Back to Journals » Risk Management and Healthcare Policy » Volume 18
Evaluation of Medical Device Aging and Replacement Decisions within Hospital Environments: A User-Centered Approach
Authors Park S , Seo G, Park Y , Kim EJ, Lee H, Lee M , Jakovljevic M
Received 17 May 2024
Accepted for publication 20 June 2025
Published 1 July 2025 Volume 2025:18 Pages 2201—2215
DOI https://doi.org/10.2147/RMHP.S478245
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Gulsum Kubra Kaya
Sewon Park,1,* Gihong Seo,2,3,* Yoonseo Park,4 Eun-Ji Kim,4 Haengjun Lee,5,* Munjae Lee,1,* Mihajlo Jakovljevic6– 8
1Department of Medical Science, Ajou University School of Medicine, Suwon, 16499, South Korea; 2MEDIPLY (Medical Device & Healthcare Solution), Seoul, 05835, South Korea; 3Department of Medical Device Management and Research, SAIHST, Sungkyunkwan University, Seoul, 06351, South Korea; 4Department of Convergence Healthcare Medicine, Ajou University, Suwon, 16499, South Korea; 5Support & Cooperation Institute for Rural Spatial Development of Gyeongsangbuk-Do, Yeungnam University, Gyeongsan, 38541, South Korea; 6UNESCO-TWAS, Trieste, Italy; 7Shaanxi University of Technology, Hanzhong, People’s Republic of China; 8Department of Global Health Economics and Policy, University of Kragujevac, Kragujevac, Serbia
*These authors contributed equally to this work
Correspondence: Haengjun Lee, Support & Cooperation Institute for Rural Spatial Development of Gyeongsangbuk-do, Yeungnam University, Gyeongsan, 38541, South Korea, Email [email protected] Munjae Lee, Department of Medical Science, Ajou University School of Medicine, Suwon, 16499, South Korea, Email [email protected]
Purpose: This study aims to evaluate the factors influencing medical device replacement decisions from a user-centered perspective: medical technicians, engineers, and healthcare professionals. We seek to prioritize criteria for medical device replacement and develop feasible strategies applicable to real-world healthcare settings.
Methods: This analysis was conducted by reviewing previous studies to identify the key factors in medical device replacements. The key variables were selected from the review, and the significant factors for analysis were determined.
Results: An analytic hierarchy process (AHP) analysis was used to compare and analyze the priority of medical device replacement, which revealed that clinical factors emerged as the crucial in medical device replacement decisions. Additionally, unlike medical technicians, doctors, nurses, and pharmacists, for whom clinical factors are the primary considerations when replacing medical devices, safety is the most important factor for medical engineers.
Conclusion: Medical device obsolescence significantly affects patient safety and healthcare operations. Therefore, the maintenance plans for medical devices should focus on clinical factors. Moreover, a more systematic medical device replacement system must be established, prioritizing the factors according to the occupations of medical workers.
Keywords: medical devices, replacement evaluation, replacement priority, performance management, decision-making
Introduction
Medical devices based on cutting-edge technologies areas essential for human health and are promising high-value-added industries with expected continuous growth. East Asia remains one of the technologically most advanced regions in this sense while Japan and China hosting the second and third largest medical device markets.1–4 Korea possesses expensive medical devices, such as Computed Tomography (CT), Positron Emission Tomography (P.E.T)/CT scanner Magnetic Resonance Imaging (MRI), Surgical Robots, Proton Beam Radiotherapy Systems, INUMAC MRI Scanner etc., most of them, in density significantly exceeding the average of those that Organization for Economic Co-operation and Development (OECD) countries have.5 According to OECD Health 2023 report and other sources South Korea surpassed the OECD average for medical imaging tests, with 229.5 CT scans and 167.8 MRI scans per 1000 people.6 Yet, approximately one in four devices superannuated by more than ten years. This means that replacement does not occur promptly during medical device evaluation. Accordingly, to manage the lifecycle of medical devices, It is necessary to establish a system through which the status of a device can be identified and evaluated periodically to determine its replacement at an appropriate time.
Preventive inspections must be conducted for medical devices used for patients in hospitals to identify and manage the risks to patient safety. Risk management during the life cycle is essential for maintaining the quality of medical devices and managing their performance.7,8 The major goals of risk management of medical devices are to control, prevent, or reduce patient illnesses, injuries, loss of life, property damage, indirect losses, and environmental impacts. To this end, the ISO 14971 standard, an international standard for medical device risk management, was developed, which covers the overall risk management procedures applied to the life cycles of medical devices.9,10 To prevent performance degradation owing to aging, according to medical device risk management procedures, medical devices require continuous replacement to ensure operation, maintenance, and stability, and at this time, economic problems occur.11 The replacement of medical devices is a problem faced by all medical institutions; however, the replacement time and costs tend to vary depending on the increase in maintenance costs and technological advancements in medical devices.12,13 Similar challenges were largely observed even among wealthiest South-East Asia ASEAN countries whose domestic manufacturing processes remain highly dependent of foreign imports.14
Taylor K. and Jackson (2005) proposed the medical equipment replacement score (MERS) to identify and prioritize medical devices eligible for replacement.15 Faisal M. and Sharawi (2015) suggested that the priority of medical device replacement can be determined using a multi-criteria decision-making model (MCDM).16 Seo et al (2022) developed a life cycle of high-risk medical devices by deriving evaluation criteria and items for medical device replacement from major medical institutions in Korea, the United States, and others.13 However, existing researches primarily consider only the performance of the device in determining priorities for its replacement, while the factors, such as cost-benefit to medical institutions, clinical effectiveness, etc., are ignored. In particular, there is a serious shortage of studies analyzing the priorities of medical device replacement from the user perspective. Therefore, it is necessary to consider the limitations to the performance of medical devices, the characteristics of medical institutions, and the priorities of medical device replacement from the user perspective.
Efficient management of medical devices is essential to maintain the normal operation of healthcare institutions and enhance their competitiveness. Repeated overuse of medical devices can place an invisible burden on patients, while defective devices can cause operational shutdowns, leading to serious injuries or accidents for patients. In healthcare institutions, there is a tendency for medical device replacement to focus more on fixed maintenance rather than preventive management, resulting in 5–80% of equipment being nonfunctional. Additionally, it is necessary to implement appropriate evaluation methods and set priorities for medical device replacement to foster clinical development in healthcare institutions. If equipment replacement is not carried out in a timely manner or is delayed, it can hinder clinical progress within the institution and ultimately have a negative impact on patient experience and safety.17–19
The life cycle of a medical device generally follows the shape of a bathtub curve. Initially, the failure rate increases, gradually decreases after a stabilization period, and then increases owing to wear. Medical devices directly related to a patient’s life and with high replacement costs require preventive management. However, currently, medical devices are replaced primarily when their performance deteriorates, which can be seen as follow-up management.20 Accordingly, in this study, we present priorities for medical device replacement by evaluating the obsolescence evaluation index of medical devices and the factors affecting replacement decisions in a user-centered manner. Through this, we can understand user perspectives on replacing medical devices to enable proactive management and prepare medical device replacement plans applicable in actual medical institutions. Any such long-term replacement strategies refering to medical imaging diagnostics devices, oncology radiotherapy cabinets, invasive vascular radiology cabinets and robotics assisted orthopedic and laparoscopic surgery devices, however have to be included into the national and provincial equipment acquisition plans.21 For such a purpose sustainable and reliable long – term budgeting is necessary not only for the initial acquisition but as well for the purpose of maintenance.22 As already witnessed the quality of such maintenance largely reflects to the radiation leakage in the radiology imaging cabinets with potentially significant health consequences for the patients and radiology specialty physicians and radiology technicians alike.23 These facts have already led to a certain degree of professional medical staff shortages in Korea and wider East Asia.24
Literature Review
Risk Management of Medical Devices
The risk management of medical devices was mentioned in 1997 when the European Committee for Standardization (CEN) developed EN 1441 as the “Medical Device-Risk Analysis”. ISO 14971–1, the first standard, was published in 1998 and revised to ISO 14971:2000 (1st edition) in 2000. In 2007, the second revision of ISO 14971:2007 (2nd Edition) was published.25,26 In 2012, CEN released EN ISO 14971:2012, the European version, in harmony with the healthcare sectors of 93/42/EEC, 98/79/EC, and 90/385/EEC. In 2019, it was revised to ISO 14971:2019 (3rd Edition), the most recent version.27–29
ISO 14971 stipulates the principles and processes for managing risks in medical devices (including software and IVD(In Vitro Diagnostic) products as medical devices) and is an important standard for manufacturers and suppliers of medical devices. The risk management process of medical devices defined in ISO 14971 has a total of six steps, including a risk management plan, risk evaluation, risk control, evaluation of overall residual risk, risk management review, and production and post-production activities.30 The first phase, the risk management plan, identifies medical device safety-related properties and hazard sources and predicts the risk for each hazard source. The second phase, the risk evaluation, determines whether risk reduction is required for the identified situations. The third phase, risk control, involves performing risk reduction activities at an acceptable level. In the fourth phase, the evaluation of the overall residual risk must assess whether there is an unacceptable level of residual risk to determine how residual risks will be handled and disclosed. The fifth phase, risk management review, involves reviewing the risk management process to record and store the results as a risk management report. Finally, the sixth phase, production and post-production activities, establishes and maintains a system for collecting information about medical devices (Figure 1). 25,31
 |
Figure 1 ISO 14971 risk management process overview.32 |
In addition, ISO 14971 is closely related to ISO 13485, an international quality management standard for medical devices. ISO 13485 was standardized based on ISO 9001 by adding related key factors to suit the characteristics of medical devices. It specifies the requirements for the quality management system of medical devices, including their installation and sterilization, management review, customer satisfaction, design and development, and manufacturing and improvement activities for medical devices.33–35 This standard defines the requirements that manufacturers must meet, ranging from the design of medical devices to production and improvement activities. Companies can establish quality management systems to reduce defects through continuous product improvement activities. ISO 13485 provides the requirements for quality management systems for organizations to design, develop, manufacture, and provide medical devices that comply with the regulations and standards specified in ISO 13485.36 Risk analysis and record keeping specified in ISO 13485 are required for all medical device-related risks. Thus, all processes related to risk identification, risk evaluation, and risk analysis required by ISO 14971 are provided, and the overall content of risk management is managed.35,37
Life Cycle of Medical Devices
Durability refers to the life expectancy of a product or as the period during which the product is useful in its original condition.38 Kotler (1979) viewed the product life cycle as the process in which a product is made, introduced in the market, increases its sales, and then decreases its demand. Fabiano and McCarthy (1981) defined it as the stage between the product launch and disappearance.37,39 Every product has a life cycle, and medical devices have unique life cycles. Medical devices used to diagnose or treat patients directly or indirectly affect human life and health. Therefore, unlike general industrial products, medical devices are managed by laws and regulations during production, distribution, and sales processes.40
In Korea, regulations on medical device durability are under Article 16–2 of the Commodity Management Act and No. 2018–14 of the Public Procurement Service Notification. The Public Procurement Service determines the fundamental matters regarding the acquisition, storage, usage, and disposal of national commodities and stipulates the durability life to manage national commodities efficiently and appropriately. Durability life is defined for frequently used items with a large inventory, and the durability life of these commodities is stipulated to be applicable to similar commodities with an undetermined durability life.41 In the case of medical devices, the requirements for performance, safety, and inspection cycles are specified only for 16 types of special medical equipment. Apart from this, no specific durability regulations exist for the replacement times of other medical devices. Medical devices must be replaced periodically according to established standards and procedures to provide optimal medical services to patients. Since only some devices have stipulated durability, regulations are required to plan and manage the use, maintenance, and replacement of medical devices. In recent years, the Green Agenda and Sustainable Development requirements, largely led by the specialized UN agencies, have led to standardization of potential environmental damage originating from a disposable medical devices and tests after the expiration of their due date.42,43 Such requirements are now increasingly embedded into related medical device legislation, marketing and reimbursement approval pathways and ultimately into the decision-making process on research and innovation funding across East Asia, Europe and North America.44,45
In contrast, in the United States, the American Hospital Association (AHA) suggests estimated figures of the durable life of medical devices for the accounting purposes of medical institutions.46 In addition, the American Society for Health Care Engineering has developed a calculation method to estimate the durability of medical devices to support replacement plans for medical devices.13 In Canada, the Diagnostic Imaging Equipment Replacement and Upgrade has developed life cycle guidelines for medical imaging equipment to determine the upgradation or replacement time of existing devices.47 According to these guidelines, radiology devices, including fluoroscopic devices, must be utilized for 5–10 years, angiography devices for 7 years, computed tomographic devices for 7 years, magnetic resonance imaging devices and ultrasound devices for 7 years, mammography devices for 5–7 years, and single photon computed tomography devices for 10 years.48
Indicators for Evaluating the Life Cycle of Medical Devices
Examining existing studies related on medical device replacement factors, Fennigkoh (1992) developed a simple model to identify and prioritize the replacement of medical devices.49 Subsequently, Taylor K. and Jackson (2005) proposed an automated MERS system that considered technology, safety, and important rules.15 He analyzed the potential risks of using medical devices in medical institutions and applied a pairwise comparison technique based on six criteria: function, mission criticality, age, risk, recall and hazard alerts, and maintenance requirements. Figure 2 presents the decision-making hierarchy for prioritizing medical device replacement using the pairwise comparison method. This hierarchical framework is structured to define evaluation criteria for replacement prioritization and to systematically allocate them to appropriate levels within the hierarchy. The evaluation criteria are positioned at the second level, with their respective weights determined through pairwise comparisons. In this process, the relative importance of each criterion is assessed to derive the necessary weights for establishing replacement priorities. Specifically, the system assigns weights to each criterion; thus, devices with higher final scores are prioritized for replacement.50
 |
Figure 2 Hierarchy to determine priorities. |
Rajasekaran (2005) developed a program that could automatically generate relative replacement numbers (RRNs) according to safety, technical aspects, and financial status for devices requiring replacement.51 Mummolo et al (2007) utilized a model to determine medical device replacement priorities based on indicators such as repair rate, failure rate, durable years, etc.52 Taghipour, Banjevic, and Jardine (2011) emphasized the necessity of replacement prioritization to minimize malfunctions and maintain the stable operation of medical devices.50 Faisal M. and Sharawi (2015) classified support, availability, performance, maintenance cost, age, equipment function, operational impact, and clinical acceptability as dominant factors in determining replacement priority and proposing decision-making processes.16
Materials and Methods
AHP Analysis
In this study, an analytic hierarchy process (AHP) analysis was conducted to determine the importance of replacement evaluation items in medical devices. The AHP MCDM was developed by Saaty (1977) in 1971. The AHP has been known as a decision-making technique that systematically combines information about tangible and intangible standards and alternatives in the decision-making process.53,54 The primary and eminent phase in the AHP involves stratifying the items related to decision-making, in which key factors are determined through a pairwise comparison between the elements that compose the decision-making hierarchy.55 The hierarchy of AHPs involves structuring the components of decision-making problems with various elements, and the decision-making analyst stratifies various interrelated decision-making items.56 At the top of the hierarchy is the purpose of decision-making, and the bottom layer consists of factors that can influence the decision-making process. These elements become more specific at lower levels, and each element within a layer must be comparable.
Research Framework and Indicators Selection
For the analysis, previous studies were reviewed to select the major factors that must be considered in medical device replacement evaluation. The frequency of each factor is shown in Table 1.
 |
Table 1 Key Factors for Evaluating Medical Device Replacement |
A survey was conducted among 86 medical device-related experts (doctors, nurses, medical technicians, pharmacists, and medical engineering staff) based on 21 variables derived to select indicators of deterioration. The survey period was from October 6 to 14, 2020, and 71 survey results were analyzed, excluding 15 inappropriate questionnaires among those returned via email. Based on the survey results, 16 variables were selected from the 21 variables: discontinuation of parts, age, number of failures, life-support equipment, potential risk, daily inspection, maintenance cost, device status, call response, serviceability, device reliability, frequency of use, major devices, model discontinuation, availability of backup devices, and technological advancements Subsequently, five variables - technology, safety, cost, support, and clinical - were selected through brainstorming as the final evaluation indicators. The reduction process was conducted through group discussions involving experts from various clinical and technical fields. This process focused on identifying the key factors influencing the prioritization of medical device replacement. Experts systematically reviewed the variables and underwent an iterative classification and factor extraction process to select the most critical and broadly applicable factors. The reduction process was carried out in a stepwise manner, with the outcomes of each meeting summarized and re-evaluated in subsequent discussions. The brainstorming and consensus process was conducted in two rounds, ultimately leading to the selection of five key evaluation criteria. The selected five factors represent fundamental considerations that are broadly applicable across diverse medical equipment categories. The weight of each factor can be adjusted based on specific clinical settings, ensuring flexibility in prioritization without compromising the robustness of the model.
The study framework was established by applying the MERS system to conduct the AHP analysis. The MERS system served as the foundation for structuring the decision-making hierarchy and deriving priority weights for medical device replacement. Through this approach, the study integrated both methodologies to enhance the robustness of the prioritization model. Figure 3 presents the research model that determines the priority of medical device replacement by applying the MERS system. It illustrates the process in which relative importance is assessed through pairwise comparisons, with the final scores determining the replacement priority. This means that the device with the highest final score is given priority for replacement by applying weights to five factors: technology, safety, cost, support, and clinical application.
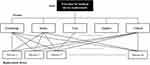 |
Figure 3 Research model for selecting replacement priorities. |
The definitions of the key factors used in this study are listed in Table 2. Technology includes the total period of use after installation and the number of annual failures, referring to the fact that no repairs were possible because the necessary parts were discontinued by the manufacturer. Safety includes daily inspections before use and potential risks to the human body, meaning that the risk to the human body increases in cases of malfunction. Cost, having the same meaning as maintenance costs, refers to the total cost used to maintain the device. Support includes the overall deterioration degree of the device, the response time from the request for repair until the repair, etc., referring to the time to restore the device after breakdown. Clinical refers to the devices that play a key role in a department, including device reliability, frequency of use, model discontinuation, devices with new technology applied, and the availability of substitute devices that can be used in case of failure.
 |
Table 2 Definition of Evaluation Factors |
Analysis Methods
Frequency analysis was conducted using SPSS 22.0 to confirm the general characteristics of the study subjects. In addition, AHP analysis was conducted to select priorities for each factor by utilizing RStudio’s AHP package. First, for the pairwise comparison of AHPs, the 9-point scale proposed by Aczél J. and Saaty (1983) was used.62 On this scale, the respondent’s choice does not significantly affect the matrix calculation in quantitative judgment through pairwise comparison, and accordingly, respondents can reduce the psychological burden of choosing accurate values, thus leading to results closest to the actual number.63 The 9-point scale was defined by 1 (similar), 3 (slightly important), 5 (important), 7 (very important), and 9 (extremely important) according to their relative importance. The Consistency Indicator (C.I) was measured and divided by the Random Index (R.I), obtaining the Consistency Ratio (C.R), calculated to ensure the consistency of the relative comparison results between components. This was intended to increase the reliability of the decision-making process. The C.R must have a value less than 0.1 to ensure the consistency of the pairwise comparison matrix.64 The C.R value in this study was less than 0.1, ensuring consistency. Therefore, the relative importance was calculated using the geometric mean, obtained by multiplying the relative importance from the evaluation perspective and the relative importance of the key performance indicators.
In AHP analysis, it is important to identify the most prioritized factors among numerous variables using an expert group. However, since the judgments may vary depending on the personal preferences of the participants, consistency ratios are calculated to assess the reliability of the analysis results. The goal is to derive consistent judgments from individuals with specialized knowledge or experience in the relevant field. Therefore, there is no clear standard for the optimal sample size, but it is generally considered that a sample of at least seven participants can provide sufficient consistency.54 In this study, to ensure the reliability and validity of the research, experts with extensive experience in medical device evaluation were selected to identify the factors, and consistency ratios were calculated to confirm the logical consistency of their responses. Additionally, the results were cross-verified by comparing them with existing literature. This approach minimized subjective bias and ensured the validity of the research outcomes.
Table 3 shows the consistency verification results of the analysis factors and the ranking of each factor. All C.R values were less than 0.1, ensuring the reliability of the AHP analysis results. The weight and ranking of each factor were as follows: clinical (0.3405), safety (0.2651), technology (0.1739), support (0.1568), and cost (0.0636).
 |
Table 3 Weight and Importance of Each Factor |
Results
General Characteristics of Research Subjects
This study included 71 subjects: 46 males (66.2%) and 25 females (33.8%). There were 24 medical technicians (33.8%), 20 medical engineering staff (28.2%), 16 doctors (22.5%), 8 nurses (11.3%), and 3 pharmacists (4.2%). In addition, 31 (43.7%) of the research subjects were in charge (see Table 4).
 |
Table 4 Characteristics of Research Subjects |
Differences in Priorities for Occupations
The results of the analysis of medical device replacement priorities according to occupation are presented in Table 5. Medical technicians appeared in the order of clinical (0.33), safety (0.29), support (0.16), technology (0.15), and cost (0.07); medical engineering staff in the order of safety (0.32), clinical (0.30), technology (0.18), support (0.14), and cost (0.06); doctors in the order of clinical (0.38), safety (0.20), technology (0.18), support (0.18), and cost (0.06); and nurses in the order of clinical (0.33), safety (0.25), technology (0.19), support (0.13), and cost (0.10). For pharmacists, these were in the following order: clinical (0.33), safety (0.31), support (0.16), technology (0.15), and cost (0.05). Clinical was the priority factor for medical technicians, doctors, nurses, and pharmacists, whereas, for medical engineering staff, safety was the priority factor for replacing medical devices. Additionally, cost was the lowest priority for all occupations.
 |
Table 5 Factor Weight Results by Occupation |
Differences in Priorities for Professional Experience
Table 6 shows the results of the comparative analysis of medical device replacement priorities according to professional experience. The priority factors for replacing the medical devices for those who had 1–3 years of experience appeared in the order of clinical (0.35), safety (0.24), support (0.18), technology (0.17), and cost (0.06); for those with 4–6 years of experience, the order was clinical experience (0.32), safety (0.29), technology (0.18), support (0.15), and cost (0.06); for those with 6–10 years of experience, the order was clinical experience (0.35), safety (0.26), technology (0.18), support (0.14), and cost (0.07). Additionally, for those with more than 10 years of experience, it was in the following order: clinical (0.34), safety (0.28), technology (0.15), support (0.15), and cost (0.08). As a result of the analysis, the key factor for replacing medical devices in all subjects was clinical, followed by safety, technology, and support in order.
 |
Table 6 Factor Weight Results by Professional Experience |
Differences in Priorities for Educational Background
The results of the comparative analysis of priority factors for medical device replacement according to educational background are presented in Table 7. For Junior college graduates the priority factors were in the following order: clinical (0.33), safety (0.32), technology (0.17), support (0.12), and cost (0.06); for University graduates: clinical (0.34), safety (0.26), technology (0.18), support (0.16), and cost (0.06); for postgraduates: clinical (0.33), safety (0.25), technology (0.17), support (0.17), and cost (0.18). In replacement priority factors based on educational background, clinical setting showed the highest weight, followed by safety, technology, support, and costs.
 |
Table 7 Factor Weight Results by Educational Background |
Discussion
Safety management of medical devices and their target performance maintenance through regular inspections are essential because superannuated medical devices have a high potential for malfunction or errors during treatment. To this end, relevant medical device-related risks are currently identified, calculated, and evaluated under ISO 14971 and ISO 13485. However, these are regulations that medical device manufacturers must follow, and thus, there are limitations to their application in medical institutions. Furthermore, no specific replacement guidelines are currently available for calculating durability to manage the life cycle of medical devices, except for particular devices. It is necessary to select replacement priorities from the user perspective through which medical institutions can proactively manage medical devices to ensure patient safety.
Therefore, in this study, we composed medical device obsolescence evaluation indexes from the perspective of medical workers who use medical devices and analyzed the priorities of medical device replacement factors through AHP analysis. Additionally, a comparative analysis of the importance of replacement factors was conducted, classifying the respondents by occupation, experience, and educational level, which was not implemented in previous studies. In previous studies on medical device replacement priorities, support availability was found to be the most important factor, followed by performance, maintenance cost, age, function, operational impact, and clinical acceptability in order.16 However, the results of this study showed that clinical factors had the highest weight among the medical device replacement factors. This suggests that clinical aspects must be considered when replacing medical devices, ie, when a medical device becomes superannuated, it cannot be repaired, and the device has more priority for replacement. These results are different from previous studies because of the different categories of medical devices used in the analyses. In this study, the priorities of the replacement factors were analyzed for all medical devices; however, previous studies have mainly analyzed medical devices in intensive care units. The intensive care unit consists of 3rd or 4th-grade medical devices with hazardous properties. Thus, in the event of a malfunction, the longer it takes to restore, the greater the risk to the patient’s life. Therefore, support appears to be an important factor among medical device replacement factors.
As a result of conducting a comparative analysis of the priorities of medical device replacement factors according to occupation, clinical is the most important factor for medical technicians, doctors, nurses, and pharmacists. However, for medical engineering staff, safety was the most important factor. Medical engineering staff must directly inspect medical devices to manage them and are responsible for their safety. Due to their roles and responsibilities, safety is a top priority. In other words, when replacing medical devices, the importance of factors may vary depending on the occupation. Thus, appropriate decisions must be made considering the position of each occupation.65 For the medical device replacement priority factors based on occupational experience and education levels clinical was the most important factor. This is similar to existing research findings that it is important to ensure the availability of backup equipment to mitigate potential risks to patients and medical staff while maintaining and managing medical devices.66 Medical devices used more frequently by patients must be produced more continuously, and in the case of a breakdown, a replacement device must be available. When the medical device becomes superannuated, the unavailability of an applicable new technology, or a backup, will reduce the reliability of the device, limiting its application to patients. With the increase in standards of career or education, the tendency to prioritize clinical factors becomes stronger. Thus, it is expected that medical devices with low clinical factors will be prioritized when replacing medical devices.
Meanwhile, the cost factor, which is the total cost of maintaining medical devices, was not found to be an important factor in replacing medical devices. This appears to be somewhat different from existing research results, which show that medical devices equipped with new medical technologies are needed owing to the increase in patients and that maintenance costs will increase as medical institutions introduce new medical devices and replace the medical devices that have reached the end of their life cycle.67 Improper maintenance of medical devices can affect their performance and patient safety, significantly impacting the expenses of medical institutions. In particular, if a medical institution is unable to secure manpower to manage medical devices, its failure to monitor the performance and safety levels of medical devices may reduce the availability of the medical devices, which can increase maintenance costs. Because this study was conducted in tertiary hospitals in Korea, manpower and resources were secured for the maintenance of medical devices; therefore, cost factors did not appear to be a major factor in medical device replacement. Such fact is largely due to prioritization of large university tertiary hospitals against prefectoral and municipal hospitals positioned much lower within the national health system hierarchy.68 This is clearly visible in terms of prioritization of technical, financial and human resource allocation.69 Overall, the lack of maintenance of medical devices may reduce their reliability. Therefore, it is necessary to establish a medical device maintenance plan that focuses on clinical factors to ultimately manage patient safety and the operating costs of medical institutions. In this study, we derived important indicators that can be used as a reference in decisions related to replacing medical devices, and a more efficient and systematic plan to replace medical devices, by considering the factors prioritized by the occupation of medical workers, will be prepared in the future.
In order to better understand consequences of the global medical device replacement landscape it is also notably important to understand vast scale demand and supply of medical devices imposed by the leading BRICs Emerging Markets.70 Given the fact that these economies are driving world’s real GDP growth for almost three decades, they do shape medical device manufacturing and supply routes throughout Asia.71,72 The same case although with certain lag in development stage is visible among other vast Emerging Markets such as Indonesia, Pakistan, Mexico, Nigeria.73 Their ability to increase investment in healthcare is substantial, unlike rather stable health and pharmaceutical expenditures in wealthy OECD markets.74 Such regional and global disparities also drive increasing demand for medical imaging, robotics assisted surgery, oncology related radiotherapy because of the fact of rapid growth of the middle citizen class in these LMICs nations.75 These layers of population count in hundreds of millions and experience sudden rise in overall purchasing power which inevitably transfers to increasing demand for technologically advanced medical care. Another more neglected driver of rapidly growing demand is also accelerated population aging with Korean, Chinese and Japanese societies all finding themselves in advanced stage of third demographic transition.76,77 The “Silver Cunami” reflects to the gradual extinction of traditional Asian family caregiving which was very much feasible in the high fertility era preceeding two world wars.78 Nowadays in single child families, and widespread burden of dementia, the demand for home-born robotics and artificial intelligence assisted medical care is likely to exponentially grow further.79 Some health expenditure forecasts and ongoing AI projections implicate the necessity to prepare for a profound transformation of contemporary societies in decades to come.76
Conclusion
The limitations of this study were as follows. First, medical devices were not divided by class to determine the reasons for medical device replacement. This study analyzed all medical devices used in medical institutions. Medical devices, classified from 1st–4th grades based on potential risks, may have different priorities for replacement depending on the purpose of use and the degree of risk to the human body. Future studies should derive replacement priorities based on the classification of medical devices in order to establish priorities according to the types of medical devices. Second, the classification of the participants as medical device users, providers, patients, and others was not considered. Medical device ecosystems can be broadly divided into medical institutions, medical device companies, and patients. The priorities for medical device replacement may vary according to the characteristics of the groups, including medical device companies that provide medical devices, medical institutions that use medical devices, and patients. Third, there is a need for an analysis according to the type of medical institution. The medical devices used, number of patients, and manpower of medical workers differ depending on the classification of medical institutions into 1st, 2nd, and 3rd institutions, therefore, factors that must be considered when replacing medical devices may vary. Additionally, this study was limited to data from Korean medical institutions, which may pose limitations in generalizing the findings to other regions. Including international data would help assess the applicability of medical device replacement priorities in various regional contexts. In particular, applying replacement priority methods tailored to regional characteristics could provide optimized replacement strategies for medical institutions in each region. A more systematic replacement plan can be developed through a detailed analysis of factors such as medical device grades, medical device users, and types of medical institutions.
There are various reasons for replacing medical devices, but a replacement must be based on an appropriate evaluation plan by each medical institution. Therefore, it is possible to establish a practical replacement program by utilizing different replacement factors for each occupation, focusing on clinical factors. Ultimately, integrating a user-centered approach when determining the priority for medical device replacement is crucial. This study identifies clinical factors as the most important element in determining replacement priorities and emphasizes the direct impact on patient safety when a device is no longer functional or becomes obsolete. This approach suggests that healthcare institutions should prioritize replacing clinically important devices, regardless of cost or technical specifications. Specifically, this study derives a wide range of medical device replacement priorities within healthcare institutions, enabling the management of the device lifecycle from a clinical perspective. Therefore, by establishing appropriate repair and replacement policies for not only currently used medical devices but also digital health and IoT-based medical devices, healthcare institutions can minimize operational costs and contribute to extending the lifespan of devices. Furthermore, applying this replacement strategy to healthcare institutions in countries with a healthcare ecosystem similar to that of Korea could improve the efficiency of medical device replacement.
Abbreviations
CT, Computed Tomography; P.E.T, Positron Emission Tomography; MRI, Magnetic Resonance Imaging; OECD, Organization for Economic Co-operation and Development; AHA, American Hospital Association; AHP, Analytic hierarchy process; C.I, Consistency Indicator; C.R, Consistency Ratio; MCDM, Multi-criteria decision-making model; MERS, Medical equipment replacement score; R.I, Random Index.
Acknowledgments
Sewon Park and Gihong Seo are co-first authors for this study. Haengjun Lee and Munjae Lee are co-correspondence authors for this study. This work was supported by the Ministry of Education of the Republic of Korea and the National Research Foundation of Korea (NRF-2023S1A3A2A05095298), and by the Bio-convergence Technology Education Programme through the Korea Institute for Advancement of Technology (KIAT), funded by the Ministry of Trade, Industry and Energy (No. P0017805).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Park S, Kim H-K, Lee H-J, Choi M, Lee M, Jakovljevic M. Strategic management and organizational culture of medical device companies in relation to corporate performance. J Med Economics. 2023;26(1):781–792. doi:10.1080/13696998.2023.2224168
2. Jakovljevic M, Chang H, Pan J, et al. Successes and challenges of China’s health care reform: a four-decade perspective spanning 1985—2023. Cost Eff Resour Allocation. 2023;21(1):59. doi:10.1186/s12962-023-00461-9
3. Sapkota B, Palaian S, Shrestha S, Ozaki A, Mohamed Ibrahim MI, Jakovljevic M. Gap analysis in manufacturing, innovation and marketing of medical devices in the Asia-Pacific region. Expert Rev Pharmacoecon Outcomes Res. 2022;22(7):1043–1050. doi:10.1080/14737167.2022.2086122
4. Jakovljevic M, Wu W, Merrick J, Cerda A, Varjacic M, Sugahara T. Asian innovation in pharmaceutical and medical device industry–beyond tomorrow. J Med Economics. 2021;24(sup1):42–50. doi:10.1080/13696998.2021.2013675
5. Martella M, Lenzi J, Gianino MM. Diagnostic technology: trends of use and availability in a 10-year period (2011–2020) among sixteen OECD countries. MDPI. 2023;11:2078.
6. An in-depth study of South Koreas diagnostic imaging equipment market landscape.
7. Sittig DF, Wright A, Coiera E, et al. Current challenges in health information technology–related patient safety. Health Informatics J. 2020;26(1):181–189. doi:10.1177/1460458218814893
8. Singh K, Selvam P. Medical device risk management. Trends Development Med Devices. 2020;65–76.
9. Safety MoFaD. Guidelines for risk management and preparation of risk analysis and management summary in international standardization documents for medical devices. Med Device Eval Department Korea Food Drug Administration. 2014.
10. Park H-J. Enhancement of Risk Management Traceability of Medical Devices and Their Management. Ajou University Graduate School; 2019.
11. Verheyen P. Economic interpretation of models for the replacement of machines. Eur J Oper Res. 1979;3(2):150–156. doi:10.1016/0377-2217(79)90101-2
12. Kronenfeld M, Stephenson PL, Nail-Chiwetalu B, et al. Review for librarians of evidence-based practice in nursing and the allied health professions in the United States. J Med Lib Assoc. 2007;95(4):394. doi:10.3163/1536-5050.95.4.394
13. Seo G, Park S, Lee M. How to calculate the life cycle of high-risk medical devices for patient safety. Front Public Health. 2022;10:989320. doi:10.3389/fpubh.2022.989320
14. Jakovljevic M, Liu Y, Cerda A, et al. The Global South political economy of health financing and spending landscape–history and presence. J Med Economics. 2021;24(sup1):25–33. doi:10.1080/13696998.2021.2007691
15. Taylor K, Jackson S. A medical equipment replacement score system. J Clin Engineering. 2005;30(1):37–41. doi:10.1097/00004669-200501000-00046
16. Faisal M, Sharawi A. Prioritize medical equipment replacement using analytical hierarchy process. IOSR J Electric Electronics Engineering. 2015;10(3):55–63.
17. Liao H-Y, Cade W, Behdad S. Markov chain optimization of repair and replacement decisions of medical equipment. Resour Conserv Recycl. 2021;171:105609. doi:10.1016/j.resconrec.2021.105609
18. Abd Rahman NH, Ibrahim AK, Hasikin K, Abd Razak NA. Critical device reliability assessment in healthcare services. J Healthcare Engineering. 2023;2023(1):3136511. doi:10.1155/2023/3136511
19. Huang L, Lv W, Huang Q, et al. Transforming medical equipment management in digital public health: a decision-making model for medical equipment replacement. Front Med. 2024;10:1239795. doi:10.3389/fmed.2023.1239795
20. Vokinger KN, Hwang TJ, Kesselheim AS, et al. Lifecycle regulation and evaluation of artificial intelligence and machine learning-based medical devices. 2022.
21. Petrou S, Jakovljevic M. Reimagining the relationship between economics and health–WHO ‘Health for all’provisions. Cost Eff Resour Allocation. 2024;22(1):5. doi:10.1186/s12962-024-00512-9
22. Wang J, Xu DR, Zhang Y, et al. Development of the China’s list of ambulatory care sensitive conditions (ACSCs): a study protocol. Global Health Res Policy. 2024;9(1):11. doi:10.1186/s41256-024-00350-5
23. Lee WJ, Choi Y, Ko S, et al. Projected lifetime cancer risks from occupational radiation exposure among diagnostic medical radiation workers in South Korea. BMC Cancer. 2018;18(1):1–10. doi:10.1186/s12885-018-5107-x
24. Oh Y. Prospective supply and demand of medical technologists in Korea through 2030. Korean J Clin Laboratory Sci. 2018;50(4):511–524. doi:10.15324/kjcls.2018.50.4.511
25. Jung YA, Kim YJ. Comparative study of ISO standards for an effective implementation of the domestic medical device GMP system. J Korean Soc Quality Manag. 2018;46(2):211–224.
26. Riess A, Lepmets M, McKechnie S, Walker A. Verification of the Effectiveness of Risk Management in the Medical Device Industry. Springer; 2018:380–386.
27. Chan T, Tong RK. ISO 14971: application of risk management to medical devices. Med Regulatory Affairs. 2022;191.
28. Luthra G, Sharma A. Importance of risk communication and risk analysis in medical device industry. J Pharm Res Int. 2021;33(31A):32–41.
29. Orleoglo A, Ciobanu N. Risk management in medicinal products and medical device manufacturing. 2020.
30. Sharma A, Luthra G. A comprehensive review of risk management in the medical device industry. J Pharm Res Int. 2023;35(6):14–23. doi:10.9734/jpri/2023/v35i67330
31. Knag IK. ISO14971:2019 detailed analysis and post market surveillance application method - focusing on IVDR requirements. J Biomed Engineering Res. 2022;43(4):199–213.
32. Odaibo SG. Risk management of AI/ML software as a medical device (SaMD): on ISO 14971 and related standards and guidances. arXiv preprint arXiv:210907905. 2021.
33. Bills E, Mastrangelo S, Wu F. Documenting medical device risk management through the risk traceability summary. Biomed Instrumentation Technol. 2015;49(s1):26–33. doi:10.2345/0899-8205-49.s1.26
34. Geremia F. Quality aspects for medical devices, quality system and certification process. Microchem J. 2018;136:300–306. doi:10.1016/j.microc.2017.04.018
35. Wehde M. System design approach to medical device development. IEEE. 2020;1–3.
36. Das M, Vogt-Ardatjew R, van den Berg B, Leferink F. Risk management plan for the hospital environment. IEEE. 2022;828–833.
37. Schaffarczyk D. Regulatory Affairs in device development–how to design medical devices capable to enter the market. Principles Biomed Sci Industry. 2022;203–223.
38. Han kwang-hee KH-S, Juyoung L, Seung-ki R. Estimating a lifetime of ITS devices. Estimating a lifetime of ITS devices. The Korea Institute Intelligent Transport Systems. 2009;2009(1):423–426.
39. Kotler P, Turner RE. Marketing Management: Analysis, Planning, and Control. Prentice-Hall Canada; 1979.
40. Seo Gi-hong PE-K, Dong-il C. Development of simple evaluation method for determining the priority of medical device replacement in hospitals. J Biomed Engineering Res. 2020;41(6):256–263.
41. Kim D. A study on public procurement law in response to COVID-19. Public Law. 2021;49(3):295–323. doi:10.38176/PublicLaw.2021.02.49.3.295
42. Unger SR, Hottle TA, Hobbs SR, et al. Do single-use medical devices containing biopolymers reduce the environmental impacts of surgical procedures compared with their plastic equivalents? J Health Services Res Policy. 2017;22(4):218–225. doi:10.1177/1355819617705683
43. Wu W, Zhang P, Zhu D, Jiang X, Jakovljevic M. Environmental pollution liability insurance of health risk and corporate environmental performance: evidence from China. Front Public Health. 2022;10:897386. doi:10.3389/fpubh.2022.897386
44. MacNeill AJ, Hopf H, Khanuja A, et al. Transforming the medical device industry: road map to a circular economy: study examines a medical device industry transformation. Health Affairs. 2020;39(12):2088–2097. doi:10.1377/hlthaff.2020.01118
45. Shao M, Jin H, Tsai F-S, Jakovljevic M. How fast are the Asian countries progressing toward green economy? Implications for public health. Front Public Health. 2022;9:753338. doi:10.3389/fpubh.2021.753338
46. Association AH. Estimated useful lives of depreciable hospital assets, revised 2013 edition. 2004.
47. Topfer L, Leseleuc L. Diagnostic imaging equipment replacement and upgrade in Canada. Ottawa. 2016;56:1–32.
48. Healy GM, Ahrari A, Alkhalifah F, et al. Typology, severity, and outcomes of adverse events related to angiographic equipment—a ten-year analysis of the FDA MAUDE database. Canad Associat Radiolog Jl. 2023;08465371231167990.
49. Fennigkoh L. A medical equipment replacement model. J Clin Engineering. 1992;17(1):43–47. doi:10.1097/00004669-199201000-00019
50. Taghipour S, Banjevic D, Jardine AK. Prioritization of medical equipment for maintenance decisions. J Oper Res Soc. 2011;62(9):1666–1687. doi:10.1057/jors.2010.106
51. Rajasekaran D. Development of an automated medical equipment replacement planning system in hospitals. IEEE. 2005;52–53.
52. Mummolo G, Ranieri L, Bevilacqua V, Galli P, Menolascina F, Padovano G. A fuzzy approach for medical equipment replacement planning. 2007;229–235.
53. Kim SY. Development of a quality competitiveness evaluation index for Korean medical device companies. Department of Medical Device Industry Graduate School of Dongguk University. 2018.
54. Park S, Kim H-K, Lee M. An analytic hierarchy process analysis for reinforcing doctor–patient communication. BMC Primary Care. 2023;24(1):24. doi:10.1186/s12875-023-01972-3
55. Saaty TL. A scaling method for priorities in hierarchical structures. J math psychol. 1977;15(3):234–281. doi:10.1016/0022-2496(77)90033-5
56. Saaty TL. The analytic hierarchy process Mcgraw Hill, New York. Agricultural Economics Rev. 1980;70.
57. Amromanoh OA. How well are equipment replacement prioritization scores followed? WRHA as a case study. CMBES Proceedings. 2017;40.
58. Capuano M. Prioritizing equipment for replacement: a plan based on data not perception. Biomed Instrumentation Technol. 2010;44(2):100–109. doi:10.2345/0899-8205-44.2.100
59. Dondelinger RM. A complex method of equipment replacement planning: an advanced plan for the replacement of medical equipment. Biomed Instrumentation Technol. 2004;38(1):26–31. doi:10.2345/0899-8205(2004)38[26:ACMOER]2.0.CO;2
60. Carleton KL, Enderle J, Jensen K, Zhu Q. Development of an equipment replacement planning tool for the veterans administration. IEEE. 2007;275–276.
61. Ouda BK, Mohamed AS, Saleh NS. A simple quantitative model for replacement of medical equipment proposed to developing countries. IEEE. 2010;188–191.
62. Aczél J, Saaty TL. Procedures for synthesizing ratio judgements. J Math Psychol. 1983;27(1):93–102. doi:10.1016/0022-2496(83)90028-7
63. Jeong Y-H, Chae I-S, Yang I-S, Kim H-Y, Lee H-Y. Analysis of relative importance of key performance indicators for center for child-care foodservice management through analytic hierarchy process (AHP). Korean J Community Nutrition. 2013;18(2):154–164. doi:10.5720/kjcn.2013.18.2.154
64. Saaty TL. The analytic hierarchy process for decision making. 1999.
65. Aljamali NM, Almuhana WHY. Review on biomedical engineering and engineering technology in bio-medical devices. J Adv Electrical Devices. 2021;6(2):18–24.
66. Zamzam AH, Abdul Wahab AK, Azizan MM, Satapathy SC, Lai KW, Hasikin K. A systematic review of medical equipment reliability assessment in improving the quality of healthcare services. Front Public Health. 2021;9:753951. doi:10.3389/fpubh.2021.753951
67. Ciklacandir S, Isler Y. Priority assessment of procuring medical equipment in Turkish hospitals using input-weighted fuzzy logic architecture. Expert Syst Appl. 2023;213:119195. doi:10.1016/j.eswa.2022.119195
68. Jakovljevic MB. The key role of the leading emerging BRIC markets in the future of global health care. Experimental Applied Biomed Res. 2014;15(3):139–143.
69. Semenova Y, Lim L, Salpynov Z, Gaipov A, Jakovljevic M. Historical evolution of healthcare systems of post-soviet Russia, Belarus, Kazakhstan, Kyrgyzstan, Tajikistan, Turkmenistan, Uzbekistan, Armenia, and Azerbaijan: a scoping review. Heliyon. 2024;10(8):e29550. doi:10.1016/j.heliyon.2024.e29550
70. Jakovljevic M, Lamnisos D, Westerman R, Chattu VK, Cerda A. Future health spending forecast in leading emerging BRICS markets in 2030: health policy implications. Health Res Policy Systems. 2022;20(1):23. doi:10.1186/s12961-022-00822-5
71. Jakovljevic M, Sugahara T, Timofeyev Y, Rancic N. Predictors of (in) efficiencies of healthcare expenditure among the leading asian economies–comparison of OECD and non-OECD nations. Risk Manag Healthcare Policy. 2020;Volume 13:2261–2280. doi:10.2147/RMHP.S266386
72. Stiglitz JE. An agenda for sustainable and inclusive growth for emerging markets. J Policy Modeling. 2016;38(4):693–710. doi:10.1016/j.jpolmod.2016.05.012
73. Zhou D, Ahuru RR, Yan M, Osabohien R, Jakovljevic M. Influences of women empowerment indices on demand for childcare services: evidence from the Nigeria demographic and health surveys. Afr J Reproduct Health. 2023;27(10):65–80. doi:10.29063/ajrh2023/v27i10.6
74. Ollivaud P, Guillemette Y, Turner D. Links between weak investment and the slowdown in productivity and potential output growth across the OECD. OECD Economic Department Working Papers. 2016;(1304):
75. Jakovljevic M, Vukovic M, Chen CC, et al. Do health reforms impact cost consciousness of Health care professionals? Results from a nation-wide survey in the Balkans. Balkan Med J. 2016;33(1):8–17. doi:10.5152/balkanmedj.2015.15869
76. Jakovljevic MM, Netz Y, Buttigieg SC, Adany R, Laaser U, Varjacic M. Population aging and migration–history and UN forecasts in the EU-28 and its east and south near neighborhood–one century perspective 1950–2050. Globalization Health. 2018;14(1):1–6. doi:10.1186/s12992-018-0348-7
77. Hyun KR, Kang S, Lee S. Population aging and healthcare expenditure in Korea. Health Economics. 2016;25(10):1239–1251. doi:10.1002/hec.3209
78. Chan SW-C. Family caregiving in dementia: the Asian perspective of a global problem. Dementia Geriatric Cognit Disord. 2011;30(6):469–478. doi:10.1159/000322086
79. Qi J, Wu C, Yang L, Ni C, Liu Y. Artificial intelligence (AI) for home support interventions in dementia: a scoping review protocol. BMJ open. 2022;12(9):e062604. doi:10.1136/bmjopen-2022-062604
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

