Back to Journals » Journal of Inflammation Research » Volume 18
Electroacupuncture Improves Learning and Memory Impairment in Rats with Cerebral Ischemia/Reperfusion Injury by Promoting Microglia/Macrophage M2 Polarization Through Nrf2/HO-1 Pathway
Authors Xiao Y , Bai Y, Sun K, Wan J, Chen L, Chen S, Wang Y, Li W, Liu A
Received 15 November 2024
Accepted for publication 18 February 2025
Published 26 February 2025 Volume 2025:18 Pages 2925—2941
DOI https://doi.org/10.2147/JIR.S504670
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Yuqian Xiao,1,2 Yanjie Bai,1 Kexin Sun,2 Jun Wan,2 Limin Chen,2 Shuying Chen,2 Yan Wang,1 Wenjing Li,2 An Liu2
1Rehabilitation Centre of the First Affiliated Hospital of Henan University of Chinese Medicine, Zhengzhou, Henan Province, People’s Republic of China; 2Rehabilitation Medicine College, Henan University of Chinese Medicine, Zhengzhou, People’s Republic of China
Correspondence: Yanjie Bai, Rehabilitation Centre of the First Affiliated Hospital of Henan University of Chinese Medicine, Zhengzhou, 450003, People’s Republic of China, Email [email protected]
Objective: While electroacupuncture (EA) has shown effectiveness in treating learning and memory deficits associated with ischemic stroke (IS), the specific mechanisms involved remain unclear. The goal of this study was to investigate whether EA improves learning and memory deficits in MCAO rats by regulating microglia/macrophage polarization through the nuclear factor red lineage 2-related factor 2 (Nrf2)/heme oxygenase-1 (HO-1) signaling pathway.
Methods: Sprague-Dawley rats were subjected to MCAO modeling and treated with EA 24 hours post-MCAO for a period of two weeks. To investigate the involvement of Nrf2/HO-1 in the effects of EA, tin protoporphyrin (SnPPIX), an inhibitor of HO-1, was injected into the left ventricle of rats before initiating EA treatment. Neurological function in MCAO rats was evaluated using a neurological deficit score. The effects of EA on learning and memory deficits were assessed using the Morris water maze (MWM) and open field test (OFT). Hematoxylin-Eosin (HE) staining was used to observe hippocampal structural morphology, and 2,3,5-Triphenyltetrazolium Chloride (TTC) staining was used to assess the infarct volume. Protein expression levels of the Nrf2/HO-1 signaling pathway and microglial/macrophage polarization were determined using ELISA, immunofluorescence double-labeling, Western blotting (WB), and real-time quantitative polymerase chain reaction PCR (qRT-PCR).
Results: EA significantly enhanced learning and memory function in rats by upregulating NRF2/HO-1 expression and promoting M2 polarization of microglia/macrophages. However, administration of SnPPIX, an HO-1 inhibitor, counteracted the beneficial effects of EA on memory improvement in MCAO rats, while also worsening cerebral infarct volume and inflammatory response.
Conclusion: EA effectively improved learning and memory impairments in MCAO rats by activating the Nrf2/HO-1 signaling pathway, leading to the promotion of M2 polarization in microglia/macrophages.
Keywords: electroacupuncture, ischemia-reperfusion injury, Nrf2/HO-1, microglia/macrophages, polarization
Introduction
Ischemic stroke (IS) remains one of the leading causes of global mortality and morbidity. Post-stroke cognitive impairments, particularly in learning and memory, are common complications, manifesting as cognitive dysfunctions such as memory loss, impaired attention, executive dysfunction, or disturbed thought processes.1 These cognitive deficits often exacerbate the condition of IS patients.2 Over the years, research into the underlying mechanisms of learning and memory deficits after stroke has predominantly focused on factors such as cerebral ischemia, neurodegeneration, neuroinflammation, blood-brain barrier disruption, and oxidative stress.3,4 Among these, neuroinflammation has emerged as a central mediator of cognitive deficits following stroke.5,6 Consequently, effective management of inflammation plays a crucial role in treating post-stroke learning and memory impairments.
Microglia are the resident macrophages in the central nervous system. Despite having different origins, microglia and infiltrating macrophages share similar morphological and functional characteristics, which is why they are collectively referred to as microglia/macrophages. Both play key roles in regulating the inflammatory response following IS.7,8 Activated microglia/macrophages are categorized into two major phenotypes based on their cytokine and protein expression: the “classically activated” M1 phenotype and the “alternatively activated” M2 phenotype.9 M1 microglia/macrophages are marked by surface proteins such as cluster of differentiation (CD) 16, CD86, and major histocompatibility complex class II (MHCII), and they secrete pro-inflammatory factors like interleukin (IL)-6, IL-1β, and tumor necrosis factor (TNF)-α, which contribute to exacerbating brain tissue damage. In contrast, M2 microglia/macrophages primarily express surface markers like CD206 and Arginase-1 (Arg-1) and secrete anti-inflammatory factors such as IL-10, IL-4, and transforming growth factor-β (TGF-β), which promote anti-inflammatory responses and aid in the repair of damaged brain tissue.10 The dual roles of polarized microglia/macrophages have been well-documented in various neurological and neurodegenerative diseases, including IS, traumatic brain injury, and Alzheimer’s disease.11–13 As a result, promoting the shift from M1 to M2 polarization of microglia/macrophages has emerged as a crucial strategy to inhibit neuroinflammation and improve learning and memory deficits following stroke.
Nuclear factor erythroid 2-related factor 2 (Nrf2) is a critical transcription factor involved in regulating and preserving redox balance, and it is regarded as a promising therapeutic target for counteracting the detrimental effects in the ischemic cascade.14 Under normal physiological conditions, Nrf2 is sequestered in the cytoplasm by its binding to Kelch-like ECH-associated protein 1 (Keap1), which facilitates the ubiquitination of Nrf2, maintaining its homeostasis within the cell.15 During ischemia-reperfusion injury, Nrf2 translocates to the nucleus where it activates heme oxygenase-1 (HO-1), which in turn inhibits the expression of pro-inflammatory proteins.16 Previous studies have demonstrated that in IS, reduced levels of Nrf2 and HO-1 exacerbate brain damage, resulting in increased infarct size and worsened neurological deficits.17 Activation of the Nrf2/HO-1 pathway promotes the polarization of microglia/macrophages from the pro-inflammatory M1 phenotype to the anti-inflammatory M2 phenotype, thereby contributing to the suppression of neuroinflammation.18,19 Electroacupuncture (EA), a key component of traditional Chinese medicine, is widely used in the clinical management of IS.20 Clinical trials and meta-analyses have shown that EA can improve various central nervous system injuries, including post-stroke cognitive impairments, without significant adverse effects.21 More recently, studies have indicated that EA can enhance neurogenesis in brain-injured rats via the Nrf2/HO-1 signaling pathway.22 However, whether EA can regulate microglia/macrophage polarization in IS through the activation of Nrf2/HO-1 remains unclear.
In conclusion, we hypothesize that EA facilitates the polarization of microglia/macrophages to the M2 phenotype, thereby improving post-stroke cognitive deficits, through the activation of the Nrf2/HO-1 signaling pathway. To test this hypothesis, we established a middle cerebral artery occlusion (MCAO) rat model and assessed cognitive function using the Morris water maze (MWM) and open field test (OFT). Furthermore, to explore the molecular mechanisms underlying microglia/macrophage polarization in post-stroke cognitive impairment, we measured the expression levels of neuroinflammatory markers and the Nrf2/HO-1 signaling pathway. This approach aims to provide insights into the role of EA in alleviating learning and memory deficits in MCAO rats.
Materials and Methods
Animals
Seventy-five male SD rats, clean-grade, weighing 260±20 g, were supplied by Jinan Ponyue Laboratory Animal Company. The experiments were authorized by the Animal Ethics Committee of Henan University of Traditional Chinese Medicine (approval number: IACUC-20230601). All procedures involving animals adhered to the guidelines outlined in the Guide for the Care and Use of Laboratory Animals. The rats were housed under standardized conditions in individually ventilated cages in the clean-grade animal room at Henan University of Traditional Chinese Medicine, with natural light and unrestricted access to water and food.
Grouping of Animals
A total of 12 SD rats were randomly assigned to the sham surgery (Sham) group, while the remaining 63 rats underwent MCAO modeling using the suture occlusion technique. Neurological function was assessed using the neurological deficit score, with scores ranging from 1 to 3 indicating successful modeling. During the procedure, 12 rats died, and 3 rats that did not develop a successful MCAO/R-induced cognitive impairment model were excluded. The remaining 48 successfully modeled rats were randomly divided into four groups: MCAO, EA, EA + SnPPIX (GLPBIO, USA), and SnPPIX, with 12 rats in each group.
Moulding Method
The rats were anesthetized with 2% isoflurane (RWD Life Science, Shenzhen, China) using a small animal anesthesia machine (RWD Life Sciences, Shenzhen, China) placed on the operating table The skin was then prepared and disinfected. A midline incision was made on the neck, and the neck tissues were carefully separated to expose the common carotid artery (CCA), internal carotid artery (ICA), and external carotid artery (ECA), which were isolated. Arterial clips were applied to the proximal ends of the CCA and ECA, and the distal end of the ICA. A small incision was made at the proximal bifurcation of the CCA using fiber scissors. A prepared wire plug (BEIJING CINONTECH, Beijing, China) was inserted into the ICA via the carotid bifurcation, advancing until resistance was felt at approximately 18–20mm, at which point the proximal end of the ICA was ligated. After the procedure, the wound was sterilized and sutured. A 1 cm thread plug was left outside the skin during suturing and was carefully retracted to the CCA after 2 hours for reperfusion.
In the Sham group, only a neck skin incision was made to expose the CCA, ICA, and ECA, after which the incision was disinfected and sutured. For the EA+SnPPIX group, the MCAO rat model of learning and memory impairment was induced, followed by the administration of SnPPIX to the left lateral ventricle after cerebral ischemia. EA was performed the following day. In the SnPPIX group, the MCAO rat model of learning and memory impairment was induced, and SnPPIX was administered to the left lateral ventricle after cerebral ischemia.
HO-1 Inhibitor SnPPIX Injection Methods
SnPPIX (20 μg/kg) was administered into the left lateral ventricle the day following successful MCAO modeling, after the rats were anaesthetized with 2% isoflurane using a small animal anaesthesia machine. The rats were then placed in a rat brain stereotaxic apparatus, and the left lateral ventricle was located at 0.8 mm behind the bregma, 1.5 mm transversely, and 4.0 mm in depth. The HO-1 inhibitor, SnPPIX, was injected at the target site where the needle tip was positioned. A total of 6 μL was injected at each site (at a rate of 1 μL/min), and the needle was held in place for 5 minutes after the injection. The needle was then slowly withdrawn, and the skin incision was sutured. The rats were then returned to their cages.
Modes of Intervention
EA group: EA was performed at Baihui (GV20) and Shenting (GV24) acupoints (for the positioning of acupoints in rats, refer to the method described in Experimental Acupuncture). GV20 is located at the midpoint of the parietal bone, while GV24 is positioned at the anterior midline of the frontal-parietal junction. The GM101 EA device was used with a peak voltage of 6 V, providing gentle needle body vibrations with a frequency range of 1–20 hz, and a sparse-dense wave pattern. The first electroacupuncture treatment started on the second day after reperfusion, with each session lasting 30 minutes, once daily for 14 consecutive days until the animals were euthanized. All EA treatments were performed by the same operator.
Neurological Deficit Score
Scoring criteria: 0 points: No neurological damage symptoms. 1 point: Inability to fully extend the contralateral forepaw. 2 points: Circling to the right. 3 points: Tilting to the contralateral side. 4 points: Inability to walk spontaneously and loss of consciousness. The higher the score, the more severe the neurological dysfunction.
Morris Water Maze Testing
MWM Test: This test is designed to assess the rats’ ability to learn spatial memory and their orientation awareness.
(1) Localization and Navigation Experiment: The water maze was divided into four equal quadrants, with a fixed platform placed 1 cm below the surface in the third quadrant. Rats were placed in the water from one of the four quadrant entry points. If a rat climbed onto the platform within 60 seconds and remained there for more than 3 seconds, it was considered to have found the platform, and this time was recorded as the latency period. If the rat did not find the platform within 60 seconds, it was guided to the platform by its tail and allowed to stay there for a familiarization period of 10 seconds. In this case, the latency was recorded as 60 seconds. The interval between each training session was 5 minutes, with training conducted for 5 consecutive days.
(2) Spatial Exploration Experiment: On day 6, after the platform was removed, the rats’ movements within the water maze were video-recorded for 60 seconds. The number of times the rats crossed the location where the platform had been and the time spent in the target quadrant were recorded using Behavioral Analysis Software.
Open Field Test
After 14 days of EA intervention, rats in each group underwent the OFT to evaluate their spontaneous behavior, exploratory behavior, and anxiety levels in an unfamiliar environment. Prior to the experiment, the environment was kept dark, and the open field box was thoroughly cleaned to eliminate any contaminants. A black open box measuring 100 cm × 100 cm × 40 cm was used, and one rat was randomly selected and placed at the center of the box. The rat’s activity was recorded for 3 minutes using a camera. After the test, the box was cleaned and sprayed with 75% alcohol to remove any odors. Behavioral analysis software was used to analyze the total distance traveled, the average speed, and the number of times the rat crossed the grids during the 3-minute period.
TTC Staining Was Performed to Assess the Volume of Cerebral Infarction in Rats
Three rat brain tissues from each group were collected, rinsed with saline, and stored at −20°C for 20 minutes. The brain tissues were then sliced into 2-mm-thick sections along the coronal plane, starting 2 mm from the frontal part of the brain. Each brain tissue sample was cut into 5 slices, which were immersed in 2% TTC staining solution. The slices were covered with tin foil and incubated in a 37°C water bath for 15–30 minutes, with occasional turning to ensure uniform exposure to the staining solution. After staining, infarcted areas appeared white, while uninfarcted areas remained red. The stained brain sections were carefully arranged on a uniform background for photography, and Image J software was used to calculate the total infarction volume for each brain tissue sample.
ELISA Was Used to Measure the Concentrations of Serum Inflammatory Factors IL-4, IL-10, TNF-α, and IL-1β
After anesthetizing the rats, blood was collected from the abdominal aorta, allowed to rest, and then centrifuged. The supernatant was harvested, and serum concentrations of IL-4, IL-10, TNF-α, and IL-1β were measured using ELISA kits (Elabscience, Wuhan, China).
Hematoxylin-Eosin Staining to Observe the Morphology of Rat Hippocampal Neurons
After 14 days of intervention, 3 rats were randomly selected from each group. After deep anesthesia, the brains were harvested and hippocampal tissue was collected. The tissue was fixed with paraformaldehyde, embedded in paraffin, and sectioned to 4 μm thickness. After drying, the brain tissue sections were stained using an HE staining kit (Solarbio, Beijing, China) following the kit’s instructions, in a fume hood. The stained sections were then mounted with neutral gum, and the morphology of the hippocampal CA1 region of the left hemisphere was observed under a microscope (Nikon, Shanghai, China).
Immunofluorescence Double-Labelling Assay Was Used to Co-Label M1 and M2 Microglia/Macrophage Surface Markers, CD16 and CD206, with Ionized Calcium-Binding Adaptor Molecule-1 (Iba-1)
Rat hippocampal tissue sections were deparaffinized and dehydrated according to standard procedures. The sections were then immersed in citrate buffer for antigen retrieval and blocked with 5% goat serum (Solarbio, Beijing, China) at 37°C for 30 minutes. After incubation, the serum was removed, and the primary antibodies—diluted Iba-1 (1:1000, Gene Tex, GTX635400), CD16 (1:250, Proteintech, 16559-1-AP), and CD206 (1:500, Santa Cruz, SC-58986)—were added individually according to the manufacturer’s instructions. The sections were incubated overnight at 4°C. The next day, the primary antibodies were removed, and secondary antibodies were applied in the dark for 1 hour at room temperature. Afterward, DAPI staining solution (Solarbio, Beijing, China) was added dropwise for nuclear counterstaining. The sections were sealed with an anti-fluorescence quenching agent (Solarbio, Beijing, China). Images were captured using an inverted fluorescence microscope (Nikon, Shanghai, China), and the number of cells co-labeled with CD16/Iba1 or CD206/Iba1 was quantified using Image analysis software.
RT-qPCR Was Performed to Assess the mRNA Expression Levels of IL-4, IL-10, TNF-α, IL-1β, Nrf2, and HO-1 in Rat Hippocampal Tissue
Frozen hippocampal tissue was collected in EP tubes, and RNA was extracted using the Trizol method. The RNA was then reverse transcribed into cDNA using the RNA reverse transcription kit (Monad, Wuhan, China). Next, an RT-PCR system was set up based on the reverse-transcribed cDNA, and PCR amplification was performed. The reaction conditions included: pre-denaturation at 95°C for 5 minutes, denaturation at 95°C for 10 seconds, annealing at 60°C for 34 seconds, and extension at 72°C for 30 seconds, repeated for a total of 40 cycles. At the end of the reaction, the amplification and melting curves were analyzed, and the data were calculated using relative quantification by the 2-ΔΔCt method. The PCR primers were synthesized by Guangzhou Weijia Biotechnology Co. The primer sequences were as follows: IL-4: forward CTCATCTGCAGGGCTTCCAG, reverse AGTGTTGTGAGCGTGGACTC; IL-10: forward CGAGATGCCTTCAGCAGAGT, reverse CGCCTTGATGTCTGGGTCTT; TNF-α: forward CGTCAGCCGATTTGCCATTT, reverse TCCCTCAGGGGTGTCCTTAG; IL-1β: forward CAGGATGAGGACCCAAGCAC, reverse CAGGTCGTCATCATCCCACG; Nrf2: forward ATGCCTTCCTCTGCTGCCAT, reverse CCGTGCCTTCAGTGTGCTTC; HO-1: forward GCCCACGCATATACCCGCTA, reverse GTCGATGCTCGGGAAGGTGA.
Western Blotting Was Used to Detect the Relative Expression Levels of Nrf2, HO-1, CD16, and CD206 Proteins in Rat Hippocampal Tissues
Approximately 100 g of hippocampal tissue from the left side of the rat brain was collected and added to lysate. The protein concentration was determined using the BCA method, and the protein content for each group was adjusted to the same level. The supernatant was then collected and mixed with protein loading buffer to prepare a sample storage solution, which was denatured for 10 minutes at 100°C in a metal bath. Following SDS-PAGE electrophoresis (Epizyme Biotech, Shanghai, China), proteins were transferred to a PVDF membrane. A 5% skimmed milk solution was used for blocking, and primary antibodies, including β-actin (1:5000, Proteintech, 66009-1-lg), Nrf2 (1:5000, Gene Tex, GTX635826), HO-1 (1:5000, Gene Tex, GTX635826), CD16 (1:700, Proteintech, 16559-1-AP), and CD206 (1:1000, Santa Cruz, SC-58986), were applied dropwise and incubated overnight at 4°C. After washing, the secondary antibody was added and incubated for 60 minutes at room temperature with shaking. After washing, ECL Plus ultrasensitive luminescent solution (Solarbio, Beijing, China) was used for image development and fixation, and the image was scanned. ImageJ software was used to analyze the grey value of each protein band, with β-actin serving as the internal reference for semi-quantitative analysis of the target proteins.
Statistical Methods
All experimental data were verified for accuracy and statistically analyzed using SPSS 25.0 software, with results expressed as mean ± standard deviation. Statistical graphs were generated using GraphPad Prism 9.0 software. The normality of data distribution was assessed using the Shapiro–Wilk test. For data following a normal distribution with homogeneity of variance, one-way analysis of variance (ANOVA) was used to compare differences between multiple groups. When data did not meet normality or homogeneity of variance assumptions, differences were analyzed using Dunnett’s T3 method. A p-value of less than 0.05 was considered statistically significant.
Results
The MCAO Model results in a Marked Increase in the Neurological Deficit Scores of Rats
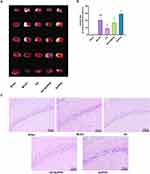
Increased neurological deficit scores suggest the presence of neurological impairments. Rats in the Sham group exhibited no signs of neurological deficits. Compared to the Sham group, the MCAO, EA, EA + SnPPIX, and SnPPIX groups showed significantly higher neurological deficit scores. No statistically significant differences were observed between these groups, except when compared to the Sham group (Figure 1).
 |
Figure 1 Effects of cerebral ischemia-reperfusion modeling on neurological function in rats. (A) Zea-Longa score: Statistical comparison of all other groups with the Sham group. **P < 0.01. |
EA Alleviates Learning and Memory Impairments in MCAO Rats
The results of the MWM test (Figure 2A-D) revealed that, compared to the Sham group, the MCAO group exhibited a significant reduction in the number of platform crossings and the time spent in the target quadrant, as well as a marked increase in escape latency, indicating severe learning and memory impairments caused by MCAO injury. In contrast, EA-treated rats showed significant improvements compared to the MCAO group, with increased platform crossings, more time spent in the target quadrant, and reduced escape latency over the course of treatment. Notably, by day 6, a significant reduction in escape latency was observed, reflecting improved learning, memory, and spatial orientation abilities. The SnPPIX group, however, showed a significant decrease in platform crossings and time spent in the target quadrant, along with an increase in escape latency. Compared to the SnPPIX group, the EA + SnPPIX group exhibited improved performance, including more platform crossings, more time in the target quadrant, and a progressive reduction in escape latency throughout the treatment period.
The results of the OFT (Figure 2E-H) revealed that the MCAO group showed a significant reduction in the number of cross-frames, average speed, and total distance traveled compared to the Sham group. However, following EA treatment, the number of cross-frames, mean speed, and total distance traveled were significantly higher than those in the MCAO group. In the SnPPIX group, a decrease in these parameters was observed. When compared to the SnPPIX group, the acupuncture + SnPPIX group exhibited a significant increase in the number of cross-frames, mean speed, and total distance traveled.
In conclusion, the above data indicate that EA effectively improves learning and memory deficits in MCAO rats.
EA Reduces Cerebral Infarct Volume and Enhances Neuronal Repair in the Hippocampal Tissue of MCAO Rats
At 14 days post-MCAO, TTC staining (Figure 3A and B) revealed no infarct damage in the Sham group, whereas the MCAO group exhibited a significant increase in cerebral infarct volume. The infarct volume was significantly reduced in the EA group compared to the MCAO group, while the SnPPIX group showed an increased infarct volume. The acupuncture + SnPPIX group demonstrated a reduction in infarct volume compared to the SnPPIX group. These results suggest that EA significantly reduces cerebral infarct volume following IS. Additionally, HE staining was used to observe the morphological changes in hippocampal neurons in the ischemic region, as shown in Figure 3C. In the Sham group, hippocampal neurons were well-aligned with normal morphology, clear nuclei, and no obvious inflammation. In contrast, the MCAO group showed neuron atrophy, disorganization, and loose arrangement, with ill-defined edges, coalesced nuclei, darker staining, and loss of cytoplasm. In the EA group, neuronal damage was significantly alleviated, with tightly packed neurons and reduced inflammation. The SnPPIX group exhibited more severe damage than the MCAO group, with even looser neuronal arrangement, coalesced nuclei, darker staining, and a significantly higher number of necrotic cells. The EA + SnPPIX group showed improved histopathological abnormalities compared to the SnPPIX group. These findings indicate that EA can significantly ameliorate neuronal damage in the hippocampal tissue of MCAO rats.
EA Induces the Polarization of Microglia/Macrophages from the M1 to the M2 Phenotype
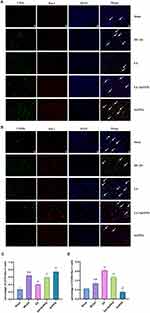
Upon activation, resting microglia/macrophages adopt an amoeboid shape, engage in phagocytosis, and express the specific surface marker Iba1.23 The WB analysis shown in Figure 4A-C reveals that the protein levels of CD16 and CD206 in the MCAO group were significantly higher than those in the Sham group. In contrast, the EA group exhibited a marked decrease in CD16 levels and an increase in CD206 expression compared to the MCAO group. In the SnPPIX group, CD16 levels were elevated, while CD206 expression was significantly reduced. Additionally, the EA+SnPPIX group showed reduced CD16 levels and enhanced CD206 expression when compared to the SnPPIX group.
The results from the immunofluorescence double-labeling experiment further corroborated these observations. As illustrated in Figure 5A-D, microglia/macrophages in the Sham group exhibited minimal activation, while those in the MCAO group showed significant activation, characterized by a marked increase in CD16+/Iba-1+ cells and a decrease in CD206+/Iba-1+ cells. Compared to the MCAO group, EA treatment notably reduced the number of CD16+/Iba-1+ cells and increased the number of CD206+/Iba-1+ cells. In contrast, the SnPPIX group demonstrated an increase in CD16+/Iba-1+ cells and a reduction in CD206+/Iba-1+ cells. Furthermore, the EA+SnPPIX group displayed decreased CD16+/Iba-1+ cell numbers and increased CD206+/Iba-1+ cells when compared to the SnPPIX group. These findings suggest that EA facilitates the polarization of microglia/macrophages from the M1 to the M2 phenotype in MCAO rats.
EA Effectively Reduces Neuroinflammation in MCAO Rats
Microglia/macrophage M1 polarization-induced neuroinflammation is a key mechanism contributing to learning and memory deficits in MCAO rats. To investigate whether EA inhibits the inflammatory response by promoting a shift in the microglia/macrophage polarization phenotype, we assessed the levels of IL-4, IL-10, TNF-α, and IL-1β using RT-qPCR and ELISA assays. As shown in Figure 6A-H, EA treatment reduced the production of pro-inflammatory cytokines TNF-α and IL-1β while increasing the production of anti-inflammatory cytokines IL-4 and IL-10 in MCAO rats. In the SnPPIX group, IL-4 and IL-10 levels were significantly lower, while TNF-α and IL-1β levels were significantly higher compared to the MCAO group. In contrast, the EA + SnPPIX group showed increased IL-4 and IL-10 levels and decreased TNF-α and IL-1β levels compared to the SnPPIX group. These findings suggest that EA induces a shift in microglia/macrophage polarization toward an anti-inflammatory M2 phenotype, leading to an increase in anti-inflammatory factors and a reduction in pro-inflammatory factors, thereby alleviating neuroinflammation in MCAO rats.
EA Modulates Microglia/Macrophage Polarization by Enhancing the Expression of the Nrf2/HO-1 Pathway in the Hippocampus, Thereby Improving Learning and Memory Deficits in MCAO Rats
The Nrf2/HO-1 pathway plays a crucial role in regulating microglia/macrophage polarization.19,24 Previous results have clearly demonstrated the positive impact of EA on alleviating learning and memory deficits in MCAO rats, but the potential involvement of the Nrf2/HO-1 pathway warrants further investigation. To explore this, the protein expression of Nrf2 and HO-1 was analyzed in this study. The WB results (Figure 7A-C) revealed a significant reduction in Nrf2 and HO-1 protein levels in the MCAO group compared to the Sham group. After 14 days of EA treatment, Nrf2 and HO-1 protein expression were notably upregulated. In contrast, SnPPIX treatment led to a significant downregulation of both Nrf2 and HO-1. The EA + SnPPIX group demonstrated a partial rescue of Nrf2 and HO-1 protein expression compared to the SnPPIX group. RT-qPCR analysis of mRNA levels of Nrf2 and HO-1 showed a similar trend to the Western blot data (Figure 7D and E). Furthermore, behavioral tests and cerebral infarct volume analysis indicated that SnPPIX significantly reduced the therapeutic efficacy of EA (Figures 2 and 3). Figures 4–6 also illustrated that SnPPIX reversed EA-induced changes in the expression of microglia/macrophage phenotypic proteins CD16, CD206, and inflammatory factors. In conclusion, EA improves learning and memory deficits in MCAO rats by promoting microglia/macrophage M2 polarization and reducing inflammation through activation of the Nrf2/HO-1 pathway.
Discussion
IS remains one of the leading causes of death worldwide, and to date, no drug has demonstrated clinical efficacy in IS trials. Individuals with IS often experience persistent impairments in learning, memory, and mobility, which impose a significant burden on their families.25 Therefore, finding new treatment options is of paramount importance. In recent years, EA has emerged as a promising treatment for IS. Liu et al showed that combining the stimulation of GV20 and ST36 acupoints with medication successfully inhibited the activation of the Ras homologous gene family member A (RhoA)/Rho-associated coiled-coil containing protein kinase (ROCK-2) pathway, leading to a marked reduction in striatal damage in ischemic rats.26 Previous studies have demonstrated that EA significantly improves learning and memory impairments in MCAO rats.27 This experiment also confirmed that EA alleviated chronic neurological damage following ischemia-reperfusion injury in cerebral infarction rats. Compared to the MCAO group, EA reduced the cerebral infarction volume, restored neuronal morphology, and partially improved learning and memory deficits in the rats.
Neuroinflammation plays a crucial role in neurological damage in patients with IS, as the inflammatory cascade is activated following blood flow interruption. This cascade leads to neuronal death, increased blood-brain barrier permeability, and brain edema, all of which further exacerbate brain injury.28,29 Numerous studies have shown that inhibiting excessive neuroinflammation in the brain can effectively improve the impairment and prognosis of learning and memory deficits following stroke.30 After MCAO, neuroglial cells are rapidly activated and play a dual role in regulating neuroinflammation. They can be identified by specific surface markers, which define their phenotype.31 Rawlinson et al demonstrated that appropriate activation of microglia/macrophages supports neural repair and promotes tissue and vascular remodeling for neuronal recovery.32 Gastrodin combined with EA has been shown to significantly reduce ischemic brain injury in rats, likely through facilitating the polarization of activated microglia toward the M2 phenotype.33 Some studies have suggested that promoting the M2 phenotype of microglia/macrophages and inhibiting the M1 phenotype are beneficial for improving learning and memory deficits following stroke.34 In conclusion, exploring therapies that promote the M2 phenotype in microglia/macrophages could offer a promising strategy for enhancing recovery after stroke. The current study investigated the effect of EA on the expression of CD16, the M1 marker, and CD206, the M2 marker, in microglia/macrophages in the hippocampus of MCAO rats. Immunofluorescence staining revealed that EA treatment reduced the number of Iba1/CD16 cells and increased the number of Iba1/CD206 cells in the CA1 region of the hippocampus 14 days after MCAO, compared to the MCAO group. The WB results for both polarization phenotypes were consistent with the immunofluorescence findings. Changes in microglia/macrophage polarization are critical in modulating inflammatory responses.35 To further confirm the link between microglia/macrophage polarization and neuroinflammation, we used RT-qPCR and ELISA to assess the expression of relevant factors. The results showed that EA treatment increased the release of anti-inflammatory factors such as IL-10 and IL-4, while reducing the secretion of pro-inflammatory factors such as TNF-α and IL-1β, in comparison to the MCAO group. These findings suggest that the mechanism by which EA alleviates the neuroinflammatory response in the hippocampal ischemic region and improves learning and memory deficits after stroke may be related to promoting microglia/macrophage polarization from the M1 to M2 phenotype.
This study further investigates the molecular mechanisms by which EA shifts microglia/macrophage polarization from the M1 to the M2 phenotype. Recent studies have highlighted that EA activates the Nrf2 signaling pathway through various mechanisms. Normally, Nrf2 is bound by its inhibitory protein Keap1. However, when oxidative stress occurs, Nrf2 is activated, translocates to the nucleus, binds to antioxidant response elements (ARE), and activates the expression of downstream antioxidant genes. EA has been shown to enhance this regulatory process significantly.36 Furthermore, research by Ni et al demonstrated that the neuroprotective effects of EA were significantly diminished in Nrf2-knockout mice, emphasizing Nrf2’s critical role as a mediator of EA’s therapeutic effects. Interestingly, the Keap1-independent Nrf2 pathway, regulated by Glycogen Synthase Kinase 3β (GSK-3β), has also been identified as a potential mechanism through which EA exerts its therapeutic effects in cerebral ischemia.37 The Nrf2/HO-1 pathway has been shown to be crucial in protecting various neurons from inflammatory damage.38 HO-1, a well-known antioxidant enzyme regulated by Nrf2, and its metabolites exhibit significant antioxidant and anti-inflammatory properties.39 EA treatment has been found to improve learning and memory in diabetic encephalopathy rats by modulating the Nrf2/HO-1 pathway within the hippocampal CA1 region, thereby reducing oxidative stress.40 Activation of the Nrf2/HO-1 pathway has also been shown to promote the polarization of microglia from the pro-inflammatory M1 phenotype to the anti-inflammatory M2 phenotype, which can enhance cognitive function and reduce neuroinflammation in lipopolysaccharide (LPS)-induced mice.41 Additionally, dexmedetomidine (Dex) has been shown to prevent cerebral ischemic injury by activating the Nrf2/HO-1 pathway, thereby promoting M2 polarization of microglia and alleviating inflammation.42 To determine whether EA’s enhancement of microglia/macrophage M2 polarization is associated with the Nrf2/HO-1 pathway, this study employed WB and RT-qPCR to evaluate the protein and mRNA expression levels of Nrf2 and HO-1, along with assessments of learning and memory performance in rats. The results revealed that compared to the MCAO group, EA treatment significantly upregulated both the protein and mRNA expression levels of Nrf2 and HO-1, with consistent results obtained from both methods. This upregulation of Nrf2 and HO-1 expression was associated with an increased proportion of M2 microglia/macrophages and improved cognitive abilities in the rats. In contrast, treatment with the HO-1 inhibitor SnPPIX resulted in reduced Nrf2 and HO-1 expression, a decrease in M2 microglia/macrophages, and worsened learning and memory impairments. In conclusion, this study demonstrates that EA enhances microglia/macrophage M2 polarization and reduces neuroinflammation via activation of the Nrf2/HO-1 pathway, thereby improving post-stroke cognitive function in rats.
In conclusion, EA may enhance the polarization of microglia/macrophages toward the M2 phenotype through activation of the Nrf2/HO-1 signaling pathway, thereby improving learning and memory deficits following a stroke. However, this study was limited to examining the effects of EA on microglia/macrophage polarization after 14 days of treatment and did not assess its effects at various time points. Additionally, Zhong et al found that EA significantly reduced microglial activation, which may have been due to decreased ROS levels following IS injury, thereby inhibiting the activation of NLRP3 inflammasome-related proteins.27 Similarly, Yao et al demonstrated that EA promotes microglial polarization toward the M2 phenotype by activating the Signal Transducer and Activator of Transcription 6 (STAT6)/Peroxisome Proliferator-Activated Receptor γ (PPARγ) pathway, which alleviates neuroinflammation in MCAO rats.43 These findings underscore that EA operates through multiple signaling pathways to induce M2 polarization of microglia/macrophages, contributing to the reduction of neurological damage caused by ischemic stroke. Nevertheless, further research is needed to explore the potential interactions among these pathways. Moreover, the precise mechanism through which EA drives microglial/macrophage polarization toward the M2 phenotype via the Nrf2/HO-1 pathway remains unclear. Future studies should focus on investigating the upstream and downstream molecular pathways of Nrf2/HO-1 to further elucidate the mechanisms by which EA improves learning and memory deficits after stroke.
Conclusion
Our study demonstrated the protective effect of EA in alleviating learning and memory impairments in MCAO rats. The anti-inflammatory benefits of EA are likely mediated through the promotion of microglia/macrophage M2 polarization, facilitated by modulation of the Nrf2/HO-1 signaling pathway. These findings highlight EA as a promising therapeutic strategy for IS.
Funding
This study was funded by the Key Scientific Research Project of Henan Universities (25A360018), the Science and Technology Tackling Program of Henan Province (222102310529), the Scientific Research Special Project of the National Clinical Research Base of Traditional Chinese Medicine of the Health Commission of Henan Province (2022JDZX005), the Special Project of Cultivation Program for Top Talents in Traditional Chinese Medicine of Henan Province (2022ZYBJ07), the Henan Province Traditional Chinese Medicine Inheritance and Innovation Talent Program (Zhongjing Project) – Leading Talent in Traditional Chinese Medicine (Research-Oriented)(CZ0262-08), and the Mechanistic Study on Acupuncture Combined with Moxibustion Ameliorating Post-Stroke Cognitive Impairment through Regulation of the TLR4 Signaling Pathway (20-21ZY1009).
Disclosure
All authors declare that there are no potential conflicts of interest in this work.
References
1. Rost NS, Brodtmann A, Pase MP, et al. Post-stroke cognitive impairment and dementia. Circ Res. 2022;130(8):1252–1271. doi:10.1161/CIRCRESAHA.122.319951
2. Jokinen H, Melkas S, Ylikoski R, et al. Post-stroke cognitive impairment is common even after successful clinical recovery. Eur J Neurol. 2015;22(9):1288–1294. doi:10.1111/ene.12743
3. Tack RWP, Amboni C, van Nuijs D, et al. Inflammation, anti-inflammatory interventions, and post-stroke cognitive impairment: a systematic review and meta-analysis of human and animal studies. Transl Stroke Res. 2023. doi:10.1007/s12975-023-01218-5
4. Yi CA, Jiang YH, Wang Y, et al. Black bamboo rhizome extract improves cognitive dysfunction by upregulating the expression of hippocampal BDNF and CREB in rats with cerebral ischaemia-reperfusion injury. Neuropsychiatr Dis Treat. 2021;17:2257–2267. doi:10.2147/ndt.S314162
5. Liu LR, Liu JC, Bao JS, Bai QQ, Wang GQ. Interaction of microglia and astrocytes in the neurovascular unit. Front Immunol. 2020;11:1024. doi:10.3389/fimmu.2020.01024
6. Cheng Y, Zhu H, Liu C, et al. Systemic immune-inflammation index upon admission correlates to post-stroke cognitive impairment in patients with acute ischemic stroke. Aging. 2024;16(10):8810–8821. doi:10.18632/aging.205839
7. Xiong XY, Liu L, Yang QW. Functions and mechanisms of microglia/macrophages in neuroinflammation and neurogenesis after stroke. Prog Neurobiol. 2016;142:23–44. doi:10.1016/j.pneurobio.2016.05.001
8. Yeh CF, Chuang TY, Hung YW, et al. Soluble epoxide hydrolase inhibition enhances anti-inflammatory and antioxidative processes, modulates microglia polarisation, and promotes recovery after ischemic stroke. Neuropsychiatr Dis Treat. 2019;15:2927–2941. doi:10.2147/ndt.S210403
9. Xu S, Lu J, Shao A, Zhang JH, Zhang J. Glial cells: role of the immune response in ischemic stroke. Front Immunol. 2020;11:294. doi:10.3389/fimmu.2020.00294
10. Wolf SA, Boddeke HW, Kettenmann H. Microglia in physiology and disease. Annu Rev Physiol. 2017;79(1):619–643. doi:10.1146/annurev-physiol-022516-034406
11. Hu X, Li P, Guo Y, et al. Microglia/macrophage polarisation dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke. 2012;43(11):3063–3070. doi:10.1161/strokeaha.112.659656
12. Chu F, Shi M, Zheng C, et al. The roles of macrophages and microglia in multiple sclerosis and experimental autoimmune encephalomyelitis. j Neuroimmunol. 2018;318:1–7. doi:10.1016/j.jneuroim.2018.02.015
13. Song GJ, Suk K. Pharmacological modulation of functional phenotypes of microglia in neurodegenerative diseases. Front Aging Neurosci. 2017;9:139. doi:10.3389/fnagi.2017.00139
14. Fadoul G, Ikonomovic M, Zhang F, Yang T. The cell-specific roles of Nrf2 in acute and chronic phases of ischemic stroke. CNS Neurosci Ther. 2024;30(3):e14462. doi:10.1111/cns.14462
15. Cui B, Zhang S, Wang Y, Guo Y. Farrerol attenuates β-amyloid-induced oxidative stress and inflammation through Nrf2/Keap1 pathway in a microglia cell line. Biomed Pharmacother. 2019;109:112–119. doi:10.1016/j.biopha.2018.10.053
16. Yamamoto M, Kensler TW, Motohashi H. The KEAP1-NRF2 system: a thiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiol Rev. 2018;98(3):1169–1203. doi:10.1152/physrev.00023.2017
17. Duan C, Wang H, Jiao D, et al. Curcumin restrains oxidative stress of after intracerebral hemorrhage in rat by activating the Nrf2/HO-1 pathway. Front Pharmacol. 2022;13:889226. doi:10.3389/fphar.2022.889226
18. Osama A, Zhang J, Yao J, Yao X, Fang J. Nrf2: a dark horse in alzheimer’s disease treatment. Ageing Res Rev. 2020;64:101206. doi:10.1016/j.arr.2020.101206
19. Subedi L, Lee JH, Yumnam S, Ji E, Kim SY. Anti-inflammatory effect of sulforaphane on LPS-activated microglia potentially through JNK/AP-1/NF-κB inhibition and Nrf2/HO-1 activation. Cells. 2019;8(2):194. doi:10.3390/cells8020194
20. Qin S, Zhang Z, Zhao Y, et al. The impact of acupuncture on neuroplasticity after ischemic stroke: a literature review and perspectives. Front Cell Neurosci. 2022;16:817732. doi:10.3389/fncel.2022.817732
21. Hung CY, Wu XY, Chung VC, Tang EC, Wu JC, Lau AY. Overview of systematic reviews with meta-analyses on acupuncture in post-stroke cognitive impairment and depression management. Integr Med Res. 2019;8(3):145–159. doi:10.1016/j.imr.2019.05.001
22. Zhou CH, Xue F, Xue SS, et al. Electroacupuncture pretreatment ameliorates PTSD-like behaviors in rats by enhancing hippocampal neurogenesis via the Keap1/Nrf2 antioxidant signaling pathway. Front Cell Neurosci. 2019;13:275. doi:10.3389/fncel.2019.00275
23. Womble T, Green S, Shahaduzzaman M, et al. Monocytes are essential for the neuroprotective effect of human cord blood cells following middle cerebral artery occlusion in rat. mol Cell Neurosci. 2014;59:76–84. doi:10.1016/j.mcn.2014.01.004
24. Sha W, Zhao B, Wei H, et al. Astragalus polysaccharide ameliorates vascular endothelial dysfunction by stimulating macrophage M2 polarisation via potentiating Nrf2/HO-1 signaling pathway. Phytomedicine. 2023;112:154667. doi:10.1016/j.phymed.2023.154667
25. Dordoe C, Chen K, Huang W, et al. Roles of fibroblast growth factors and their therapeutic potential in treatment of ischemic stroke. Front Pharmacol. 2021;12:671131. doi:10.3389/fphar.2021.671131
26. Liu M, Wang W, Zhang Y, Xu Z. Effects of combined electroacupuncture and medication therapy on the RhoA/ROCK-2 signaling pathway in the striatal region of rats afflicted by cerebral ischemia. Brain Res Bull. 2023;205:110828. doi:10.1016/j.brainresbull.2023.110828
27. Zhong X, Chen B, Li Z, et al. Electroacupuncture ameliorates cognitive impairment through the inhibition of NLRP3 inflammasome activation by regulating melatonin-mediated mitophagy in stroke rats. Neurochem Res. 2022;47(7):1917–1930. doi:10.1007/s11064-022-03575-3
28. Przykaza Ł. Understanding the connection between common stroke comorbidities, their associated inflammation, and the course of the cerebral ischemia/reperfusion cascade. Front Immunol. 2021;12:782569. doi:10.3389/fimmu.2021.782569
29. Liang E, Li X, Fu W, Zhao C, Yang B, Yang Z. COP9 signalosome subunit 3 restricts neuroinflammatory responses during cerebral ischemia/reperfusion injury through stabilizing suppressor of cytokine signaling 3 protein. injury through stabilizing suppressor of cytokine signaling 3 protein. Neuropsychiatr Dis Treat. 2021;17:1217–1227. doi:10.2147/ndt.S298966
30. Liu L, Yang C, Lavayen BP, Tishko RJ, Larochelle J, Candelario-Jalil E. Targeted BRD4 protein degradation by dBET1 ameliorates acute ischemic brain injury and improves functional outcomes associated with reduced neuroinflammation and oxidative stress and preservation of blood-brain barrier integrity. integrity. J Neuroinflammation. 2022;19(1):168. doi:10.1186/s12974-022-02533-8
31. Qin C, Zhou L-Q, Ma X-T, et al. Dual functions of microglia in ischemic stroke. Neurosci Bull. 2019;35(5):921–933. doi:10.1007/s12264-019-00388-3
32. Rawlinson C, Jenkins S, Thei L, Dallas ML, Chen RJBS. Post-ischaemic immunological response in the brain: targeting microglia in ischaemic stroke therapy. Brain Sci. 2020;10(3):159. doi:10.3390/brainsci10030159
33. Liu M, Gong R, Ding L, et al. Gastrodin combined with electroacupuncture prevents the development of cerebral ischemia via rebalance of brain-derived neurotrophic factor and interleukin-6 in stroke model rats. Neuroreport. 2024;35(10):664–672. doi:10.1097/wnr.0000000000002050
34. Yang HC, Zhang M, Wu R, et al. C-C chemokine receptor type 2-overexpressing exosomes alleviated experimental post-stroke cognitive impairment by enhancing microglia/macrophage M2 polarization. World J Stem Cells. 2020;12(2):152–167. doi:10.4252/wjsc.v12.i2.152
35. Morimoto M, Nakano T, Egashira S, et al. Haptoglobin regulates macrophage/microglia-induced inflammation and prevents ischemic brain damage via binding to HMGB1. J Am Heart Assoc. 2022;11(6):e024424. doi:10.1161/JAHA.121.024424
36. Yang XC, Jin YJ, Ning R, et al. Electroacupuncture attenuates ferroptosis by promoting Nrf2 nuclear translocation and activating Nrf2/SLC7A11/GPX4 pathway in ischemic stroke. Chin Med. 2025;20(1):4. doi:10.1186/s13020-024-01047-0
37. Ni C, Huang B, Huang Y, Wen Z, Luo S. Keap1-independent GSK-3β/Nrf2 signaling mediates electroacupuncture inhibition of oxidative stress to induce cerebral ischemia-reperfusion tolerance. Brain Res Bull. 2024;217:111071. doi:10.1016/j.brainresbull.2024.111071
38. Bai Y, Sui R, Zhang L, Bai B, Zhu Y, Jiang H. Resveratrol improves cognitive function in post-stroke depression rats by repressing inflammatory reactions and oxidative stress via the Nrf2/HO-1 pathway. reactions and oxidative stress via the Nrf2/HO-1 pathway. Neuroscience. 2024;541:50–63. doi:10.1016/j.neuroscience.2024.01.017
39. Zhang B, Zhao J, Wang Z, Xu L, Liu A, Du GJII. DL0410 attenuates oxidative stress and neuroinflammation via BDNF/TrkB/ERK/CREB and Nrf2/HO-1 activation. Int Immunopharmacol. 2020;86:106729. doi:10.1016/j.intimp.2020.106729
40. Wang W, Liu M, Miao H, et al. Electroacupuncture improves learning and memory deficits in diabetic encephalopathy rats by regulating the Nrf2/HO-1 pathway. Brain Res. 2025;1847:149309. doi:10.1016/j.brainres.2024.149309
41. Wang L, Ding YY, Wu YQ, et al. Koumine ameliorates neuroinflammation by regulating microglia polarization via activation of Nrf2/HO-1 pathway. Biomed Pharmacother. 2023;167:115608. doi:10.1016/j.biopha.2023.115608
42. Wang N, Nie H, Zhang Y, et al. Dexmedetomidine exerts cerebral protective effects against cerebral ischemic injury by promoting the polarisation of M2 microglia via the Nrf2/HO-1/NLRP3 pathway. Inflamm Res. 2022;71(1):93–106. doi:10.1007/s00011-021-01515-5
43. Yao Z, Cai L, Zhao A, et al. Electroacupuncture alleviates neuroinflammation by regulating microglia polarization via STAT6/PPARγ in ischemic stroke rats. Neuroscience. 2023;532:23–36. doi:10.1016/j.neuroscience.2023.09.007
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.