Back to Journals » Journal of Inflammation Research » Volume 17
ETS1 Expression in Diabetic Foot Ulcers: Implications for Fibroblast Phenotype and Wound Healing Through the PP2A/YAP Pathway
Authors Yi W, Bao Q, Xu D, Long C, Fang R, Cheng W, Song J, Feng H
Received 9 May 2024
Accepted for publication 29 August 2024
Published 16 October 2024 Volume 2024:17 Pages 7373—7388
DOI https://doi.org/10.2147/JIR.S477470
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tara Strutt
ETS1 Regulation in Diabetic Foot Ulcers – Video abstract [477470]
Views: 52
Wenjuan Yi,1 Qionglin Bao,2 Dingkun Xu,3 Chenyu Long,1 Ruixin Fang,1 Wenlin Cheng,4 Jiquan Song,1 Huiting Feng1
1Department of Dermatology, Zhongnan Hospital of Wuhan University, Wuhan, People’s Republic of China; 2Wound Repair Center, Chronic Wound and Diabetic Foot Clinical Medical Research Center, Liyuan Hospital Affiliated to Tongji Medical College, Huazhong University of Science and Technology, Wuhan, People’s Republic of China; 3Hubei Key Laboratory of Cell Homeostasis, College of Life Sciences, TaiKang Center for Life and Medical Sciences, Wuhan University, Wuhan, People’s Republic of China; 4Department of Cardiology, Zhongnan Hospital, Wuhan University, Wuhan, People’s Republic of China
Correspondence: Huiting Feng; Jiquan Song, Email [email protected]; [email protected]
Objective: Diabetic foot ulcers (DFUs) are a serious complication of diabetes, characterized by impaired wound healing and high morbidity and mortality risks. While ETS1 is known to influence fibroblast pathological remodeling, its specific role in DFU and fibroblast wound healing remains unclear.
Methods: Skin tissue samples from DFU patients were categorized by Wagner grades to analyze ETS1 expression. Primary fibroblasts derived from diabetes mellitus wound (DMFBs) were collected from wound margins to test migration ability and analyze cell phenotype by immunofluorescence; they were further treated with siETS1 and the ETS1 inhibitor YK-4-279. Techniques including Western blotting, quantitative Real-Time PCR (qRT-PCR), and immunofluorescence were used to assess the expressionof ETS1, Collagen I, and phenotype in DMFBs. Additionally, the binding sites between human ETS1 and the PP2A promoter were predicted by the UCSC and JASPAR databases. It intended to explore the negative transcriptional regulation of PP2A by ETS1 and its implications in fibroblast function and wound healing.
Results: Fibroblasts derived from Wagner Grades II–IV exhibit differences in cell morphology, migratory ability, and phenotype. Our findings indicate a significant upregulation of ETS1 in Wagner III and IV. The downregulation of ETS1 was observed to enhance DMFB migration and increase the expression of Collagen I and α-SMA. These changes suggest a potential mechanism by which PP2A regulates the YAP/Hippo pathway in diabetic wound healing.
Conclusion: ETS1 appears to impede the repair processes in DFUs, likely through the negative regulation of PP2A, affecting fibroblast function and wound healing.
Keywords: diabetic wound, diabetic foot ulcer, ETS1, PP2A, YAP
Introduction
Diabetes mellitus (DM) is a metabolic disorder characterized by high blood sugar levels. According to the 2019 International Diabetes Federation, approximately 463 million adults were affected by diabetes, and it is estimated to reach 700 million by 2045.1 With the changes in economic development and dietary habits, the number of diabetes cases in China has been steadily increased each year, accompanied by a growing number of complications. Diabetic foot ulcers (DFUs) are one of the common complications, characterized by chronic, non-healing wounds. Approximately 20% of diabetes patients develop foot ulcers and non-healing wounds.2 It has been reported that approximately 50% to 70% of amputations are diabetes-related, with one leg amputated globally due to diabetic wounds every 30 seconds.3 Diabetic wounds have a significant impact on patients’ quality of life and long-term survival, imposing a heavy burden on both patients and healthcare systems.1 The pathogenesis of diabetic non-healing wounds involves complex network regulation at multiple levels, including cellular, genetic, and protein factors.4 The latest advancements in single-cell RNA-sequencing (scRNA-seq) technologies have revealed the remarkable variety and essential functions of fibroblasts in both normal and pathological conditions.5 This study, focuses on fibroblasts derived from diabetic wounds, examining their morphology, migratory capabilities, collagen expression, and phenotype.
Erythroblast Transformation Specific 1 (ETS1) is a member of the ETS (E-twenty-six) transcription factor family, located on chromosome 11q24, and encoded by 8 exons, featuring a distinctive ETS domain.6 ETS1 serves as a transcriptional activator or inhibitor for numerous genes and plays a role in proliferation, differentiation, migration, apoptosis, and angiogenesis. It also regulates tissue development and contributes to cancer progression.7 ETS1 controls downstream targets such as matrix metalloproteinases (MMPs) and vascular endothelial growth factor (VEGF), playing a significant role in epithelial-mesenchymal transition and cancer cell invasion.8 Additionally, ETS1 is an effective oncogene in the skin, particularly in the progression of human melanoma.9 ETS1 plays a pivotal role as a transcription factor in the development and functioning of immune and endothelial cells.8,10 ETS1 can be considered a regulator of human health aging, as its deletion reduces ribosomal protein expression in fibroblasts, thereby slowing down cellular aging processes.11 However, its role in fibroblasts is still not well comprehended. A recent study published in Nature Immunology has garnered attention, highlighting ETS1 as a crucial transcription factor believed to control the pathological remodeling processes in fibroblasts. It was noted that the specific loss of ETS1 in fibroblasts can improve joint damage associated with both bone and cartilage in arthritis.12 Currently, research on ETS1 predominantly focuses on its roles in development and cancer, with little reporting on its role in diabetic wound healing.
Here, we analyzed the expression of ETS1 in non-healing DFUs and found that ETS1 is upregulated in Wagner III/IV. It demonstrated that ETS1 regulates the migration, collagen formation, and phenotype of DMFB. Consequently, it suggests that the ETS1-centered transcriptional regulation in fibroblasts plays a crucial role in the pathogenesis of DFUs, and targeting ETS1 may offer a potential avenue for controlling the wound healing.
Materials and Methods
Cell Cultures
Tissue samples were obtained from the wound edges of discarded specimens of diabetic foot ulcers (DFUs), and from healthy individuals at our institution, following the provision of informed consent. This study was approved by the Medical Ethics Committee of Zhongnan Hospital of Wuhan University, in compliance with the ethical guidelines of the declaration of Helsinki. DMFBs from patients with Wagner grades II-IV, and fibroblasts from healthy controls were isolated. Each sample was dissected into approximately 5mm² fragments and placed in sterile Petri dishes containing 5ml of dispase-I (Roche) at 4℃. After overnight digestion, the epidermis was separated from the dermis by two sets of forceps. Mince the dermis into small pieces with scissors. Digest the minced dermis with 5ml of collagenase (2 mg/ml in RPMI 1640 medium) at 37°C for 2.5 h. Filter the cell suspension with a 70μM cell strainer. RPMI 1640 medium (Gibco, Invitrogen) supplemented with 10% fetal bovine serum (FBS) was added to stop the enzymatic activity of collagenase. The mix was then centrifuged at 870 g for 5 min. Resuspend the cell pellet in 10ml of RPMI 1640 medium (supplemented with 10% FBS, 100 U/ml penicillin, and 100 U/ml streptomycin), and cultured at 37°C in a humidified 5% CO2 atmosphere. Fibroblasts for experiments were cultured to 2-4 passages.
Quantitative Real-Time PCR (qRT-PCR)
Total RNAs were extracted from fibroblast or skin tissues utilizing Trizol reagent (Invitrogen, Eugene, OR, USA) following the manufacturer’s instructions. cDNAs were synthesized from the total RNAs using the Moloney murine leukemia virus reverse transcriptase (M-MLV) first strand kit (Invitrogen). PCR was conducted in triplicate using SYBR Green PCR core reagents (Applied Biosystems, Foster City, CA, USA), incorporating 50 ng cDNA, and 1 mM forward and reverse primers for the targeted genes. Real-time PCR was executed utilizing ABI7500 with the following cycle parameters: denaturation at 95°C for 30s, followed by 40 cycles of 95°C for 5s, 60°C for 4s, and 72°C for 30s. The purity of each PCR product was validated through dissociation curve analysis and electrophoresis on 1% agarose gels. Fold change values were computed using the formula 2^ΔΔCt and normalized to the internal reference gene ACTB. The following primers were used for qRT-PCR:
ETS1 forward, 5′-GAGACCACAGACTTTGAGGGA −3′ and reverse, 5′-TCTGCTCTCAGCACCTCACT-3′;
Collagen I forward, 5′-CAGCCGCTTCACCTACAGC-3′ and reverse, 5′-TTTTGTATTCAATCACTGTCTTGCC-3′;
α-SMA forward, 5′-CCGACCGAATGCAGAAGGA-3′ and reverse, 5′-ACAGAGTATTTGCGCTCCGAA-3′;
and ACTB forward, 5′-GCCGCCAGCTCACCAT-3′ and reverse, 5′-TCGTCGCCCACATAGGAATC-3′.
Western Blotting Analysis
Cell and skin tissue samples were harvested and subsequently washed with Phosphate-Buffered Saline (PBS). Lysis of these samples was performed using an extraction buffer composed of 1% Nonidet P-40, 0.01% Sodium Dodecyl Sulfate (SDS), and a protease inhibitor cocktail (Roche, Indianapolis, IN, USA). The concentration of proteins in the lysates was quantified utilizing a BCA assay kit (Pierce, Rockford, IL, USA). For electrophoretic analysis, 20μg of protein from each extract was loaded per lane and separated by 10% SDS-Polyacrylamide Gel Electrophoresis (SDS-PAGE). Following electrophoresis, the proteins were transferred to Immobilon-P membranes (Millipore, Bedford, MA, USA). The membranes were then blocked using a solution of 5% nonfat milk in saline buffer to prevent nonspecific binding. Subsequently, the membranes were probed with primary antibodies, including anti-ETS1 antibody (Cat#: ab307672, Abcam), anti-collagen I antibody (Cat#: PA5-95137, ThermoFisher), anti-Alpha Smooth Muscle Actin (α-SMA) antibody (Cat#: ab124964, Abcam) at a 1:1500 dilution, or with an anti-GAPDH antibody (Cat#: EPR16891, Santa Cruz) at a dilution of 1:1000, for 1h at room temperature. After a thorough washing step, the membranes were incubated with HRP-conjugated anti-rabbit IgG at a dilution of 1:2000 for 1h at room temperature. Following another round of washing, specific bands were visualized through a chemiluminescent reaction (ECL; Amersham, Piscataway, NJ, USA).
Immunofluorescent Staining
Fibroblasts were seeded at a density of 1x10⁴cells/ml onto 12-well plates with sterile cover slips. Post-overnight incubation, fibroblasts were fixed in 4% paraformaldehyde at 4°C for 2h. Subsequently, the coverslips were washed thrice with PBS and blocked with 10% normal goat serum for 1h at 37°C. Primary antibodies, including anti-ETS1 antibody (Cat# ab307672, Abcam), anti-collagen I antibody (Cat# PA5-95137, ThermoFisher), or anti-Alpha Smooth Muscle Actin (α-SMA) antibody (Cat# ab124964, Abcam), or anti-Vimentin antibody (Cat# 10366-1-AP, Proteintech), or anti-Fibronectin antibody (Cat# 15613-1-AP, Proteintech), were diluted in antibody diluent buffer and applied to coverslips at 4°C for 16–18 hours . The secondary antibody, goat anti-rabbit IgG (H+L) conjugated with Alexa-488 (Invitrogen), or goat anti-rabbit IgG (H+L) conjugated with Alexa-647 (Invitrogen), was diluted to 1:200 with antibody diluent buffer and incubated with the cells on coverslips for 1h at 37°C. After removing the secondary antibody and washing thrice with PBS in the dark, counterstaining with DAPI (1.5 mg/ml) (Vector Laboratories, Burlingame, CA, USA). Images were captured using a microscope (BZX800E, Keyence, Japan), with at least ten images per coverslip. Mean intensity quantification for ETS1, Collagen I, and other markers was done using Image J software (NIH, Bethesda, MD, USA) in triplicate experiments.
Masson’s Staining
Masson’s trichrome staining is a significant method for visualizing fibers in tissues. The tissues were initially fixed in Bouin’s solution and then underwent routine dehydration and embedding. The sections were stained sequentially with Weigert’s iron hematoxylin, Biebrich scarlet-acid fuchsin, and aniline blue. Following staining, the slides were sealed with neutral gum. Microscopic analysis revealed collagen fibers in blue, and cytoplasm, muscle fibers, cellulose, and red blood cells in red, while nuclei appeared in blue-black.
Wound Healing Assay
Fibroblasts were seeded at a density of 1×105 cells/well in a 12-well plate, or those subjected to siETS1 incubation for 24h were cultured until the cell monolayer achieved nearly 100% confluence. Subsequently, the medium was replaced with serum-free RPMI 1640. A 200-µL pipette tip was used to create a scratch in the cell monolayer, which was then washed three times with PBS to eliminate cellular debris. The width and area of the wound was observed at 0 and 24h using an inverted light microscope, and ImageJ was used to analyze the percentage of wound closure.
Cell viability assay
Cell viability was evaluated through MTT assays. Initially, fibroblasts were plated at a density of 1×104 cells/well in a 96-well plate and cultured for 24h. Subsequently, the cells were treated with varying concentrations of YK-4-279 (0, 0.3, 1, or 3 µM) for 24h, 48h, or 72h at 37°C with 5% CO2. Following treatment, 20µl of MTT solution (5 mg/ml) was added to each well and incubated for an additional 4h at 37°C with 5% CO2. After removing the supernatant, 100µl of DMSO was added to dissolve the formazan crystals in each well. Absorbance was then measured at 450nm using a microplate reader (INFINITE200PRO, Tecan, Switzerland). The results of cell viability are presented as the mean±SD from three independent experiments.
siRNA-Induced ETS1 mRNA Knockdown
Small interfering RNA (siRNA) duplexes specific to ETS1 (Cat#: sc-29309) and a non-targeting scrambled control (Cat#: sc-37007) were procured from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Fibroblasts were plated in 6-well or 12-well plates. In accordance with the manufacturer’s guidelines, ETS1 siRNA or the scrambled control was combined with siRNA transfection medium (Cat#: sc-29528, Santa Cruz) and applied to the fibroblasts. The efficiency of ETS1 knockdown by these siRNAs was assessed by qRT-PCR and Western blotting.
Statistical Analysis
Statistical analyses were carried out by SPSS software (version 27.0). Multiple group comparisons were assessed through one-way ANOVA followed by Tukey’s post hoc test, while two-group comparisons were examined using the T-test. The data is reported as the mean±SD from three independent experiments at least. A significance threshold of P<0.05 denotes statistical significance.
Results
The Expression of ETS1 in DFUs
A significant study published in Nature Immunology highlighted the role of Erythroblast Transformation Specific 1 (ETS1) as a key transcription factor in pathological remodeling in fibroblasts. This study found that targeting ETS1 depletion in fibroblasts reduces joint damage in arthritis, affecting both bone and cartilage.12 This discovery led to further research on ETS1 expression in DFUs.
Clinically, DFUs are categorized using the Wagner grading system, which ranges from levels I to V.13 The Wagner system classifies and manages DFUs as follows:13 Grade I involves superficial ulcers and is treated with antibiotics and glycemic control. Grade II, characterized by deep ulcers affecting bone, ligament, or joint, requires debridement in addition to antibiotics and glycemic control. Grade III, which includes deep abscesses or osteomyelitis, may necessitate debridement and possible amputation. Grade IV, presenting with gangrene in the toes or forefoot, often leads to wide debridement and amputation. Grade V, with extensive gangrene of the entire foot, typically requires below-knee amputation.
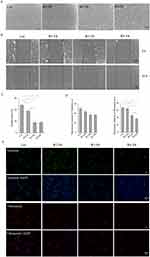
In our study, skin tissue samples from DFUs classified as Wagner II–IV were collected. H&E staining was performed on DFUsamples of Wagner II–IV to demonstrate histopathological differences (Figure 1A). For the normal skin tissue, a regular epidermis and well-ordered dermal collagen fibers were observed. In contrast, distinct pathological changes were noted across different Wagner grades of DFUs. In the W2 DFU, there was evidence of epidermal peeling, partial collagen fragmentation, and minimal inflammatory cell infiltration. The W3 DFU group exhibited an absence of epidermis, disorganized collagen, extensive neutrophil infiltration in the dermis, visible neovascularization, and lumina frequently containing thrombi. For the W4 DFU, it exhibited collagen proliferation, extensive inflammatory cell infiltration in the dermal and adipose layers, and partial necrosis of adipocytes.
Masson’s trichrome staining was used to assess collagen in Wagner II–IV DFUs, revealing a notable decrease (Figure 1A and B). To explore ETS1 in non-healing diabetic wounds, qRT-PCR was conducted, indicating increased ETS1 mRNA expression correlating with DFU severity (Figure 1C). Additionally, Western blotting and immunofluorescence analyses were used to evaluate ETS1 protein level (Figure 1D and E). Results showed a significant upregulation of ETS1 in Wagner II-IV DFUs, while no statistical difference for ETS1 mRNA and protein was observed between Wagner III and IV groups.
Culture and Phenotype of Fibroblasts in DFU
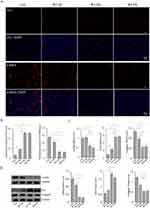
Transitioning from in vivo to ex vivo investigations, we collected skin tissues from various Wagner grades of DFUs to establish primary cultures for analyzing morphological and functional differences. Using an inverted microscope, we observed notable morphological changes in the second-generation fibroblasts derived from Wagner III/IV DFUs (Figure 2A). Normal fibroblasts displayed a well-organized structure, characterized by tight, radiate, or woven arrangements, overlapping growth, and distinct cell shapes. In contrast, fibroblasts from DFUs exhibited several irregularities, such as increased cell volume, flattened morphology, enlarged cytoplasmic areas, and irregular protuberances. Additionally, these DFU-derived fibroblasts contained intracellular particles and vacuoles, along with indistinct boundaries and irregular shapes, aligning with findings from a previous study.14 Moreover, fibroblasts originating from Wagner III or IV DFUs demonstrated a slower growth rate compared to those from Wagner II and normal control groups. In wound healing assays, fibroblasts from Wagner III/IV showed significantly reduced migratory capabilities (Figure 2B and C). These observations collectively underscore distinct morphological and functional characteristics of fibroblasts from different Wagner grades of DFU.
Immunofluorescence was further utilized to detect two markers, Vimentin and Fibronectin, to analyze fibroblasts’ phenotype, with quantified by ImageJ (Figure 2D and E). It revealed that Fibronectin expression decreased with increasing severity of DFU, while Vimentin did not show significant differences among the groups. Fibronectin is a crucial protein involved in wound healing, particularly in the formation of the extracellular matrix, which is essential for the development of granulation tissue. It has shown that Fibronectin’s soluble form circulating in the plasma (pFN), is incorporated into the fibrin clot that forms immediately after injury.15 The healing of excision wounds in the skin of STZ-diabetic rats is significantly accelerated by the local application of human pFN. It enhances the infiltration of fibroblasts into the wound, increases collagen synthesis, and promotes re-epithelialization.16 Vimentin plays a key role in maintaining cell shape, integrity, and the ability to withstand mechanical stress. It is suggested that differential expression of cell-specific markers can be attributed to the pathophysiological status and chronicity of the wound, with Vimentin being crucial for in vitro cell proliferation.17 No significant difference in Vimentin expression was observed among groups, which may be due to the 10% FBS environment reducing the differences in proliferation between groups.
ETS1 Upregulated in DFU
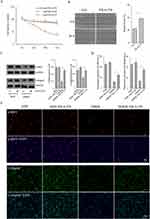
Upon prior research and our own findings indicating an elevated ETS1 expression in DFU tissues, particularly correlated with the severity of DFU, we conducted a comprehensive examination of ETS1 mRNA and protein in DMFB. The evident upregulation of ETS1 in Wagner II–IV was substantiated by results from qRT-PCR and Western blotting (Figure 3C and D). Notably, no statistically significant difference was observed between Wagner III and IV groups. Fibroblasts derived from both the healthy control and DFU exhibited weak or negligible expression of α-SMA (data not shown).18 To induce α-SMA expression, TGFβ1 (Thermo Fisher) intervention was used in alignment with existing study.18,19 In contrast, downregulated collagen I and α-SMA were noted in DFU compared to the healthy control group. It correlates with the clinical progression of DFUs, where an increase in Wagner grading is associated with exacerbated symptoms, heightened susceptibility to tissue loss, and aggravated impairment of cell migration.20 Immunofluorescence analysis further unveiled an upregulation of nuclear ETS1 expression and a downregulation of cytoplasmic α-SMA expression (Figure 3A and B).
In both DFU tissues and primary fibroblasts, no statistically significant difference for ETS1 was observed between Wagner III and IV. Consequently, for subsequent experiments, primary fibroblasts from Wagner III were selected for further in-depth investigation. This nuanced exploration of ETS1 in DFUs sheds light on its potential role in the underlying molecular mechanisms of diabetic wound pathology.
Effects of ETS1 on Migration and Collagen Proliferation in DMFB
To elucidate the role of ETS1 in DMFBs, primary fibroblasts were subjected to transfection with ETS1 siRNA. The efficacy of siRNA knockdown was rigorously validated through qRT-PCR and Western blotting, confirming a significant downregulation of both ETS1 mRNA and protein (Figure 4A and B). Subsequent scratch assays were used to assess migratory capability following ETS1 knockdown in DMFBs, with wound closure percentage analyzed by ImageJ (Figure 4C and D). It revealed an initial decrease in migratory capability, followed by a subsequent recovery after ETS1 knockdown. Additional qRT-PCR and Western blot analyses were conducted to evaluate collagen production and α-SMA induced by TGFβ1 in DMFB after ETS1 silencing (Figure 4E and F).It indicated an upregulation in collagen I and α-SMA mRNA and proteinafter ETS1 knockdown, albeit still marginally lower than the healthy control group.
Impact of YK-4-279 on Migration and Collagen Production in DMFB
YK-4-279 is a small molecule inhibitor of ETS, known for its ability to target and inhibit ETS transcription factors by disrupting protein-protein interactions with RNA helicase.21 Recent studies have indicated the potential of YK-4-279 in impeding the progression of thyroid cancer and the occurrence of melanoma.22,23 Therefore, the next step is to verify whether YK-4-279 can positively influence DMFB. YK-4-279 was dissolved in dimethyl sulfoxide (DMSO) with an original concentration of 10 mM and stored at −80°C. Fibroblasts were seeded in 12-well or 96-well plates, and different concentrations (0.3, 1, 3μM) of YK-4-279 were added for 24h, 48h, and 72h. Cell viability was assessed using MTT assays, revealing no significant changes in cell viability with 0.3μM or 1μM YK-4-279, while cell viability noticeably decreased with 3μM YK-4-279 (Figure 5A). Therefore, 1μM YK-4-279 was chosen for subsequent experiments. Following small molecule drug YK-4-279, scratch assays showed an enhance migratory ability in DMFBs (Figure 5B).
We utilized Western blotting and immunofluorescence to assess the expression of CollagenI and α-SMA in DMFBs (Figure 5C–E). Our results demonstrated a significant upregulation of Collagen I. It is particularly relevant as Collagen I plays a crucial role in the structural integrity and healing process of skin tissues. As known as TGFβ1 is pivotal in fibrosis and wound healing processes, we observed a notable induction and upregulation of α-SMA in the fibroblasts after TGFβ1. α-SMA is a marker of myofibroblast differentiation, a process vital for wound contraction and closure. The induced upregulation of α-SMA suggests that TGFβ1 effectively stimulates the fibroblasts towards a myofibroblast phenotype, which is essential in the context of wound healing and tissue repair.
ETS1 Regulation of YAP Through Targeting PP2A
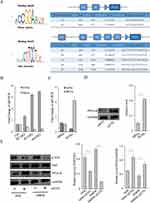
Utilizing bioinformatics techniques, predictions from the UCSC and JASPAR databases, two binding sites were identified between human ETS1 and the PP2A promoter, and five binding sites between murine ETS1 and the PP2A promoter were discovered. The binding intensity scores, binding segment start and end points, as well as gene sequences are illustrated in Figure 6A.
In our study, qRT-PCR analysis was conducted on tissues obtained from DFUs (Figure 6B). We observed ETS1 upregulation correlated with higher Wagner grades. Conversely, Protein Phosphatase 2A (PP2A) decreased, suggesting a negative correlation between PP2A and ETS1 mRNA. As the severity of DFUs increased, as indicated by higher Wagner grades, there was a notable decline in PP2A. In normal fibroblasts, siRNA-mediated silencing of ETS1 (siETS1) led to an increase in both mRNA and protein levels of PP2A (Figure 6C and D). It preliminarily supports the hypothesis of negative transcriptional regulation of PP2A by ETS1. It extensively highlights the significance of the Yes-Associated Protein (YAP)/Hippo signaling pathway in wound healing and tissue regeneration.24 It has been proposed that the B subunit PR55α of PP2A can either directly dephosphorylate and activate YAP or indirectly activate YAP by destabilizing the upstream kinase LATS, thereby influencing YAP/Hippo pathway activity.25 In our study, by qRT-PCR and WB, we aimed to elucidate the interplay between ETS1, PP2A, and phosphorylated YAP (p-YAP) in both healthy control and diabetic fibroblasts (Figure 6E).Using siRNA to silence ETS1 in NFBs and DMFBs resulted in decreased p-YAP and increased PP2A expression. It indicated that ETS1 may exert regulatory effects on DMFBs through the PP2A/YAP/Hippo axis, thereby influence their function and behavior.
Discussion
The successful healing of diabetic wounds is a multifaceted molecular biology process, intricately involving three interlinked phases: inflammation, proliferation, and tissue remodeling. Among the key players in this intricate process, fibroblasts emerge as crucial functional cells. Their pivotal roles encompass migration and proliferation, which contribute to the generation and regulation of the extracellular matrix (ECM). Additionally, fibroblasts actively participate in neovascularization, facilitating the filling of tissue defects and creating a conducive environment for the coverage of the wound by epidermal cells. Notably, fibroblasts can undergo a phenotypic transformation into myofibroblasts, thereby introducing contractile properties to the tissue.4,26 Despite these advancements in our understanding, the precise mechanisms governing diabetic wound repair remain incompletely elucidated.
Functioning as a transcriptional activator or inhibitor for a multitude of genes, ETS1 plays a pivotal role in diverse biological processes, including proliferation, differentiation, migration, apoptosis, and angiogenesis. Its regulatory influence extends to tissue development and contributes significantly to the progression of cancer.7 Notably, glutamine-induced expression of ETS1 has been linked to the promotion of migration and invasion in ovarian cancer cells.8 The absence of ETS1 in endothelial cells has been shown to impact downstream target VEGF secretion, leading to compromised coronary vascular development.7 ETS1 emerges as a key transcription factor governing pathological remodeling processes in fibroblasts. The specific loss of ETS1 in fibroblasts has demonstrated notable associations with improved joint damage in both bone and cartilage, particularly in cases of arthritis.12 While current research on ETS1 predominantly emphasizes its roles in development and cancer, scant attention has been given to its potential involvement in diabetic wound healing.
In this study, we conducted an analysis of ETS1 expression in non-healing DFUs, revealing an upregulation of ETS1 particularly in Wagner III/IV grading. Our study elucidated the regulatory function of ETS1 in modulating proliferation, migration, and collagen synthesis in DMFBs. It exhibited a noteworthy decrease in both proliferation and migration abilities, particularly in cases classified as Wagner III/IV. Through ETS1 siRNA and YK-4-279, the downregulation of ETS1 was found to enhance the expression of α-SMA induced by TGFβ1 and collagen I in DMFBs. Additionally, utilizing bioinformatics databases, identified a high-affinity binding site between ETS1 and the PP2A promoter. Experimental validation indicated that ETS1 might regulate PP2A/YAP through the Hippo pathway, thereby potentially playing a role in the intricate process of diabetic wound repair.
Hippo is an evolutionarily conserved signaling pathway that primarily regulates organ development, vascular remodeling, stem cell renewal, tumor suppression, and metastasis, playing a crucial role in wound healing and tissue regeneration.27,28 The Hippo pathway is regulated by a series of kinase phosphorylation cascades and is ultimately mediated by the downstream transcriptional co-activator Yes-associated protein (YAP), making YAP a pivotal component.27 Dysregulation of the Hippo pathway results in the dephosphorylation of YAP, allowing its translocation to the nucleus, where it forms functional hybrid transcription factors with TEA domain transcription factors (TEAD) and promotes the expression of genes related to cell proliferation and tissue regeneration29 (Figure 7). In the basal layer of the skin, YAP protein localization is crucial for maintaining epidermal homeostasis and wound healing.30 Ectopic expression of mutated YAP or dysregulation of upstream negative regulators, such as cell adhesion molecule α-catenin, leads to uncontrolled skin injury responses.31 High expression of dephosphorylated YAP has been found in skin tissues of systemic sclerosis patients, mediating fibrosis.32 Research also suggests that YAP induces dermal fibroblast conversion into myofibroblasts, which is critical for wound repair.33 Specific knockout of YAP in adult mice results in hair loss, skin damage, reduced epidermal cell proliferation, and significantly impairs wound regeneration.30 In mouse skin wounds, elevated dephosphorylated YAP expression in fibroblast nuclei is associated with delayed wound healing and downregulation of TGFβ1, Smad-2, and Smad-7 expression.28 Hence, the YAP/Hippo pathway is closely related to skin wound repair.
Protein phosphatase type 2A (PP2A) is a member of the serine/threonine-specific protein phosphatase family, leading to dephosphorylation and influencing various biological processes such as cell proliferation, apoptosis, vascular remodeling, and tumor metastasis.34,35 PP2A is a heterotrimeric protein consisting of scaffolding subunit A, regulatory subunit B, and catalytic subunit C. Subunit B specifically targets and regulates numerous critical pathways controlling cell phenotypes.36 Recent studies have shown that the B subunit PR55α of PP2A directly dephosphorylates and activates YAP, or indirectly activates YAP by reducing the stability of upstream kinase LATS, thereby activating YAP/Hippo signaling.25,37,38 Skin oxidative stress can downregulate PP2A methylation in normal human fibroblasts, suggesting that PP2A acts as a key regulator in oxidative stress signaling and skin aging.39 Decreased PP2A activity leads to impaired actin processing, disrupting skin homeostasis.40
The small molecule YK-4-279 targets and inhibits ETS transcription factors by blocking protein-protein interactions with RNA helicases. Recent research indicates that YK-4-279 can impede the progression of thyroid cancer and the onset of melanoma.22,23 In our study,YK-4-279 led to an upregulation of collagen I and TGFβ1 induced α-SMA in DMFB, offering a novel target to accelerate wound repair. Further research is needed to explore whether the small molecule inhibitor YK-4-279 can become a potential new drug for the treatment of diabetic wounds.
Conclusions
Our research posits that ETS1 hinders DFUs healing, potentially through the negative regulation of PP2A. Upregulated ETS1 transcriptionally downregulates PP2A, which would impede the dephosphorylation of YAP, thereby hindering YAP’s nuclear translocation. Consequently, this could suppress critical cellular processes such as proliferation, migration, and tissue regeneration, leading to the persistence of chronic non-healing wounds in DFUs. However, further research is required to validate these findings and fully understand the underlying mechanisms.
Data Sharing Statement
The data that supports the findings of this study are available in the “Materials and methods” section of this article.
Ethics Approval
Written informed consents were obtained from the patient or family members, and the study were approved by the Institutional Ethics Board of Zhongnan Hospital of Wuhan University (Wuhan, Hubei, China).
Funding
This research received funding from Hubei Provincial Natural Science Foundation of China (Grant No. 2023AFB235).
Disclosure
The authors declare no conflicts of interest.
References
1. Teo ZL, Tham YC, Yu M, et al. Global prevalence of diabetic retinopathy and projection of burden through 2045: systematic review and meta-analysis. Ophthalmology. 2021;128(11):1580–1591. doi:10.1016/j.ophtha.2021.04.027
2. Patel S, Srivastava S, Singh MR, Singh D. Mechanistic insight into diabetic wounds: pathogenesis, molecular targets and treatment strategies to pace wound healing. Biomed Pharmacother. 2019;112:108615. doi:10.1016/j.biopha.2019.108615
3. Vijayakumar V, Samal SK, Mohanty S, Nayak SK. Recent advancements in biopolymer and metal nanoparticle-based materials in diabetic wound healing management. Int J Biol Macromol. 2019;122:137–148. doi:10.1016/j.ijbiomac.2018.10.120
4. Talbott HE, Mascharak S, Griffin M, Wan DC, Longaker MT. Wound healing, fibroblast heterogeneity, and fibrosis. Cell Stem Cell. 2022;29(8):1161–1180. doi:10.1016/j.stem.2022.07.006
5. Shi SY, Luo X, Yamawaki TM, Li CM, Ason B, Furtado MB. Recent advances in single-cell profiling and multispecific therapeutics: paving the way for a new era of precision medicine targeting cardiac fibroblasts. Curr Cardiol Rep. 2021;23(7):82. doi:10.1007/s11886-021-01517-z
6. Wu Z, Jiang Y, Huang X, et al. In the tumor microenvironment, ETS1 is an oncogenic immune protein: an integrative pancancer analysis. Evid Based Complement Alternat Med. 2022:2022.
7. Wang L, Lin L, Qi H, Chen J, Grossfeld P. Endothelial loss of ETS1 impairs coronary vascular development and leads to ventricular non-compaction. Circ Res. 2022;131(5):371–387. doi:10.1161/CIRCRESAHA.121.319955
8. Di M, Zhang Y, Zeng R, et al. The pro-angiogenesis effect of miR33a-5p/Ets-1/DKK1 signaling in ox-LDL induced HUVECs. Int J Bio Sci. 2021;17(15):4122. doi:10.7150/ijbs.60302
9. Yang LX, Guo HB, Liu SY, Feng HP, Shi J. ETS1 promoted cell growth, metastasis and epithelial–mesenchymal transition process in melanoma by regulating miR-16-mediated SOX4 expression. Melanoma Res. 2021;31(4):298–308. doi:10.1097/CMR.0000000000000743
10. Prasad P, Roy SS. Glutamine regulates ovarian cancer cell migration and invasion through ETS1. Heliyon. 2021;7(5):e07064. doi:10.1016/j.heliyon.2021.e07064
11. Xiao FH, Yu Q, Deng ZL, et al. ETS1 acts as a regulator of human healthy aging via decreasing ribosomal activity. Sci Adv. 2022;8(17):eabf2017. doi:10.1126/sciadv.abf2017
12. Yan M, Komatsu N, Muro R, et al. ETS1 governs pathological tissue-remodeling programs in disease-associated fibroblasts. Nat Immunol. 2022;23(9):1330–1341. doi:10.1038/s41590-022-01285-0
13. Syauta D, Hendarto J, Mariana N, Kusumanegara J, Faruk M. Risk factors affecting the degree of diabetic foot ulcers according to Wagner classification in diabetic foot patients. Medicina Clínica Práctica. 2021;4:100231. doi:10.1016/j.mcpsp.2021.100231
14. Li B, Zhou Y, Chen J, et al. Long non-coding RNA H19 contributes to wound healing of diabetic foot ulcer. J Mol Endocrinol. 2020;65(3):69–84. doi:10.1530/JME-19-0242
15. Kanta J, Zavadakova A, Sticova E, Dubsky M. Fibronectin in hyperglycaemia and its potential use in the treatment of diabetic foot ulcers: a review. Int Wound J. 2023;20(5):1750–1761. doi:10.1111/iwj.13997
16. Qiu Z, Kwon A, Kamiyama Y. Effects of plasma fibronectin on the healing of full thickness skin wounds in streptozotocin-induced diabetic rats. J Surg Res. 2007;138(1):64–70. doi:10.1016/j.jss.2006.06.034
17. Monika P, Chandraprabha MN, Murthy KC, Rangarajan A, Waiker PV, Sathish M. Human primary chronic wound derived fibroblasts demonstrate differential pattern in expression of fibroblast specific markers, cell cycle arrest and reduced proliferation. Exp Mol Pathol. 2022;127:104803. doi:10.1016/j.yexmp.2022.104803
18. Goldberg MT, Han YP, Yan C, Shaw MC, Garner WL. TNF-α suppresses α-smooth muscle actin expression in human dermal fibroblasts: an implication for abnormal wound healing. J Invest Dermatol. 2007;127(11):2645–2655. doi:10.1038/sj.jid.5700890
19. Liu J, Wang Y, Pan Q, et al. Wnt/β-catenin pathway forms a negative feedback loop during TGF-β1 induced human normal skin fibroblast-to-myofibroblast transition. J Dermatological Sci. 2012;65(1):38–49. doi:10.1016/j.jdermsci.2011.09.012
20. Akkus G, Sert M. Diabetic foot ulcers: a devastating complication of diabetes mellitus continues non-stop in spite of new medical treatment modalities. World J Diabetes. 2022;13(12):1106. doi:10.4239/wjd.v13.i12.1106
21. Spriano F, Chung EY, Gaudio E, et al. The ETS inhibitors YK-4-279 and TK-216 are novel antilymphoma agents. Clin Cancer Res. 2019;25(16):5167–5176. doi:10.1158/1078-0432.CCR-18-2718
22. Xue J, Li S, Shi P, et al. The ETS inhibitor YK-4-279 suppresses thyroid cancer progression independent of TERT promoter mutations. Front Oncol. 2021;11:649323. doi:10.3389/fonc.2021.649323
23. Huang L, Zhai Y, Fajardo CD, Lang D. YK-4-279 attenuates progression of pre-existing pigmented lesions to nodular melanoma in a mouse model. Cancers. 2022;14(1):143. doi:10.3390/cancers14010143
24. Dey A, Varelas X, Guan KL. Targeting the hippo pathway in cancer, fibrosis, wound healing and regenerative medicine. Nat Rev Drug Discov. 2020;19(7):480–494.
25. Hein AL, Brandquist ND, Ouellette CY, et al. PR55alpha regulatory subunit of PP2A inhibits the MOB1/LATS cascade and activates YAP in pancreatic cancer cells. Oncogenesis. 2019;8(11):63. doi:10.1038/s41389-019-0172-9
26. Wan R, Weissman JP, Grundman K, Lang L, Grybowski DJ, Galiano RD. Diabetic wound healing: the impact of diabetes on myofibroblast activity and its potential therapeutic treatments. Wound Repair Regener. 2021;29(4):573–581. doi:10.1111/wrr.12954
27. Koo JH, Plouffe SW, Meng Z, et al. Induction of AP-1 by YAP/TAZ contributes to cell proliferation and organ growth. Genes Dev. 2020;34(1–2):72–86. doi:10.1101/gad.331546.119
28. Lee MJ, Byun MR, Furutani-Seiki M, Hong JH, Jung HS. YAP and TAZ regulate skin wound healing. J Invest Dermatol. 2014;134(2):518–525. doi:10.1038/jid.2013.339
29. Faraji F, Ramirez SI, Anguiano Quiroz PY, Mendez-Molina AN, Gutkind JS. Genomic hippo pathway alterations and persistent YAP/TAZ activation: new hallmarks in head and neck cancer. Cells. 2022;11(8):1370. doi:10.3390/cells11081370
30. Elbediwy A, Vincent-Mistiaen ZI, Spencer-Dene B, et al. Integrin signalling regulates YAP and TAZ to control skin homeostasis. Development. 2016;143(10):1674–1687. doi:10.1242/dev.133728
31. Schlegelmilch K, Mohseni M, Kirak O, et al. Yap1 acts downstream of α-catenin to control epidermal proliferation. Cell. 2011;144(5):782–795. doi:10.1016/j.cell.2011.02.031
32. Toyama T, Looney AP, Baker BM, et al. Therapeutic targeting of TAZ and YAP by dimethyl fumarate in systemic sclerosis fibrosis. J Invest Dermatol. 2018;138(1):78–88. doi:10.1016/j.jid.2017.08.024
33. Kim CL, Choi SH, Mo JS. Role of the hippo pathway in fibrosis and cancer. Cells. 2019;8(5):468. doi:10.3390/cells8050468
34. Wlodarchak N, Xing Y. PP2A as a master regulator of the cell cycle. Crit Rev Biochem Mol Biol 2016;51(3):162–184. doi:10.3109/10409238.2016.1143913
35. Jiang X, Hu J, Wu Z, et al. Protein phosphatase 2A mediates YAP activation in endothelial cells upon VEGF stimulation and matrix stiffness. Front Cell Develop Biol. 2021;9:675562. doi:10.3389/fcell.2021.675562
36. Tang Y, Fang G, Guo F, et al. Selective inhibition of STRN3-containing PP2A phosphatase restores hippo tumor-suppressor activity in gastric cancer. Cancer Cell. 2020;38(1):115–128. doi:10.1016/j.ccell.2020.05.019
37. Hein AL, Brandquist ND, Ouellette CY, et al. PR55α regulatory subunit of PP2A inhibits the MOB1/LATS cascade and activates YAP in pancreatic cancer cells. Oncogenesis. 2019;8(11):1–13.
38. Hein AL, Seshacharyulu P, Rachagani S, et al. PR55alpha subunit of protein phosphatase 2A supports the tumorigenic and metastatic potential of pancreatic cancer cells by sustaining hyperactive oncogenic signaling. Cancer Res. 2016;76(8):2243–2253. doi:10.1158/0008-5472.CAN-15-2119
39. Huber KL, Fernández JR, Webb C, et al. AGSE: a novel grape seed extract enriched for PP2A activating flavonoids that combats oxidative stress and promotes skin health. Molecules. 2021;26(21):6351. doi:10.3390/molecules26216351
40. O’Shaughnessy RF, Welti JC, Sully K, Byrne C. Akt-dependent Pp2a activity is required for epidermal barrier formation during late embryonic development. Development. 2009;136(20):3423–3431. doi:10.1242/dev.037010
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.