose at 4 mL, with a lockout time of 15 minutes.
In the post-anesthesia care unit (PACU), shoulder and incisional pain were assessed by an independent anesthetist using visual analogue scales (VAS). If the shoulder pain score exceeded 4, the patient was enrolled in the study. For other patients with pain scores less than 5, oxycodone (2–5 mg) and flurbiprofen were administered to relieve shoulder pain.
Randomization and Blinding
Randomization was achieved through a computer-generated list. Since the brachial plexus block might alter both motor and sensory functions of the arm, the blinding method was not robust in this trial. However, to reduce the immediate placebo effect after the intervention, the blinding method was still employed for patients and the anesthetist who assessed the pain score. An independent nurse prepared the injection.
Study Intervention
The intervention was conducted in the PACU with routine monitoring, including ECG, pulse oximetry, and non-invasive blood pressure. Based on the allocation, 5mL of 0.5% ropivacaine + 5 mg dexamethasone or 5mL of normal saline was administered.
After skin disinfection, a linear array ultrasound probe was positioned parallel to the clavicle at the level of the cricoid cartilage to identify the anterior and middle scalene muscles. A 22G 80mm needle (Uniplex Nanoline, Germany) was advanced under ultrasound guidance using the in-plane technique until the needle tip was located between the C5 and C6 nerve roots (Supplementary File 1). Subsequently, the prepared injection was administered slowly at a rate of 0.5 mL per second.
Arterial blood gases were tested 15 minutes after the intervention. The rescue l analgesia was administered by using titrated sufentanil according to patients’ need. Adverse reactions were recorded in the PACU. Mask oxygen inhalation was applied to patients whose pulse oxygen saturation fell below 90%. Routinely, all patients remained in the PACU until their Steward Scores reached 4. In this trial, all participants were required to stay in the PACU for at least 30 minutes after the intervention.
Measured Outcomes
Pain intensity was assessed by an anesthetic nurse in the PACU 15 minutes after the intervention. Effective pain relief was defined as a reduction in pain of at least 75%. This study’s primary outcome was the incidence of effective pain relief.
Secondary outcomes included: (1) the timing of the first rescue analgesic. (2) Arterial oxygen saturation tested 15 minutes after the intervention. (3) Total analgesic use within 48 hours. (4) Pain intensity at 24 and 48 hours after the intervention assessed by an anesthetist. (5) Adverse reactions related to ISB. (6) Patient satisfaction for shoulder pain and the treatment, rated from 0 (very dissatisfied) to 10 (very satisfied).
Sample Size Calculation
Sample size calculation was conducted using PASS software (Version 2021, USA). A previous study reported an effective rate of 62% for ultrasound-guided brachial plexus block in managing shoulder pain following thoracic surgeries.12 We assumed a 15% effective rate in the control group. The significance level was set at 0.05 with a power of 90%. The minimal sample size required was 18 in each group. Considering a potential drop-out rate of 20%, the final sample size was set at 22 patients per group.
Statistical Analysis
Data normality was assessed using the Kolmogorov–Smirnov test. Continuous data were presented as mean ± standard deviation or median (interquartile range), as appropriate. The independent sample t-test or the Mann–Whitney test was used to analyze continuous data between groups, based on their distribution. Data not conforming to a normal distribution within groups were analyzed using the Wilcoxon test. The comparison of arterial oxygen saturation within the group was performed using the Wilcoxon signed-rank test. Categorical data were analyzed using the chi-square test. The significance level was set at 0.05 in all analyses. All statistical analyses were performed using SPSS (Version 26.0, USA).
Results
A total of 227 patients undergoing hepatectomy were screened for postoperative shoulder pain score > 4. Among them, 44 patients were identified to meet the inclusion criteria. All patients completed their follow-up. The study’s flow chart is shown in Figure 1.
 |
Figure 1 The flow chart of the study. Notes: Forty-four patients were ultimately enrolled in this study. The follow-up was completed for all patients. |
The demographic data of the enrolled patients are shown in Table 1. No significant differences were found in the baseline data between the two groups. After the intervention, significant differences in shoulder pain intensity were observed between the treatment and control groups at all time points (Figure 2). Shoulder pain-related data were shown in Table 2. In the treatment group, a higher proportion of patients reported effective shoulder pain relief (15 (68.2%) vs 2 (9.1%), P<0.001). The timing of the first rescue analgesics is shown in Figure 3. ISB extended the time to the first use of rescue analgesics compared with placebo, within 48 hours after the intervention. Additionally, fewer patients in the treatment group required rescue analgesics compared to those in the control group, in both the PACU and ward.
 |
Table 1 Demographics of Enrolled Patients |
 |
Table 2 Shoulder Pain-Related Data After Intervention |
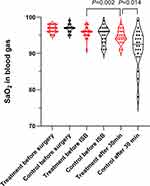
Regarding adverse reactions, perioperative SaO2 data are summarized in Figure 4. ISB was associated with a slight but statistically significant decrease in SaO2 15 minutes after the intervention within the treatment group (median 94.5% vs 96%, P=0.002). Regarding the comparison between groups after the intervention, SaO2 was significantly lower in the control group compared to the treatment group (median 92.5% vs 94.5% P=0.014). Four patients (18.2%) in the control group required oxygen treatment for hypoxemia via face mask. Patients treated with ISB were more satisfied with the shoulder pain treatment. However, one patient in the treatment group was dissatisfied due to the numbness in the right arm.
Discussion
In this study, 44% of patients reported shoulder pain after hepatectomy, with 19.4% reporting moderate to severe shoulder pain. ISB significantly relieved shoulder pain within 48 hours after hepatectomy without any clinical adverse reactions.
Epidural anesthesia provided better analgesia than bilateral paravertebral blocks during open liver resections.13 Furthermore, numerous studies have shown that postoperative epidural analgesia offers better pain relief than intravenous analgesia following hepatectomy.14,15 Although previous studies have indicated that epidural anesthesia might increase the risk of postoperative shoulder pain in patients undergoing general anesthesia due to its opioid-sparing effect,1,16 combined epidural analgesia was still employed in this trial for better incisional pain management. Additional intravenous flurbiprofen was also administered to ensure multimodal analgesia.
The phrenic nerve is positioned nearly indistinguishably close to the C5 ventral ramus at the level of the cricoid cartilage.17 Urmey et al reported that ISB inevitably caused a phrenic nerve block.11 Although the puncture technique without ultrasound guidance and the use of large volumes of local anesthetic are inconsistent with current clinical practice, this still indicates the close relationship between ISB and phrenic nerve block. Previous studies have shown that a phrenic nerve block may improve referred shoulder pain after hepatectomy and laparoscopic cholecystectomy.3,9 In this study, all patients reported at least 50% pain relief following ISB. Since the irritation of the diaphragm was the main etiology of post-hepatectomy shoulder pain, suggesting that phrenic nerve block caused by ISB was the therapeutic target. We did not select the C7 root as the injection site because it would diminish the efficacy of local anesthetic penetration to the phrenic nerve.18 The other advantage of ISB was the analgesia for the shoulder region. Another cause of postoperative shoulder pain is intraoperative shoulder ligament strain.4,5 The special position during hepatectomy may result in excessive shoulder extension.
Perineural dexamethasone has commonly been used as an adjuvant in brachial plexus nerve blocks in previous studies. Multiple meta-analyses have found that perineural dexamethasone prolongs the duration of the sensory blocks when compared to the use of local anesthetics alone in brachial plexus blocks.19,20 Published literature supports that peripheral dexamethasone is more effective than intravenous dexamethasone at prolonging sensory block duration at equal doses.21,22 Therefore, dexamethasone was utilized as an adjuvant in ISB in this study.
Low-volume and low-concentration local anesthetics in ISB can reduce the degree of diaphragmatic paralysis (DP).23,24 The volume of local anesthetics used in ISB has been extensively studied in the existing literature. Sinha et al found that reducing the volume from 20mL to 10mL in ISB did not decrease the incidence of DP.25 However, using 5mL of local anesthetic significantly reduces the incidences of DP and respiratory complications compared to 20mL.24,25 Therefore, we determined 5mL as the optimal volume of ISB in our study. Though diaphragm mobility was not assessed in our study, clinically, dyspnea or significant hypoxemia were not reported in patients following ISB, indicating that 5mL ropivacaine used in ISB is safe following general anesthesia. Based on previous studies, 0.5% ropivacaine in ISB was widely used.24,25 Fang et al demonstrated that the minimum effective concentration of ropivacaine in brachial plexus block was 0.257%.26 Therefore, the effectiveness and safety of lower concentration ropivacaine in ISB for postoperative shoulder pain requires further studies.
In the patients in the treatment group, arterial oxygen saturation slightly decreased after the intervention, suggesting a potential mild impairment of diaphragmatic mobility due to ISB-related DP. Nevertheless, the changes in blood oxygenation are clinically insignificant and can be disregarded in clinical practice. However, in the control group, arterial oxygen saturation markedly decreased after the use of sufentanil for rescue analgesia. These results demonstrate that ISB offers significant advantages over strong opioids in terms of respiratory function, leading to a shorter PACU stay.
This study has several limitations. First, the blinding method was not robust due to the effect of nerve block. The effects of nerve block may potentially lead to an overestimation of the evaluation of shoulder pain relief by patients in the treatment group. Second, the study utilized low volume while relatively high concentrations of local anesthetic. A lower concentration of local anesthetic may has similar pain improvement without affecting oxygenation. Third, as phrenic nerve block was not performed, a comparison between phrenic nerve blocks and ISB could not be addressed in this trial.
Conclusion
Postoperative ISB provided immediate shoulder pain relief after hepatectomy, with the analgesic effect lasting for at least two days. However, upper limb numbness caused by ISB is unavoidable. Anesthetists must carefully weigh the risk and benefits in clinical practice. Conservatively, ISB could serve as an alternative to phrenic nerve block in treating post-hepatectomy shoulder pain. When the surgical position, especially the shoulder range, needs to be adjusted intraoperatively, prioritizing ISB is advisable.
Abbreviations
ISB, interscalene brachial plexus block; PACU, post-anesthesia care unit; VAS, visual analogue scales.
Data Sharing Statement
Data is available on request from the corresponding author.
Ethics Approval and Informed Consent
Ethical approval was obtained from the ethics committees of the Fudan University Shanghai Cancer Center (No: 2302270-7). Written informed consent was obtained from all participants. The study complies with the Declaration of Helsinki.
Acknowledgment
We appreciated Anni Chen for the English language editing.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Shanghai Municipal Commission of Science and Technology Project: No. 22Y11904200.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Yang Y, Zhang Y, Dai SL, Wang L, Zhang J. Incidence and risk factors for acute shoulder pain after hepatectomy: a nested case-control study. BMC Anesthesiol. 2022;22(1):395. doi:10.1186/s12871-022-01944-7
2. Yang Y, Zhang Y, Dai S, Wang L, Zhang J. Influence of extent of surgical resection on post-hepatectomy shoulder pain: an observational study. Sci Rep. 2023;13(1):10861. doi:10.1038/s41598-023-38052-6
3. Bak TS, Bøgevig S, Christensen AP, et al. Phrenic nerve block on severe post-hepatectomy shoulder pain: a randomized, double-blind, placebo-controlled, pilot study. Acta Anaesthesiol Scand. 2021;65(9):1320–1328. doi:10.1111/aas.13928
4. Pipanmekaporn T, Punjasawadwong Y, Charuluxananan S, et al. The effectiveness of intravenous parecoxib on the incidence of ipsilateral shoulder pain after thoracotomy: a randomized, double-blind, placebo-controlled trial. J Cardiothorac Vasc Anesth. 2018;32(1):302–308. doi:10.1053/j.jvca.2017.05.048
5. Bamgbade OA, Dorje P, Adhikary GS. The dual etiology of ipsilateral shoulder pain after thoracic surgery. J Clin Anesth. 2007;19(4):296–298. doi:10.1016/j.jclinane.2006.09.010
6. Kandil TS, El Hefnawy E. Shoulder pain following laparoscopic cholecystectomy: factors affecting the incidence and severity. J Laparoendosc Adv Surg Tech A. 2010;20(8):677–682. doi:10.1089/lap.2010.0112
7. Huot MP, Chouinard P, Girard F, et al. Gabapentin does not reduce post-thoracotomy shoulder pain: a randomized, double-blind placebo-controlled study. Can J Anaesth. 2008;55(6):337–343. doi:10.1007/BF03021488
8. Chang SH, Lee HW, Kim HK, Kim SH, Kim DK. An evaluation of perioperative pregabalin for prevention and attenuation of postoperative shoulder pain after laparoscopic cholecystectomy. Anesth Analg. 2009;109(4):1284–1286. doi:10.1213/ane.0b013e3181b4874d
9. Yi MS, Kim WJ, Kim MK, et al. Effect of ultrasound-guided phrenic nerve block on shoulder pain after laparoscopic cholecystectomy-a prospective, randomized controlled trial. Surg Endosc. 2017;31(9):3637–3645. doi:10.1007/s00464-016-5398-4
10. Blichfeldt-Eckhardt MR, Laursen CB, Berg H, et al. A randomised, controlled, double-blind trial of ultrasound-guided phrenic nerve block to prevent shoulder pain after thoracic surgery. Anaesthesia. 2016;71(12):1441–1448. doi:10.1111/anae.13621
11. Urmey WF, Talts KH, Sharrock NE. One hundred percent incidence of hemidiaphragmatic paresis associated with interscalene brachial plexus anesthesia as diagnosed by ultrasonography. Anesth Analg. 1991;72(4):498–503. doi:10.1213/00000539-199104000-00014
12. Saranteas T, Alevizou A, Sidiropoulou T, et al. Ultrasound-guided interscalene brachial plexus nerve block with an ultralow volume of local anesthetic for post-thoracotomy shoulder girdle pain. J Cardiothorac Vasc Anesth. 2018;32(1):312–317. doi:10.1053/j.jvca.2017.04.043
13. Schreiber KL, Chelly JE, Lang RS, et al. Epidural versus paravertebral nerve block for postoperative analgesia in patients undergoing open liver resection: a randomized clinical trial. Reg Anesth Pain Med. 2016;41(4):460–468. doi:10.1097/AAP.0000000000000422
14. Zhang XP, Wei WT, Huang Y, et al. Efficacy and safety of patient-controlled epidural analgesia versus patient-controlled intravenous analgesia following open hepatectomy: a single-center retrospective study. Heliyon. 2024;10(1):e23548. doi:10.1016/j.heliyon.2023.e23548
15. Arslan-Carlon V, Qadan M, Puttanniah V, et al. Randomized prospective trial of epidural analgesia after open hepatectomy. Ann Surg. 2024;279(4):598–604. doi:10.1097/SLA.0000000000006205
16. Miyoshi H, Nakamura R, Hamada H, Kawamoto M. Shoulder pain after hepatectomy occurs more frequently on the right side: a retrospective observational analysis. Eur J Anaesthesiol. 2015;32(10):744–745. doi:10.1097/EJA.0000000000000281
17. Kessler J, Schafhalter-Zoppoth I, Gray AT. An ultrasound study of the phrenic nerve in the posterior cervical triangle: implications for the interscalene brachial plexus block. Reg Anesth Pain Med. 2008;33(6):545–550.
18. Renes SH, Rettig HC, Gielen MJ, Wilder-Smith OH, van Geffen GJ. Ultrasound-guided low-dose interscalene brachial plexus block reduces the incidence of hemidiaphragmatic paresis. Reg Anesth Pain Med. 2009;34(5):498–502. doi:10.1097/AAP.0b013e3181b49256
19. Choi S, Rodseth R, McCartney CJ. Effects of dexamethasone as a local anaesthetic adjuvant for brachial plexus block: a systematic review and meta-analysis of randomized trials. Br J Anaesth. 2014;112(3):427–439. doi:10.1093/bja/aet417
20. Pehora C, Pearson AM, Kaushal A, Crawford MW, Johnston B. Dexamethasone as an adjuvant to peripheral nerve block. Cochrane Database Syst Rev. 2017;11(11):Cd011770. doi:10.1002/14651858.CD011770.pub2
21. Sakae TM, Marchioro P, Schuelter-Trevisol F, Trevisol DJ. Dexamethasone as a ropivacaine adjuvant for ultrasound-guided interscalene brachial plexus block: a randomized, double-blinded clinical trial. J Clin Anesth. 2017;38:133–136. doi:10.1016/j.jclinane.2017.02.004
22. Zufferey PJ, Chaux R, Lachaud PA, et al. Dose-response relationships of intravenous and perineural dexamethasone as adjuvants to peripheral nerve blocks: a systematic review and model-based network meta-analysis. Br J Anaesth. 2024;132(5):1122–1132. doi:10.1016/j.bja.2023.12.021
23. Wong AK, Keeney LG, Chen L, et al. Effect of local anesthetic concentration (0.2% vs 0.1% Ropivacaine) on pulmonary function, and analgesia after ultrasound-guided interscalene brachial plexus block: a randomized controlled study. Pain Med. 2016;17(12):2397–2403. doi:10.1093/pm/pnw057
24. Riazi S, Carmichael N, Awad I, Holtby RM, McCartney CJ. Effect of local anaesthetic volume (20 vs 5 mL) on the efficacy and respiratory consequences of ultrasound-guided interscalene brachial plexus block. Br J Anaesth. 2008;101(4):549–556. doi:10.1093/bja/aen229
25. Sinha SK, Abrams JH, Barnett JT, et al. Decreasing the local anesthetic volume from 20 to 10 mL for ultrasound-guided interscalene block at the cricoid level does not reduce the incidence of hemidiaphragmatic paresis. Reg Anesth Pain Med. 2011;36(1):17–20. doi:10.1097/AAP.0b013e3182030648
26. Fang G, Wan L, Mei W, Yu HH, Luo AL. The minimum effective concentration (MEC90) of ropivacaine for ultrasound-guided supraclavicular brachial plexus block. Anaesthesia. 2016;71(6):700–705. doi:10.1111/anae.13445
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.